Jaafari Mohamed1* , Nadia Cherradi2
, Nadia Cherradi2 , Hafsa El ouazzani2
, Hafsa El ouazzani2 , Sanae ouadghiri3,4
, Sanae ouadghiri3,4 , Alae Koraichi5
, Alae Koraichi5 , Razine Rachid6,7
, Razine Rachid6,7 , Samia El hilali6,7
, Samia El hilali6,7 and Laila Hesseissen8
and Laila Hesseissen8
1Doctoral Study Center for Life and Health Sciences, Faculty of Medicine and Pharmacy of Rabat, Impasse Souissi, Rabat 10100, Morocco
2Pathology Department of Specialties Hospital –University Hospital of Rabat - Faculty of Medicine and Pharmacy-Mohammed V University in Rabat, Morocco
3Blood Transfusion, Immunology and Cell Therapy Department, Ibn Sina University Hospital, Rabat, Morocco.
4Department of Education and Research in Immunology, Faculty of Medicine and Pharmacy, Mohamed V University, Rabat, Morocco
5Department of Anesthesia and Intensive care in Children's Hospital of Rabat, Ibn Sina University Hospital, Rabat, Morocco.
6Laboratory of Biostatistics, Clinical, and Epidemiological Research, Department of Public Health, Faculty of Medicine and pharmacy-Mohamed V University in Rabat, Morocco.
7Laboratory of Community Health, Department of Public Health, Faculty of Medicine and Pharmacy Rabat, University Mohammed V, Rabat, Morocco.
8pediatric oncology and hematology department, Hopital d'enfant Rabat, Faculty of Medicine and pharmacy-Mohamed V University in Rabat, Morocco.
Corresponding Author E-mail: jaafarimohammed15@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2885
Abstract
Background: In Morocco, medulloblastoma (MB) is one of the most common malignant brain tumors in children. There is a deficiency of epidemiological information and typical characteristics of this pathology. Methods: In this retrospective study, we investigated 41 cases of MB diagnosed and treated at Rabat's University Hospital Ibn Sina from January 2010 until December 2019. Each patient underwent histological classification. Only 20 patients underwent molecular subgroups that were determined using immunohistochemistry (IHC) markers, including beta-catenin, GAB1, YAP1, and P53. Results: Histologically, there were only 3 instances with big cells or anaplastic types, 10 cases with nodular or desmoplasic cases, and 28 cases with classic types.. 6(30%) patients were in the nonWNT/nonSHH, 13(65%) patients and one patient(5%) were in SHH group and the WNT group respectively. After five years, patients who had radiation therapy had the highest overall survival rates (P=0.035). There was a statistically significant P value for the interval between surgery and radiation therapy. Patients who began treatment within 120 days had the highest overall survival rates when compared to those who began treatment later (P=0.002). Patients with metastases also had the lowest survival rates, according to our findings (P=0.001) Conclusions: The study provides epidemiological data about the childhood MB from a single institution in Morocco. In terms of overall survival rates, the results are quite promising. However, there is still a need to reduce the large gap between high and low-income countries and improve survival outcomes. To achieve this, expanding the network of the oncology centers is recommended as well as implementing twinning and telemedicine initiatives.
Keywords
Childhood; Medulloblastoma; NonWNT/nonSHH; SHH; Survival rates; TP53; WNT
Download this article as:| Copy the following to cite this article: Mohamed J, Cherradi N, El-ouazzani H, Ouadghiri S, Koraichi A, Rachid R, El-hilali S, Hesseissen L. Clinical, Molecular Subgroups and Survival Rates Finding of Childhood Medulloblastoma: A Ten Years Moroccan Experience in Pediatric Hematology and Oncology Center. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Mohamed J, Cherradi N, El-ouazzani H, Ouadghiri S, Koraichi A, Rachid R, El-hilali S, Hesseissen L. Clinical, Molecular Subgroups and Survival Rates Finding of Childhood Medulloblastoma: A Ten Years Moroccan Experience in Pediatric Hematology and Oncology Center. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3PsYt5i |
Introduction
MB is one of the most common malignant brain tumors of childhood, representing 20% or more of all pediatric cancers 1–4. during the last few years, a huge improvement has been made in terms of overall survival rates, the median overall survival of all subgroups is estimated to be 70%5,6. Recent studies on MB have unveiled that stratifying risk according to molecular subtypes is more precise, providing valuable guidance for clinical treatment decisions and determining clinical prognosis 5,7,8. Four primary molecular subgroups of MB patients are categorized in the 2021 World Health Organization categorization of brain Tumors: WNT and SHH with wild-type TP53, SHH and mutant TP53, and non-WNT/non-SHH 9.
According to Ellison et al, the MB may be divided into Three primary subgroups (WNT, SHH, and nonWNT/nonSHH) using antibodies(beta-catenin, Gab1, and Yap1)10. The immunohistochemistry (IHC) is rapid way, economical, and feasible on Formalin-Fixed, Paraffin-Embedded (FFPE) tumor tissues and also gives results similar to those using DNA-methylation profiling in earlier studies 11,12. in addition, this technique is reliable, easily obtained, and commonly used for analyses of MB subgroup classification by many authors 10,12–14.
To date, no studies have been conducted in Morocco to perform molecular subgroups using IHC to classify pediatric patients with MB into the three main subgroups. Therefore, in this investigation, our purpose was to establish clinical and paraclinical profiles and use beta-catenin, GAB1, YAP1, and P53 antibodies to determine the molecular subgroups. Finally evaluating the survival rates of the Moroccan with MB.
Patients and Methods
Retrospectively, we conducted a cross-sectional analysis of health records from patients who were diagnosed and treated at Rabat’s University Hospital Ibn Sina from January 2010 until December 2019. Only patients who were under 18 years old at the moment of diagnosis and had histological confirmation by the 2007 categorization of the World Health Organization (WHO)15 (figure 2) were included. From the health records, Follow-up data, demographics, clinical features, and paraclinical features were gathered.The patients were divided into two risk categories, namely standard and high. Patients without metastasis disease, residual tumor ≤1.5 cm2, classical or desmoplasic variant, and age >3 years were assigned to standard risk whereas those with Metastasis disease, large cell or anaplastic (LCA) variant, residual tumor > 1.5 cm2, and age ≤3 years were assigned to high-risk. In cases with molecular subgroups assessment, the tumors were classified into three main subgroups according to World Health Organization (WHO) classifications 201616. We were able to identify molecular subgroups By using the IHC technique. Beta-catenin, GAB1, YAP1, and P53 antibodies were applied to 5 μm sections of (FFPE) tumor tissues, and the three primary subgroups of MBwere categorized by different IHC staining to the antibodies, companies, dilutions and antibody sources for IHC studies are indicated in table 1 and figure 2.
Immunohistochemical analysis
Beta-catenin was considered as positive if The WNT subgroup exhibited nuclear staining in more than 5% of tumor cells. GAB1 was assessed as positive when cytoplasmic staining was detected in more than 10% of tumor cells and these cases were classified as SHH subgroup. YAP1 was assessed as positive when 10% or more of cytoplasmic or nuclear staining was detected, these cases were classified in the WNT or SHH subgroup. For P53 staining, If more than 50% of tumor cells exhibited strong nuclear staining, these cases were classified as the mutant type otherwise it was classified as a non-mutant (wild type). (figure 3)
Ethical statement
The research was carried out in compliance with the 2013 revision of the Declaration of Helsinki. The informed permission of all patients’ legal representatives was obtained, and the study was authorized by the ethics committee for biomedical research (CERB) of the Faculty of Medicine and Pharmacy Rabat. The serial number: AF 69/22
Statistical analysis
The quantitative variables were shown as medians with interquartile ranges or mean with standard deviations, but the qualitative factors were reported as percentages. Routine clinical and radiological exams were performed on those undergoing treatment, and statistical software was used to process the data. Statistical analysis was performed. The overall survival rates were calculated from the time of diagnosis until the final follow-up or the date of death. Using the Kaplan-Meier method, survival curves were produced, and the log-rank test was used to compare overall survival between patient subgroups. A P value of less than 0.05 was taken to be statistically significant.
Table 1: Antibodies pannel used for immunohistochemistry technique.
|
Antibodies |
Molecular subgroups |
Antibody sources |
dilution |
Companies |
immunoreactivity |
|
Beta-catenin |
WNT pathway |
Mouse monoclonal |
[1:200 – 1:500] |
GeneTex (GTX34339) |
Nuclear + cytoplasmic |
|
GAB1 |
SHH pathway |
Rabbit polyclonal |
[1:50 – 1:200] |
ABclonal(A6248) |
cytoplasmic |
|
YAP1 |
SHH and WNT pathway |
Mouse monoclonal |
[1:100 – 1:1000] |
GeneTex(GTX633541) |
Nuclear + cytoplasmic |
|
P53 |
Pronostic marker |
Mouse monoclonal |
[1:150] |
ORIGENE(OTI5E2) |
Nuclear |
Results
Clinical and paraclinical features
Eighty-two kids with MB were sent to the Ibn Sina University Hospital’s Pediatric Oncology and Hematology Department. 33 of them were eliminated for a variety of reasons from the study: Two were transferred to another hospital, six lacked medical records, eight died while receiving therapy, seven stopped receiving it, four were lost in the investigation, and six lacked histologic proof. And eight patients with tumor tissues not available. There were 41 patients with available data in all that were recruited. Patients with inaccurate IHC results were excluded from molecular subtype classification due to inadequate tissue for reliable classification. At First the initial biopsy yielded fragments with a limited representation of the tumor tissue furthermore the tissue sections from these cases exhibited features of extensive necrosis in addition the presence of potential artifacts may introduce bias or inaccuracies in molecular subgroups classification (Figure 1).
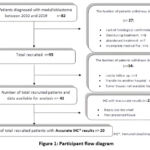 |
Figure 1: Participant flow diagram |
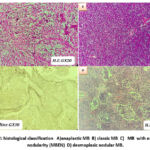 |
Figure 2: histological classification A) anaplastic MB B) classic MB C) MB with extensive nodularity (MBEN) D) desmoplasic nodular MB. |
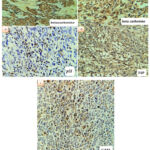 |
Figure 3: IHC staining E) nuclear and cytoplasmic positivity with beta-catenin antibody (in classic MB) F) cytoplasmic positivity with beta-catenin G) nuclear positivity with P53 antibody. |
There were forty-one children in the research, ages two to fifteen, with an average age of eight years. Thirty-three of the youngsters were older than three. With 22 males and 19 females in the research, the sex ratio (male/female) was 1.15, and a small preponderance of males was reported (53.7% vs 46.3%, respectively). The vermis was the most typical site for tumors. With almost 80.5% of cases versus 17.1 % in the cerebellar hemisphere. Hydrocephalus was reported in 26 cases. Among the histological type, there were 3 cases of large cell/anaplastic type, 10 cases of desmoplasic/nodular type, and 28 cases of the classic type. In our cohort, the MB with extensive nodular was not detected in any cases. Thirteen patients had recurrences. There were nine incidences of metastases at the time of diagnosis. Table 2 offers a summary of further findings.
Table 2: Clinical and paraclinical features. (The data are displayed as median [interquartile] or n (%)).
|
Characteristics |
Values (N=41) |
|
Sexe
· Male · Female
Sex-ratio(M/F)
|
22 (53.7) 19 (46.3)
1.15 |
|
Age (years) |
8 [6 – 10.50] |
|
Interval age : · ≤3 years · >3 years
|
4 (9.8) 37 (90.2)
|
|
Hydrocephalus : · Yes · No
|
26 (63.4) 15 (36.6) |
|
Tumor location : · Vermis · Cerebellar hemisphere · No data
|
33(80.5) 7(17.1) 1 (2.4) |
|
Metastasis disease · M+ · M0
|
22 (53.7) 19 (46.3) |
|
Histological type : · Classic · Nodular/desmoplasic /MBEN · Anaplasic/Large cell (LCA) |
28 (68.3) 10(24.4) 3(7.3) |
|
surgery : · Complete resection · Incomplete resection |
12 (29.3) 29 (70.7) |
|
Radiotherapy · Yes · no |
36 (87.8) 5 (12.2) |
|
Prognosis : · standard risk · high risk |
17 (41.5) 24 (58.5) |
|
Recurrence : · Yes · No |
13 (31.7) 28 (68.3) |
Clinicopathological characteristics and molecular subgroups
According to their molecular characteristics, 6(30%) patients were in the non-WNT/SHH, 13(65%) patients, and one patient(5%) were in the SHH group and the WNT group respectively. All cases of the SHH group were SHH wild-type (table 3). Histologically, desmoplastic/nodular/MBEN variants were observed in the SHH group, All classical variants were included in the nonWNT/nonSHH group.LCA variants were included in the SHH group Tumors, and metastasis disease were detected among 7(more than 50%) patients in (the SHH group) and four patients in the nonWNT/nonSHH group.Table 4 provides a summary of the outcomes.
Table 3: Molecular subgroups features (Data are presented as n (%))
|
Characteristics |
Values (N=20) |
|
Medulloblastoma subgroups : · Non-WNT/Non-SHH · SHH · WNT |
6(30) 13(65) 1(5) |
Table 4: Association between molecular subgroups and demographic, extent resection, metastasis disease, prognosis, and histological variant.
|
Characteristics |
WNT |
SHH |
Non-WNT/non-SHH |
Total (N=20) |
|
Sexe · Male · Female |
0 2 |
8 7 |
2 1 |
10 10 |
|
surgery : · incomplete resection · complete resection |
2 0 |
9 4 |
4 2 |
14 6 |
|
Metastasis disease: · M0 · M+ |
1 0 |
6 7 |
2 4 |
9 11 |
|
Histological type : · Classic · Nodular/desmoplasic/MBEN · Anaplasic/Large cell |
1 0 0 |
7 4 2 |
5 1 0 |
13 5 2 |
|
Prognosis : · Standard risk · High risk |
0 1 |
6 7 |
3 3 |
9 11 |
Survival analysis based on clinical and histo-molecular subgroups:
The follow-up period in this investigation was 44.48 months on average (range: 22 to 70 months). After three and five years, the overall survival (OS) rates were, respectively, 70% and 52% (Figure 4). Males had an OS rate of 42% after five years, but females had an OS rate of 63% (P=0.19). According to our research, patients who had radiation therapy outlived the other patients in terms of total five-year survival (50% versus 25%, P=0.035) (Figure 5). We found that there was no apparent difference in the overall survival rate in the total and the partial resection (58% and 50%, respectively, P=0.56). A statistically significant P was observed in the period from surgery to radiation If the interval was more than 120 days. It was found that patients who began radiation therapy before 120 days had the highest OS rates (65%), whereas patients who began therapy beyond 120 days had the lowest rates (28%; P=0.002) (Figure 5). According to the risk category, individuals at high risk had 5-year OS rates of 42% as opposed to 63% for patients at normal risk (P=0.077). Furthermore, we discovered that, patients who did not have metastases had an 80% of five-year OS, while individuals with metastases had the lowest survival rates (21%)(figure 6). Based on histology, the 5-year OS for the classic, anaplastic/large cell, and nodular/desmoplasic types was 53%, 0%, and 50%, respectively (P=0.029). OS rates were lowest for the anaplastic type (Figure 7). Table 5 displays all of the results.
Table 5: Survival based on sex, radiation, prognosis, metastasis, histology, and molecular subgroups. ( CI, confidence interval; RT, radiotherapy).
|
Category |
Os after 3 year (%) |
Os after 5 year (%) |
The Mean (months 95% CI) |
Log Rank test (Montel_cox) P |
|
sex · Male · Female |
60% 83% |
42% 63% |
[45.68 83.94] [61.90 96.89] |
P=0.19 |
|
histological type · Classic · Nodular/desmoplasic · Anaplasic/Large cell |
75% 80% 0% |
53% 50% 0% |
[59.52 95.80] [47.73 115.90] [12.28 35.72] |
P=0.029 |
|
Metastasis disease : · M0 · M+ |
86% 57% |
80% 21% |
[85.72 129.58] [31.94 59.03] |
P=0.001 |
|
Type of resection: · Complete · Incomplete |
75% 76% |
58% 50% |
[58.81 112.13] [57.62 97.58] |
P=0.56 |
|
Radiotherapy · yes · No |
77% 25% |
56% 25% |
[66.79 103.02] [5.67 51.32] |
P=0.035 |
|
The duration between RT & surgery · <120 days n=27 · >=120 days n=9 (patients didn’t undergo RT n=5) |
84% 55% |
65% 28% |
[81.47 121.97] [27.74 59.09] |
P=0.002 |
|
Molecular subgroups : (Patients with accurate IHC n=20) · WNT · SHH · Non-WNT/Non-SHH |
100% 53% 83% |
100% 36% 55% |
NO DATA NO DATA NO DATA |
P=0.38 |
|
Prognosis (Risk group) · High risk · Standard risk |
69% 100% |
42% 63% |
[41.75 71.58] [75.84 125.42] |
P=0.077 |
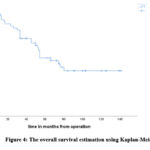 |
Figure 4: The overall survival estimation using Kaplan-Meier |
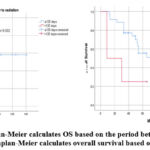 |
Figure 5: 1) Kaplan-Meier calculates OS based on the period between surgery and radiation. 2) Kaplan-Meier calculates overall survival based on the radiation. |
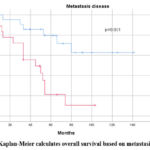 |
Figure 6: Kaplan-Meier calculates overall survival based on metastasis disease |
 |
Figure 7: Kaplan-Meier calculates overall survival based on molecular subgroup and histological type |
Discussion
The median age at the moment of diagnosistypically falls between 5 and 6 years in children 17–19, which is consistent with our results. In our series, the median age at the moment of diagnosis is 8 years, the age range is 2 to 15 years. MB demonstrates a notable male preponderance, a phenomenon extensively observed in various investigations, including the CBTRUS 2014 report, where the male-to-female sex ratio often surpasses 1.7 20–22 our study findings indeed corroborate this slight male predominance, with an incidence rate of 53.7% in male patients, resulting in a sex ratio of 1.15.
Age is one of the clinical criteria utilized to classify risk associated with MB. Infants and children diagnosed before the age of three appear to exhibit a less favorable prognosis, with reduced overall survival compared to younger patients23–25. Within our dataset, 5-year survival data indicates that the survival rate for children under 3 years of age does not exceed 50%. These findings may be attributed to the high malignancy of tumors, delayed diagnosis, and inadequate, or even the absence of, post-surgical irradiation. Nevertheless, It is pertinent to emphasize that a recent meta-analysis and a retrospective study in Brazil did not identify age as a significant prognostic factor 5,26.
Histologically, the desmoplastic/nodular type had the best mean survival (more than 80 months) (P=0.029), while the classic form was the most common (n=28). Our outcomes coincided with those of an international meta-analysis conducted by Rucktowski et al., the histological variant MBDN is an independent favorable prognostic factor, even in the presence of metastases27. Furthermore, the results published by Von Bueren et al. have shown a significantly higher OS as well as event-free survival rate after 5 years in patients with MBDN/MBEN treated exclusively with chemotherapy compared to other patients (respective values of p < 0.008 and p < 0.001.)17.
In our series, tumor resection did not appear to be associated with a better Os rate (p=0.56). There is controversy regarding the survival rate after total resection (GTR), subtotal resection (NTR) (tumor residue < 1.5 cm²), or partial resection (STR) (RT>1.5 cm²). Some authors have reported a correlation between total resection and a better survival rate, particularly demonstrating a benefit in terms of progression-free survival (PFS) for patients who underwent total resection (GTR) compared to those with partial resection (STR) p=0.0228.
In our series, radiotherapy appears to play an important role in improving survival. Indeed, the 5 years Os rate was significantly higher in patients who received chemotherapy and radiation combined compared to those who only received post-surgical chemotherapy (55.5% versus 25%) (p=0.035). These findings are consistent with previous results reported by Paulino29. Furthermore, the duration of radiotherapy (RT) is of paramount importance. Authors Back et al. observed a significant association between the duration of radiation and relapse-free survival (RFS) (p=0.049) 30.
The period between the initial radiation treatment and surgery is an important consideration in the management of MB. is advised that radiation begin 30 days after the surgery and not exceed 90 days 19. This recommendation is based on clinical evidence, including a subsequent study by Chin et al., which identified a decrease in overall survival after 5 years in patients who started radiotherapy early, within three weeks following surgery 31. Furthermore, Dietzsch et al. reported in a multicenter study that the time between radiation treatment and surgery is an independent prognostic factor. Patients who underwent radiotherapy with an interval exceeding 49 days had a lower rate of progression-free survival (PFS) compared to those with a shorter interval 32. However, authors showed a less favorable event-free survival (EFS) after 3 and 5 years for metastatic patients who started radiotherapy before 110 days compared to those with an interval exceeding 110 days (p=0.04) 33. In our study, we found that the interval between surgery and the initiation of radiotherapy had a statistically significant P value. Patients with an interval of less than 120 days had the Os after 5 years higher than those with an interval exceeding 120 days (p=0.002). This prolonged interval can be explained by several factors, including delays in patient management, Insufficient collaboration and communication among various healthcare professionals (surgical, radiological, and oncological), a high volume of patients scheduled for radiotherapy, insufficient patient information (irregular consultations and follow-up), challenging communication with parents (illiteracy, missed appointments), and logistical challenges related to rural settings, wide geographical distribution, and difficult access areas. The 5-year overall survival rates (21%) were impacted by the stage of metastasis, patients with metastasis disease had the lower OS after 5 years (21% versus 80% P=0.001). Multiple series reported similar outcomes33,34.
The majority of MB molecular subgroups were classified as SHH subtypes (13 cases), however other studies showed the nonWNT/nonSHH as the major type 8,28. histologically, all desmoplastic/nodular/MBEN variants were observed in the SHH group and the four cases of classic variants were among the nonWNT/nonSHH group which was consistent with other research10,12. the WNT group was observed in one patient which may be due to the small size of our cohort. Zhukova et al showed a significant difference in terms of five-year overall survival between the SHH group with and without tp53 mutations (81% +/- 5% and 41% +/-9% p<0.001 respectively)35. additionally, The majority of non-metastatic SHH patients are classified under the average/standard risk category, exhibiting a five-year overall survival rate exceeding 80% 8,20. In our data, the SHH group with tp53 mutation was not observed, therefore the five-year OS was lower by 45%, this may be due to the high frequency of metastatic disease in the SHH group (>50 %). the non-WNT/SHH subgroup displayed the most unfavorable outcome, primarily due to its resistance to therapy, as documented in previous studies36,37. moreover, it exhibited elevated rates of metastatic disease at the time of diagnosis 10. Wnt tumors contributed almost 14 % of all MB in these studies 10,38. The majority of WNT patients are typically located in the midline (vermian)8,14.they had long-term survival rates(>90%)10. as well Thompson et al published the results of WNT tumors with incomplete resection are considered as low risk 28. In our series, only one case of 19 patients was in the WNT group.
Limitation of the study
The study has a limited sample size and the results are from a single institution, moreover, we couldn’t identify P53 mutation by wild spread immunostain or by sequencing and other subgroups such as group 3 and group 4 (MYC amplification not available in the laboratory).
Conclusion
The study provides epidemiological data about childhood MB from a single institution in Morocco. In terms of overall survival rates, the results are quite promising. However, there is still a need to reduce the large gap between high and low-income countries and improve survival outcomes. To achieve this, expanding the network of the oncology centers is recommended as well as implementing twinning and telemedicine initiatives.
What is already known on this topic
Radiotherapy is a prognosis factor in MB management.
The metastasis disease impacts negatively the overall survival.
The desmoplasic/nodular/ MBEN variants had the best mean survival rates.
What this study adds
The overall survival rates of childhood MB in Morocco.
The duration between surgery and radiotherapy was significant.
The study provides clinical and paraclinical features of childhood MB.
Acknowledgements
All authors contributed to this study by making substantial contributions :
Conception: Mr Mohamed, Pr Hessissen, and Pr Cherradi Design: Pr ouazzani and Pr Alae. Acquisition data: Mr Mohamed Analysis and interpretation: Pr Razine and Dr El Hilali Drafting the manuscript: Mr Mohamed, Pr Hessissen, and Pr Cherradi Revising the manuscript: Pr Hessissen, Pr Cherradi, and Pr Oudghiri
Conflict of interest
All authors declare that they have no conflicts of interest
Funding Sources
there is no funding Sources
References
- Farwell, J. R., Dohrmann, G. J. & Flannery, J. T. Medulloblastoma in childhood: an epidemiological study. J. Neurosurg. 61, 657–664 (1984).
CrossRef - McKean-Cowdin, R. et al. Trends in childhood brain tumor incidence, 1973-2009. J. Neurooncol. 115, 153–160 (2013).
CrossRef - Ostrom, Q. T. et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncol. 16, x1–x36 (2015).
CrossRef - Pui, C.-H., Gajjar, A. J., Kane, J. R., Qaddoumi, I. A. & Pappo, A. S. Challenging issues in pediatric oncology. Nat. Rev. Clin. Oncol. 8, 540–549 (2011).
CrossRef - Liu, Y., Xiao, B., Li, S. & Liu, J. Risk Factors for Survival in Patients With Medulloblastoma: A Systematic Review and Meta-Analysis. Front. Oncol. 12, (2022).
CrossRef - Sursal, T. et al. Molecular Stratification of Medulloblastoma: Clinical Outcomes and Therapeutic Interventions. Anticancer Res. 42, 2225–2239 (2022).
CrossRef - Hennika, T. & Gururangan, S. Childhood medulloblastoma: current and future treatment strategies. Expert Opin. Orphan Drugs 3, 1299–1317 (2015).
CrossRef - Gajjar, A. et al. Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 39, 822–835 (2021).
CrossRef - Louis, D. N. et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 23, 1231–1251 (2021).
CrossRef - Ellison, D. W. et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. (Berl.) 121, 381–396 (2011).
CrossRef - Tauziède-Espariat, A. et al. Diagnostic Accuracy of a Reduced Immunohistochemical Panel in Medulloblastoma Molecular Subtyping, Correlated to DNA-methylation Analysis. Am. J. Surg. Pathol. 45, 558–566 (2021).
CrossRef - Taylor, M. D. et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. (Berl.) 123, 465–472 (2012).
CrossRef - Northcott, P. A., Korshunov, A., Pfister, S. M. & Taylor, M. D. The clinical implications of medulloblastoma subgroups. Nat. Rev. Neurol. 8, 340–351 (2012).
CrossRef - Kaur, K. et al. Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. Zurich Switz. 26, 334–343 (2016).
CrossRef - Louis, D. N. et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. (Berl.) 114, 97–109 (2007).
CrossRef - Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. (Berl.) 131, 803–820 (2016).
CrossRef - von Bueren, A. O. et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: Results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro-Oncol. 13, 669–679 (2011).
CrossRef - Padovani, L. et al. A common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int. J. Radiat. Oncol. Biol. Phys. 68, 433–440 (2007).
CrossRef - Taillandier, L. et al. Les médulloblastomes : revue générale. Rev. Neurol. (Paris) 167, 431–448 (2011).
CrossRef - Lannering, B. et al. Hyperfractionated Versus Conventional Radiotherapy Followed by Chemotherapy in Standard-Risk Medulloblastoma: Results From the Randomized Multicenter HIT-SIOP PNET 4 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30, 3187–93 (2012).
CrossRef - Zhang, Z.-Y. et al. Medulloblastoma in China: clinicopathologic analyses of SHH, WNT, and non-SHH/WNT molecular subgroups reveal different therapeutic responses to adjuvant chemotherapy. PloS One 9, e99490 (2014).
CrossRef - Vigneron, C. et al. [Pediatric medulloblastoma: Retrospective series of 52 patients]. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 20, 104–108 (2016).
- Rutkowski, S. et al. Treatment of Early Childhood Medulloblastoma by Postoperative Chemotherapy Alone. N. Engl. J. Med. 352, 978–986 (2005).
CrossRef - Johnston, D. L. et al. Medulloblastoma in children under the age of three years: a retrospective Canadian review. J. Neurooncol. 94, 51–56 (2009).
CrossRef - Zeltzer, P. M. et al. Metastasis Stage, Adjuvant Treatment, and Residual Tumor Are Prognostic Factors for Medulloblastoma in Children: Conclusions From the Children’s Cancer Group 921 Randomized Phase III Study. J. Clin. Oncol. 17, 832–832 (1999).
- Bleil, C. B., Bizzi, J. W. J., Bedin, A., de Oliveira, F. H. & Antunes, Á. C. M. Survival and prognostic factors in childhood medulloblastoma: A Brazilian single center experience from 1995 to 2016. Surg. Neurol. Int. 10, 120 (2019).
CrossRef - Rutkowski, S. et al. Survival and Prognostic Factors of Early Childhood Medulloblastoma: An International Meta-Analysis. J. Clin. Oncol. 28, 4961–4968 (2010).
CrossRef - Thompson, E. M. et al. Prognostic Value of Medulloblastoma Extent of Resection After Accounting for Molecular Subgroup: An Integrated Clinical and Molecular Analysis. Lancet Oncol. 17, 484–495 (2016).
CrossRef - Paulino, A. C. Current multimodality management of medulloblastoma. Curr. Probl. Cancer 26, 317–356 (2002).
CrossRef - Back, M. et al. Importance of radiation time and dose factors on outcome for childhood medulloblastoma*. Australas. Radiol. 49, 298–303 (2005).
CrossRef - Chin, A. L. et al. Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro-Oncol. 20, 1133–1141 (2018).
CrossRef - Dietzsch, S. et al. Evaluation of Prognostic Factors and Role of Participation in a Randomized Trial or a Prospective Registry in Pediatric and Adolescent Nonmetastatic Medulloblastoma – A Report From the HIT 2000 Trial. Adv. Radiat. Oncol. 5, 1158–1169 (2020).
CrossRef - Taylor, R. E. et al. Outcome for patients with metastatic (M2–3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur. J. Cancer 41, 727–734 (2005).
CrossRef - Zeltzer, P. M. et al. Metastasis Stage, Adjuvant Treatment, and Residual Tumor Are Prognostic Factors for Medulloblastoma in Children: Conclusions From the Children’s Cancer Group 921 Randomized Phase III Study. J. Clin. Oncol. 17, 832–832 (1999).
- Zhukova, N. et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 31, 2927–2935 (2013).
- Mynarek, M. et al. Nonmetastatic Medulloblastoma of Early Childhood: Results From the Prospective Clinical Trial HIT-2000 and An Extended Validation Cohort. J. Clin. Oncol. 38, 2028–2040 (2020).
CrossRef - Robinson, G. W. & Gajjar, A. Genomics Paves the Way for Better Infant Medulloblastoma Therapy. J. Clin. Oncol. 38, 2010–2013 (2020).
CrossRef - Schwalbe, E. C. et al. Rapid Diagnosis of Medulloblastoma Molecular Subgroups. Clin. Cancer Res. 17, 1883–1894 (2011).
CrossRef







