Ahmed L. Osman1* , Abd Elgadir A. Altoum1
, Abd Elgadir A. Altoum1 , Devapriya Finney Shadroch1
, Devapriya Finney Shadroch1 Asaad MA. Babker1
Asaad MA. Babker1 , Hesham Elashmouny2
, Hesham Elashmouny2  , Nourhan Khaled Hassan2
, Nourhan Khaled Hassan2 , Rania Moataz El-Dahmy3
, Rania Moataz El-Dahmy3 , Mohamed Haider4,5
, Mohamed Haider4,5 and Ibrahim Elsayed2,6
and Ibrahim Elsayed2,6
1Department of Medical Laboratory Sciences, College of Health Sciences, Gulf Medical University. Ajman, United Arab Emirates
2Department of Pharmaceutical Sciences, College of Pharmacy, Gulf Medical University, Ajman, United Arab Emirates
3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, October 6 University, Cairo, Egypt
4Department of Pharmaceutics and Pharmaceutical Technology, College of Pharmacy, University of Sharjah, 27272, Sharjah, United Arab Emirates
5Research Institute of Medical and Health Sciences, University of Sharjah, 27272, Sharjah, United Arab Emirates
6Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt.
Corresponding Author E-mail: dr.ahmed@gmu.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2840
Abstract
Fluconazole is potent triazole drug used as effectively anti-fungal for treating a variety of local and systemic fungal infections. This drug is sparingly soluble in water. The objective of this research was to statistically optimize nanovesicular formulations contain fluconazole to improve its antifungal effect. The nanovesicular formulations were composed of Tween® 80, cetyl alcohol, and chitosan. The prepared nanovesicular formulations were investigated for their zeta potential, polydispersity index, particle size, and encapsulation efficiency. The nanovesicular that has been optimized formulation was consist of tween/cetyl alcohol ratio of 15:1 and 0.1% chitosan. Additionally, the optimized formulation increased significantly in fluconazole release ratio and extent in comparison to the suspension form of the drug. The find that fluconazole nanovesicles had a better effect and lower MIC when compared to the aqueous fluconazole suspension. Finally, the optimized nanovesicles can be considered a promising nanocarrier for delivery of fluconazole to increase its systemic antifungal efficacy.
Keywords
Antifungal Activity; Fluconazole; Central Composite Design; In Vitro Release; Nanovesicles
Download this article as:| Copy the following to cite this article: Osman A. L, Altoum A. E. A, Shadroch D. F, Babker A. M, Elashmouny H, Hassan N. K, El-Dahmy R. M, Haider M, Elsayed I. Evaluation of the Antifungal Activity of Fluconazole Nanovesicles Against Aspergillus fumigatus. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Osman A. L, Altoum A. E. A, Shadroch D. F, Babker A. M, Elashmouny H, Hassan N. K, El-Dahmy R. M, Haider M, Elsayed I. Evaluation of the Antifungal Activity of Fluconazole Nanovesicles Against Aspergillus fumigatus. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3T5XU2k |
Introduction
Rhinosinusitis occurs when the mucosa of the nasal and paranasal sinuses is inflamed 1. It may be infectious or non-infectious due to immunological or non-allergic causes. Infectious rhinosinusitis is caused by fungal pathogens such as Bipolaris, Curvularia, and Aspergillus species2. The most prevalent causative agent appears to be Aspergillus. The diagnosis is histological, based on allergic mucin exhibiting fungal components in Gomori’s methenamine silver staining method. The diagnosed Aspergillus fumigatus is tested by various methods to identify the minimum inhibitory concentration (MIC) of fluconazole3. One of the most common fungal isolates causing sinusitis is Aspergillus fumigatus. Surgical debridement followed by prolonged oral antifungal therapy results in chances of attaining renal impairment.
Fungal rhinosinusitis demands immediate attention, as its urgency necessitates swift initiation of both aggressive antifungal therapy and prompt surgical intervention to effectively address and mitigate the potentially severe consequences of this condition4.
Fluconazole is a triazole antifungal medication employed for the treatment of various local and systemic fungal infections5. It functions as an inhibitor of ergosterol, disrupting the integrity and fluidity of the fungal cell membrane, thereby impeding fungal growth 6. It exhibits limited solubility in water and boasts a remarkable 90% absolute oral bioavailability. The recommended oral dosage typically ranges from 50 to 400 mg, dependent on the specific pathological conditions7.
Nanovesicles are commonly utilized as carriers or targeting vehicles for active substances within various body organs and tissues 8. These nanovesicles prepared in different forms, including micelles, cubosomes, noisomes, liposomes, transferosomes, and ethosomes 9-11. All serve as a versatile platform for enhancing the drug characteristics such as stability, solubility, and release characteristics, and overall bioavailability 12. The nanovesicle has been fine-tuned to be compatible with multiple administration routes, include ocular, transdermal, oral, parenteral and nasal routes. Furthermore, specialized adaptations have been made to create vector oriented nanovesicle designed to target drugs to specific locations such as the colon, brain, liver, or tumors13.
This study was aiming to determine the appropriate range of nanovesicles loaded with fluconazole to be directly exposed to the ATCC strains of Aspergillus fumigatus. This is to estimate the MIC of the antifungal agent to determine the minimal dosage of the drug to be administered to the infected individual rescuing them from the renal impairments that could occur.
Material and Methods
Materials
Fluconazole was provided as a gift from Julphar (Gulf Pharmaceutical Industries), United Arab Emirates. Cetyl alcohol and tween 80 was purchased form Sigma–Aldrich, Co. (St. Lois, United States). Other chemical materials of the analytical grade used were not purified further.
Fluconazole Nanovesicles Preparation
A modified thin film hydration process was used to prepare the nanovesicles 14. In a nutshell, fluconazole, Tween 80, in addition to cetyl alcohol were precisely measured, dissolved in 2/1, v/v mixture of methanol and chloroform 10 mL, and placed in 250 mL flask round bottom. The rotary evaporator machine (Heidolph, Rotavapor, VV2000) was then employed under vacuum conditions, evaporating the organic solvent mixture at 60 ºC and 150 rpm for 20 minutes. The resulting thin film on the wall was hydrated with a 10 mL of the low molecular weight chitosan solution in 0.1 M acetic acid under normal pressure. To prevent aggregation, the prepared nano-vesicles underwent sonication in an ultrasonic bath from (SH 15O-41, PCl Analytic Pvt, Ltd) for a period of 1 minute 15.
Statistical Designing
The research employed central composite design with
Design Expert® 7 software (Version 7. Stat_Ease Inc, MN) to investigate the
impact of formulation variables on the characteristics of nanovesicles. Two
independent factors, namely Tween/Cetyl with ratio (

Characterization of the prepared fluconazole nanovesicle
Analysis of particle polydispersity index, zeta potential and the size
The Zeta sizer (Nano ZS, Malvern Instrument, Malvern, United Kingdom) employed dynamic scattering of light for analyzing the polydispersity index (PDI), zeta potential (ZP), and particle size (PS) of the nano vesicular formulations. Prior to analysis, dilution of samples from each formulation were performed to the point of haziness.
Assessment of the encapsulation effectiveness of the formulated fluconazole nanovesicle
Nano-vesicles loaded with fluconazole underwent centrifugation at 20,000 revolutions per minute for 1 hour at 40°C using a high-speed cool centrifuge (Andres Hetich Gmb.H and Co. KG, Tutlingen, Germany) to separate them from the un-encapsulated drug. The fluconazole concentration in the supernatant was determined by analyzing it with a UV-spectrophotometer (Shimadzu, Tokyo, Japan) according to previously established curve of calibration.
Drug release from nanovesicular formulations in an in-vitro setting
The release of the drug from the optimized nano vesicular formulation in addition to the drug suspensions were assessed utilizing the reverse dialysis method using a USP II dissolution machine (Pharm Test, Hainburg, Germany)17. A phosphate buffer (pH 6.6) of 900 mL served as the dissolution medium. Dialysis bags having a cut-off molecular weight of 12-14 kDa were filled with 3 mL of dissolution media. The speed of the rotation was set to 50 revolutions per minute, at 37 ± 1 oC. Samples were collected at specified time intervals up to 240 minutes, and the concentration of the drug was analyzed using spectrophotometer at the predetermined λ max. To statistically compare the release profiles of fluconazole from the nano-vesicular formulation and the drug suspension, the similarity factor (f2) was calculated.
Microbiology Test
Test organism
Aspergillus fumigatus ATCC 1022 reference strain were used.
Inoculum preparation
The test organisms’ inoculants were prepared in accordance with the instructions of the National Committee for Clinical Laboratory Standards (NCCLS) document M38-A 18. These cultures were cultivated on Potato Dextrose Agar (PDA) slants at a temperature of 35°C for a period of 7 days. For preparation of the conidial inoculants, the cultures were flooded with a sterile solution consisting of 0.85% normal saline and 0.025% Tween 20 (obtained from Sigma company), and slowly agitated using a tip of the pipette. Following this, the resulting suspension was subjected to vortexing, and the heavier particles were allowed to settle for a duration of 3 to 5 minutes. Subsequently, the supernatant was adjusted to achieve a transmission reading of 80 – 82 percent spectrophotometrically, with a wavelength set at 530 nm.
Broth Microdilution Method
Out of nine fluconazole nanovesicle prepared, one (F2) is tested against aqueous suspension of the drug. Both the aqueous suspension and nanovesicle forms of fluconazole were mixed with RPMI 1640 medium containing L-glutamine lacking bicarbonate. The pH of the mixture was adjusted to 7.0 by using (0.165 M.MOPS solution by Sigma Company). Serial dilution of the fluconazole were then prepared in nine microtiter plate wells, ranging from 1.0 to 0.2 mg/ml. Each well received 100 µl of the diluted fungal suspensions along with 100 µl of the fluconazole solution, resulting in a serial of diluted fluconazole concentrations. In order to ensure the accuracy of the experiment, each set was subjected to growth and sterile controls as part of the experimental procedure.
The microtiter plate was subsequently incubated at 35°C, and after 48 hours, they were examined to determine the Minimum Inhibitory Concentration (MIC). The MICs interpretation was the drug concentration at which 50% inhibition of growth was observed. Microscopic examination was used to determine the MICs, specifically identifying the lowest fluconazole concentration that led to morphological abnormality of the fungal hyphae characterized by short, numerous branching 19.
Agar dilution technique
Fluconazole was prepared in serial dilution in a molten medium that had been equilibrated to a temperature of 50°C. This medium consisted of RPMI 1640 with 2% glucose (sourced from Sigma Company) and 1.5% Agar. The purpose of this dilution was to create a series of drug concentrations. Subsequently, one milliliter of this mixture was dispensed into each well of a 12-well cell culture plate with a flat bottom and kept for solidification. In the middle of each well, 10 µl of the conidial suspension was introduced. To serve as a control, organism-free wells were included.
Minimal Inhibitory Concentrations (MICs) for mold fungi were examined after incubation for 48 h at a temperature of 35°C. The MICs were determined as the lowest drug concentration that effectively inhibits the fungal growth on the solid agar medium 20.
Galactomannan (GM) antigen release method
Alexander Imhof devised a Galactomannan antigen release method to assess the growth of the fungi independently of colony characteristics or growth density21. This approach was selected because literature had indicated that Galactomannan is released in the growing media in quantities that correlate with the fungal burden21. The microtiter plate was prepared using antifungal substances and fungal samples following the same procedure as the broth microdilution method described earlier. After incubating for 24 hours at a temperature of 35°C. From each well, 5 µl of the liquid was added to 5 ml of saline making overall dilution 1:1,000 ratio. The released GM was quantified using an enzyme immunoassay, specifically the GM Test Fungiopert Aspergillus Galactomannan ELISA Detection Kit, following the instructions of the manufacturer.
Next, 50 µl of these dilutions were transfer to microtiter plate wells that had been sensitized with EB-A2 monoclonal antibody, which targets Aspergillus Galactomannan. The microtiter plate was then placed in the incubator at 37°C for 90 minutes. Following this incubation, the microtiter plates washed, then 100 µl of a buffer solution contain ortho-phenylenediamine dihydro-chloride was added. The microtiter plates were placed in the incubator for an additional 30 minutes in the absence of light at room temperature, before 100 µl of a 1.5 M. sulfuric acid solution was added to halt the reaction. The reading of the optical density (OD) was taken at 450 nm.
The optical density index was determined by dividing the optical density of each sample by the optical density of a control sample that contain 1 ng of Galactomannan /ml. The rate between the Galactomannan indices in the samples and those in the controls were computed, and the concentration of the drug at which this ratio approached 0.5 is determined as a Minimum Inhibitory Concentration (MIC).
Results and Discussion:
Effect of formulations factors on particle size (PS)
The particle size measurements of the fluconazole nanovesicles prepared varied between 450 and 753. The equation for calculating the particle size analysis was:
particle size (PS) = 633.38 − 114.83 X1 + 1.67 X2 (1)
Figure 1A shows that only tween/cetyl ratio (X1) significantly affected the PS values of the prepared nanovesicular formulations. Where the PS values were significantly decreased with increasing the tween/cetyl ratio. This could be attributed to the effect of tween 80 in reducing the interfacial tension between the nanovesicular surface and the surrounding aqueous medium. Additionally, the prepared nanovesicles were stabilized and protected from aggregation by the steric hindrance of the used surfactant. These findings are consistent with that published by Elsayed et al., who investigated the effect of tween 80 concentration on the vesicle size of rosuvastatin calcium elastic nanovesicles 23.
The values of the particle size (PS), polydispersity index (PDI), zeta potential (ZP), and encapsulation efficiency (EE) of the prepared nanovesicular formulations is published in table 1 (part 1 of this project) by Ahmed et al 28.
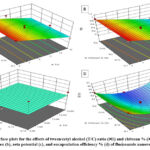 |
Figure 1: Response surface plots for the effects of tween/cetyl alcohol (T/C) ratio ( |
Effect of formulations factors on polydispersity index (PDI)
The polydispersity index (PDI) measurement of the prepared fluconazole nanovesicle varied from 0.152 to 1. As illustrated by Ahmed et al 28. The derived equation used for the PDI analysis was:
PDI = 0.66 − 0.33 X1 − 0.043 X2 (2)
Fig. 1B illustrates that only tween/cetyl ratio (X1) had a significant impact on the PDI values of the prepared nanovesicular formulations. Where the PDI values were significantly decreased with increasing the tween/cetyl ratio. This could be attributed to the efficiency of Tween 80 as a surfactant in preventing the aggregation of the prepared nanovesicles. Zambaux et al., and Ruiz et al., also assured that the increase of the surfactant concentration resulted in a considerable decrease in the PDI values 24, 25.
The PDI is used to make sure that there is no variation in particle size. The formula that has less variation in particle size will has less PDI.
Effect of formulations factors on zeta potential (ZP)
ZP for the prepared fluconazole nanovesicle varied from 7.25 to -2.46. As illustrated by Ahmed et al 27. The calculated equation for the zeta potential analysis was:
ZP = 0.6492 – 1.76 X1 – 1.49 X2 (3)
Figure 1C illustrates that both the
independent variables X1 and X2 had no
significant impact on the zeta potential of all
the prepared nanovesicular formulations.
In general, the zeta potential indicates the potential stability of the colloidal system in solution.
Effect of formulations factors on encapsulation efficiency (EE)
EE % for the prepared fluconazole nanovesicles ranged between 92.00 and 96.56 %. As illustrated by Ahmed et al 28. The calculated equation for the EE% analysis was:
EE = 92.54 – 0.753 X1 – 0.837 X2 + 0.164 X1 X2 + 1.57 X12 + 0.476 X22 (4)
Figure 1D illustrates that both the independent variables X1 and X2 had a significant impact on the EE% of all the prepared nanovesicular formulations. The EE% was decreased with increasing the tween/cetyl ratio. This could be accredited to that increasing tween concentration led to decreasing the vesicle sizes, hence decreasing the EE% due to the small inner space of the prepared vesicles. These findings are in consistency with that stated by Duong et al., who found that the EE% of the formulated vesicles were significantly decreased with increasing the surfactant concentration25. Additionally, the chitosan % (X2) significantly impacted the EE% of the nanovesicles. Where the EE% was significantly decreased with increasing the chitosan %. Increasing the chitosan concentrations could result in increasing the solution viscosity which could hinder the drug entrapment. This agrees with the findings stated by Valente et al., who found that the drug EE was significantly decreased with increasing the chitosan % 27.
Statistical analysis of central composite design
The effect of the studied formulation factors on the characteristics of the prepared nanovesicles was investigated using central composite design. Each response was investigated individually and fabricated using different order models. As displayed in Table 1, the predicted R2 values of all the examined responses were in harmony with the adjusted R2. A precision value higher than 4 was attained in all responses, assuring the suitability of the designed model to navigate the design space.
Selection of the optimized Fluconazole nanovesicular formulation
To identify the optimized nanovesicular formula, it was practically hard to provide all the needed responses at the same time., as the optimal condition met for one response may have a detrimental effect on another. However, the desirability function aggregated all desired responses in one variable to determine the optimum level of the studied factors. Figure 2 shows the highest desirability value was 0.681 for the optimized fluconazole nanovesicular formulation (F7) containing tween/cetyl ratio of 15:1 and chitosan concentration of 0.1%. This optimized formulation collectively showed the maximal PS and PDI and maximal ZP and EE%. According to the comparison of the observed and predicted values, a notable similarity was observed, as indicated in Table 1. Consequently, the nanovesicular formulation that was optimized has been selected for further investigation.
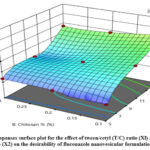 |
Figure 2: Responses surface plot for the effect of tween/cetyl (T/C) ratio (Xl) and chitosan % (X2) on the desirability of fluconazole nanovesicular formulation. |
Table 1: Output data of the central composite responses surface design and predicted and observed values of the optimized nanovesicular formula.
|
Responses |
PS (nm) |
PDI |
ZP (mV) |
EE (%) |
|
Minimum |
450.08 ± 7.66 |
0.152 ± 0.01 |
-2.46 ± 0.07 |
92.00 ± 1.02 |
|
Maximum |
753.15 ± 6.24 |
1 ± 0.00 |
7.25 ± 0.68 |
96.55 ± 2.64 |
|
Model |
Linear |
Linear |
Linear |
Quadratic |
|
P-value |
0.0003 |
< 0.0001 |
0.0697 |
0.0001 |
|
Adequate precision |
10.97 |
17.33 |
6.35 |
18.35 |
|
Adjusted R2 |
0.762 |
0.870 |
0.295 |
0.929 |
|
Predicted R2 |
0.604 |
0.795 |
-0.402 |
0.802 |
|
R2 |
0.802 |
0.892 |
0.412 |
0.959 |
|
Significant factors |
X1 |
X1 |
None |
X1, X2, X12, X22 |
|
Observed values of optimal formulation |
480.35 |
0.495 |
2.256 |
94.4262 |
|
Predicted values of optimal formulation |
516.885 |
0.376 |
2.380 |
94.505 |
Presented data mean ± SD (n = 3).
In vitro drug release from the optimized nano vesicular formulation
The optimized nano vesicular formulation significantly increased the Fluconazole release in comparison to the drug suspension. After 240 minutes, 57.14 % of the drug was released from the optimized formulation, while just 17.94 % of the drug was released from the drug suspension, as shown in Figure 3, with a similarity factor (f2) of 33. This considerable increase in fluconazole release from the optimized nanovesicular formulation could be due to the large surface area of the prepared nanovesicles and the incorporation of tween 80 that enhanced fluconazole diffusion from the prepared vesicles to the medium 23.
 |
Figure 3: Fluconazole release profiles from the optimized lipotomal formula compared to the drug suspension. |
Microbiology test
Fluconazole suspension and fluconazole nanovesicles MICs were determined by agar dilution, broth microdilution and GM antigen release against Aspergillus fumigatus ATCC 1022. Aspergillus fumigatus showed resistance to all concentrations ofregular fluconazole by using the three methods.
The MIC obtained by broth microdilution were similar to the GM antigen release (0.5 mg/ml) as shown Figure 6. On the other hand, MIC obtained by agar dilution method show little higher (0.6 mg/ml) compared to the other two methods Figure 4.
The result of the broth microdilution method was confirmed by examining the microtiter plate under microscope x40 HPF, sign of growth was observed (hyphal conidia and conidiophores) in wells containing 0.2, 0.3, and 0.4 mg/ml Figure 5.
 |
Figure 4: Agar dilution technique. Well No.1 control negative (No organism), wells from 2 to 10 fluconazole serial dilution concentration 1 to 0.2 mg/ml. |
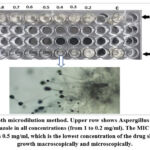 |
Figure 5: Broth microdilution method. Upper row shows Aspergillus resistance to normal fluconazole in all concentrations (from 1 to 0.2 mg/ml). |
 |
Figure 6: Range of aqueous and fluconazole nanovesicles MIC using different methods, organism is resistant to normal fluconazole, or the MIS is >1 mg/ml (range used 1 – 0.2). |
Conclusions
In this study, the optimized fluconazole nanovesicular formulation and the appropriate concentration of fluconazole nanovesicles was identified to be directly exposed to the ATCC strains of Aspergillus fumigatus. This is to determine the minimal dosage of the drug to be administered by the infected individuals rescuing them from the renal impairments that could occur. Finally, fluconazole nanovesicles had a better effect and lower MIC when compared to the aqueous fluconazole suspension. Considering these results obtained, the enhanced antifungal effect of fluconazole can be attributed to the promising nanocarriers provided by the optimized nanovesicular formulation.
Acknowledgement
None to declare
Conflict of Interest
All authors declared that there is no conflict of interest in this research.
Funding Source
This research is funded by Gulf Medical University, Ajman, UAE, grant number is GMU/COHS/GR/2019-10/003
References
- Greiner, A. N., Hellings, P. W., Rotiroti, G., & Scadding, G. K. J. T. L. Allergic rhinitis., 2011; 378(9809), 2112-2122.
CrossRef - Ciecko, S. C., Scher, R. J. E. E., Nose, & Journal, T. Invasive fungal rhinitis caused by Paecilomyces lilacinus infection: Report of a case and a novel treatment., 2010; 89(12).
- Wallace, D. V., Dykewicz, M. S., Bernstein, D. I., Blessing-Moore, J., Cox, L., Khan, D. A., immunology, c. The diagnosis and management of rhinitis: an updated practice parameter., 2008; 122(2), S1-S84.
CrossRef - Piromchai P, Thanaviratananich S. Impact of treatment time on the survival of patients suffering from invasive fungal rhinosinusitis. Clin Med Insights Ear Nose Throat. 2014; 9;7:31-4. doi: 10.4137/CMENT.S18875. PMID: 25288891; PMCID: PMC4167318.
CrossRef - Soliman, O. A. E.-A., Mohamed, E. A., Khatera, N. A. A. J. P. D., & Technology. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro–in vivo evaluation., 2019; 24(1), 48-62.
CrossRef - Cardoso NN, Alviano CS, Blank AF, Romanos MT, Fonseca BB, Rozental S, Rodrigues IA, Alviano DS. Synergism Effect of the Essential Oil from Ocimum basilicum var. Maria Bonita and Its Major Components with Fluconazole and Its Influence on Ergosterol Biosynthesis. Evid Based Complement Alternat Med. 2016;2016:5647182.
CrossRef - Moffat, A. C., Osselton, M. D., Widdop, B., & Watts, J. Clarke’s analysis of drugs and poisons., 2011 (Vol. 3): Pharmaceutical press London.
- Elsaied, E. H., Dawaba, H. M., Ibrahim, E., & Afouna, M. I. J. U. J. o. P. R. Effect of pegylated edge activator on Span 60 based nanovesicles: comparison between Myrj 52 and Myrj., 2019; 59. 4(4), 1-8.
CrossRef - Costa, R., & Santos, L. J. P. T. Delivery systems for cosmetics-From manufacturing to the skin of natural antioxidants., 2017; 322, 402-416.
CrossRef - Javadzadeh, Y., & Bahari, L. A. Therapeutic nanostructures for dermal and transdermal drug delivery. In Nano-and Microscale Drug Delivery Systems., 2017; (pp. 131-146): Elsevier.
CrossRef - Mathur, M., & Devi, V. K. J. J. o. D. T. Potential of novel drug delivery systems in the management of topical candidiasis., 2017; 25(8), 685-703.
CrossRef - Indulkar, A. S., Mo, H., Gao, Y., Raina, S. A., Zhang, G. G., & Taylor, L. S. J. P. r. Impact of micellar surfactant on supersaturation and insight into solubilization mechanisms in supersaturated solutions of atazanavir., 2017; 34(6), 1276-1295.
CrossRef - Hsu, C.-Y., Chen, C.-H., Aljuffali, I. A., Dai, Y.-S., & Fang, J.-Y. J. N. Nanovesicle delivery to the liver via retinol binding protein and platelet-derived growth factor receptors: how targeting ligands affect biodistribution., 2017; 12(4), 317-331.
CrossRef - Abdel-Hafez SM, Hathout RM, Sammour OA. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surfaces B Biointerfaces. 2018;167:63–72.
CrossRef - Xu Y, Zhang X, Zhang Y, Ye J, Wang H-L, Xia X, et al. Mechanisms of deformable nanovesicles based on insulin-phospholipid complex for enhancing buccal delivery of insulin. Int J Nanomedicine. 2018;13:7319.
CrossRef - Elsayed I, El-Dahmy RM, Elshafeey AH, El Gawad A, Abdelaziz N, Gazayerly E, et al. Tripling the Bioavailability of Rosuvastatin Calcium Through Development and Optimization of an In-Situ Forming Nanovesicular System. Pharmaceutics. 2019;11(6):275.
CrossRef - Abdel-Messih HA, Ishak RAH, Geneidi AS, Mansour S. Tailoring novel soft nano-vesicles ‘Flexosomes’ for enhanced transdermal drug delivery: Optimization, characterization and comprehensive ex vivo–in vivo evaluation. Int J Pharm. 2019;560:101–115.
CrossRef - Wayne, P. J. C. d. M.-A. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard., 2022
- Arikan, S., Lozano-Chiu, M., Paetznick, V., Rex, J. H. J. A. A., & Chemotherapy. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates., 2001; 45(1), 327-330.
CrossRef - Therese, K., Bagyalakshmi, R., Madhavan, H., & Deepa, P. J. I. j. o. m. m. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates., 2006; 24(4), 273-279.
CrossRef - Alexander Imhof, S. Arunmozhi Balajee, and Kieren A. Marr, New Methods To Assess Susceptibilities of Aspergillus Isolates to Caspofungin, Journal Of Clinical Microbiology., 2003; p. 5683–5688.
CrossRef - R. M. Winn, A. Warris, T. G. Abrahmsen, and P. Gaustad, Abstr. 6th Congr. Eur. Confed. Med. Mycol. Soc., abstr. 2000; P3-016.
- Elsayed I, El-Dahmy RM, El-Emam SZ, Elshafeey AH, Abd El Gawad NA, El-Gazayerly ON. Response surface optimization of biocompatible elastic nanovesicles loaded with rosuvastatin calcium: enhanced bioavailability and anticancer efficacy. Drug Deliv Transl Res. 2020;10:1459–1475. doi.org/10.1007/s13346-020-00761-0.
CrossRef - Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E,
Alonso MJ, et al. Influence of experimental parameters on the
characteristics of poly (lactic acid) nanoparticles prepared by a
double emulsion method. J Control Release Elsevier. 1998;50:
31–40 - Ruiz CC, Hierrezuelo JM, Peula-García JM, Aguiar J. Interaction
between n-octyl-b-D-thioglucopyranoside and bovine serum albumin. Open Macromol J. 2008; 2: 6–18. - Duong V-A, Nguyen T-T-L, Maeng H-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules. 2020; 25(20):4781. https://doi.org/10.3390/molecules25204781.
CrossRef - Valente JFA, Pereira P, Sousa A, Queiroz JA, Sousa F. Effect of Plasmid DNA Size on Chitosan or Polyethyleneimine Polyplexes Formulation. Polymers. 2021; 13(5):793. https://doi.org/10.3390/polym13050793.
CrossRef - Ahmed Luay Osman1, Salah Eldin Omar Hussein, Iqra Nizam, Deepa Dilip, Mariam Mahamadou, Jood Al Herafi, Sana Gulroz, Ibrahim Elsayed, Abd Elgadir Elamin Eltom, Devapriya Finney, Praveen Kumar Kandakurti. An In Vitro Evaluation of Anti-fungal Activity of Different Nano forms of Fluconazole Against Candida albicans, Biomedical & Pharmacology Journal., 2023; 16(3), 1421-1430.
CrossRef







