Manuscript accepted on :04-03-2024
Published online on: 14-03-2024
Plagiarism Check: Yes
Reviewed by: Dr. Pravinkumar Darji
Second Review by: Dr. Miss Zahraa Hussein Ali
Final Approval by: Dr. Anton R Kiselev
Mariia Shanaida1* , Olesia Palamar1 and Olena Holembiovska2
, Olesia Palamar1 and Olena Holembiovska2
1Department of Pharmacognosy and Medical Botany, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine.
2Department of Translational Medical Bioengineering, National Technical University of Ukraine “Igor Sikorsky Kyiv Polytechnic Institute”, v, Ukraine.
Corresponding Author E-mail: shanayda@tdmu.edu.ua
DOI : https://dx.doi.org/10.13005/bpj/2834
Abstract
During its triple extraction, the HPLC analysis revealed polyphenols' contents in the A. foeniculum herb. Several hydroxycinnamic acids (rosmarinic, chlorogenic, ferulic and caffeic) and flavonoids (apigenin, apigenin-7-O-glucoside, hyperoside, quercitrin, rutin and quercetin) were identified in the A. foeniculum herb. It was established that rosmarinic acid followed by apigenin-7-O-glucoside and apigenin were the predominant compounds of the A. foeniculum raw material. The content of rosmarinic acid as the major compound during the primary, secondary and tertiary extraction decreased in the following order: 37.563>15.435>0.642 (mg/g); the content of apigenin-7-O-glucoside was 24.508>9.107>0.945 (mg/g) and apigenin was 19.547>9.676>1.816 (mg/g), respectively. Generally, the third extraction was determined to be inefficient in terms terms of low content of polyphenols as well as excessive analysis time and solvent costs.
Keywords
Aerial part; Anise hyssop; High-performance liquid chromatography; Multiplicity of extraction; Polyphenols
Download this article as:| Copy the following to cite this article: Shanaida M, Palamar O, Holembiovska O. Chromatographic Profile of Polyphenols in the Agastache foeniculum (Pursh) Kuntze Herb: Evaluation of Optimal Extraction Efficiency. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Shanaida M, Palamar O, Holembiovska O. Chromatographic Profile of Polyphenols in the Agastache foeniculum (Pursh) Kuntze Herb: Evaluation of Optimal Extraction Efficiency. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/3va7Tvo |
Introduction
In the process of developing new medicines from herbal raw materials, it is important to determine the spectrum of major bioactive compounds. Scientists have proven a significant influence of genetic prerequisites (depending on the chosen subspecies, chemotype/variety, and age of the plant), climatic conditions and cultivation features on the accumulation of polyphenols in plants’ raw material1. Undoubtedly, the choice of the extraction method, type of solvent, the raw material-extractant ratio, time, temperature, rate, and multiplicity of extraction play key roles in the phytochemical analysis2.
The use of water-methanol and water-ethanol mixtures is quite effective due to their ability to extract both hydrophilic and hydrophobic compounds of a polyphenolic nature. Besides, these two solvents are the most compatible with the principles of green extraction among a whole range of organic solvents3. The pharmaceutical and food industries increasingly prefer green solvents (water, ethanol, deep eutectic solvents, etc.) for extraction due to their safety and recycling4,5.
It should be noted that the extremity of extraction of bioactive compounds from plant raw material can be associated with risks through various factors. Thus, the high extraction levels may elevate the concentration of active compounds, potentially increasing the risk of undesired effects or toxicity. However, the low extraction efficiency can result in inadequate isolation of beneficial compounds, limiting therapeutic potential and raising the risk of insufficient drug efficacy. Thus, achieving an optimal balance in extraction levels becomes crucial for minimizing risks and attaining the desired pharmacological effect of developed herbal substances6.
The Giant hyssop (Agastache Clayton ex Gronov, Lamiaceae Martinov family) genus comprises 22 species native mostly to North America, and only A. rugosa (Fisch. & C.A. Mey.) Kuntz) originates from East Asia7,8. Some Agastache species are used in traditional medicine as natural remedies against pain, bronchitis, hypertension, and gastrointestinal disorders 8,9. The Korean mint (A. rugosa) is the most studied species of this genus regarding its chemical composition and biological activity while other species of this genus (A. foeniculum (Pursh) Kuntze, A. mexicana (Kunth) Lint & Epling, etc.) have attracted much less attention of researchers in the area of pharmacognosy8,9. The biological activities of Agastache species are related mainly to the valuable compounds of their essential oils 7,8,10,11. Such groups of valuable secondary metabolites of the Agastache representatives as polyphenols or triterpenoids were investigated much less 7,8,12-15.
This study aimed to conduct the chromatographic analysis of polyphenols and determine the influence of extraction frequency on the efficiency of extracting hydroxycinnamic acids and flavonoids from raw material of anise hyssop (A. foeniculum) under its cultivation in Ukraine.
Materials and Methods
Plant material
The aerial part of A. foeniculum (the variety with white flowers) was harvested during the flowering period from the plots (Fig. 1, a) in the Ternopil region (Ukraine). The collected flowering shoots were cut into pieces up to 15 cm long and dried at 25–35°C. Dried raw materials (Fig. 1, b) were sifted through a sieve with a hole diameter of 2.5 mm before the extraction.
HPLC analysis
The chromatographic analysis of phenolic compounds was performed by the validated method of high-performance liquid chromatography (HPLC)16 in 70% ethanol extracts of the ground raw material. The triple extraction of raw materials was used. The raw material-solvent ratio was 1:10. The extractions were performed in an ultrasonic bath for 30 min each time (at 40°C).
A Shimadzu LC20 Prominence chromatograph with column Phenomenex Luna C18 (250 mm×4.6 mm with silica gel as sorbent) was used for HPLC analysis. The UV absorption spectra of the reference standards of polyphenols and the test samples were recorded in the range of 190–400 nm. The gradient elution was carried out with two solvents: 1) 0.1% aqueous solution of trifluoroacetic acid; 2) 0.1% solution of trifluoroacetic acid in acetonitrile17. The time of HPLC analysis was 60 min. The HPLC analysis was carried out in triplicate and results were expressed as mean value ± standard deviation.
 |
Figure 1: Appearance of Agastache foeniculum plants on the experimental plot (during flowering) (a) and dried raw material (b).
|
Results and Discussion
Several hydroxycinnamic acids (rosmarinic, ferulic, caffeic and chlorogenic) and flavonoids (apigenin, apigenin-7-O-glucoside, hyperoside, quercitrin, rutin, and quercetin) were revealed in the A. foeniculum herb by HPLC method (Table 1, Fig. 2-4).
It was established that the content of rosmarinic acid as the main identified dominant compound during the primary, secondary and tertiary extraction of the A. foeniculum raw material decreased in the following order: 37.563>15.435>0.642 (mg/g). Regarding the predominant flavonoids, the content of apigenin-7-O-glucoside was 24.508>9.107>0.945 (mg/g) and apigenin 19.547>9.676>1.816 (mg/g), respectively.
Table 1: The content of phenolic compounds in the Agastache foeniculum herb (HPLC analysis)
|
Compound |
Retention time, min |
Content, mg/g |
||
|
First extraction |
Second extraction |
Third extraction |
||
|
Chlorogenic acid |
19.9 |
2.124 |
0.935 |
0.096 |
|
Caffeic acid |
22.3 |
5.563 |
2.872 |
0.301 |
|
Rutin |
31.5 |
0.926 |
0.319 |
0.045 |
|
Ferulic acid |
32.3 |
1.296 |
0.456 |
0.060 |
|
Hyperoside |
32.8 |
9.713 |
5.483 |
0.432 |
|
Quercitrin |
34.9 |
0.469 |
0.128 |
<0.01 |
|
Rosmarinic acid |
37.8 |
37.563 |
15.435 |
0.642 |
|
Apigenin-7-O-glucoside |
38.2 |
24.508 |
9.107 |
0.846 |
|
Quercetin |
46.6 |
0.317 |
0.143 |
<0.01 |
|
Apigenin |
52.4 |
19.547 |
9.676 |
1.816 |
 |
Figure 2: HPLC chromatogram of hydroxycinnamic acids and flavonoids in the A. foeniculum herb after the first extraction (at 280 nm, 330 nm and 350 nm). |
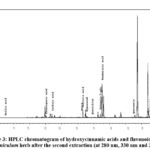 |
Figure 3: HPLC chromatogram of hydroxycinnamic acids and flavonoids in the A. foeniculum herb after the second extraction (at 280 nm, 330 nm and 350 nm) |
 |
Figure 4: HPLC chromatogram of hydroxycinnamic acids and flavonoids in the A. foeniculum herb after the third extraction (at 280 nm, 330 nm and 350 nm). |
Thus, it was revealed that during the secondary extraction of A. foeniculum raw material, a 2-3 times lower quantitative content of dominant polyphenols was extracted compared to the primary extraction. As for the tertiary extraction of plant raw materials, it was determined to be inefficient in terms of excessive time and solvent consumption because HPLC analysis showed that an order of magnitude less polyphenolic compounds are compared to primary extraction.
Rosmarinic acid, the predominant component of the studied A. foeniculum herb, possesses noticeable antioxidant, anti-inflammatory, antiviral, antimicrobial hepatoprotective, anti-nociceptive and immunomodulatory properties5,18-20. Apigenin and apigenin-7-O-glucoside as other predominant compounds of the investigated raw material demonstrate the prominent antioxidant, anti-inflammatory and anticancer effects21,22. As it is known, among a wide range of exogenous antioxidants, polyphenols are one of the most effective classes of compounds possessing antioxidant properties .
Numerous data of scientific literature regarding the polyphenolic profiles of the different Lamiaceae species demonstrated that rosmarinic acid is quite often their common major compound14, 23-30. Thus, the ultra-performance liquid chromatography analysis of methanolic extracts from A. rugosa roots revealed the predominance of rosmarinic acid among 24 identified polyphenols25. Its level was 3.82–9.16 mg/g, depending on the used in vitro culture system. The content of rosmarinic acid in the 70% ethanolic extract from A. foeniculum herb grown in Romania fluctuated in the range of 6.45-8.12 mg/g, depending on the harvesting period of the plant raw material26. The other study revealed that the content of rosmarinic acid in the ethanolic extract of Origanum vulgare (Lamiaceae) herb was 12.40 mg/g27. Rosmarinic acid (21.42 mg/g) was also the main predominant hydroxycinnamic acid of the Betonica peraucta (Lamiaceae) herb collected in Ukraine28. The experimental results showed that the content of rosmarinic acid in the aerial parts of several Lamiaceae species from different genera collected in Ukraine was in the ranges of 12.61–24.83 mg/g in the methanolic extracts obtained by maceration29,30 which is consistent with our data regarding A. foeniculum herb.
The experimental studies demonstrated that the concentrations of polyphenols were higher in the methanolic extract of A. rugosa compared to the ethanolic one24. The content of flavon genistein as the main predominant compound of A. rugosa was 3.17 mg/g in methanolic extract and 2.23 mg/g in ethanolic extract which is much less than in the A. foeniculum herb studied by us. Researchers found significant correlations between the total phenolic contents and the antioxidant activity of the studied extracts.
Recently, it was revealed by Korean researchers31 the high bioactive potential of rosmarinic acid and flavons tilianin and acacetin as key phenolic compounds of A. rugosa in humans and a Caco-2 cell model. Another validated bioanalytical method was developed for the simultaneous quantification of the dominated bioactive phenolic compounds (rosmarinic acid and flavons) from the A. rugosa aerial part in human plasma using UHPLC-MS/MS32. These clinical studies showed that the concentration of rosmarinic acid was the highest among other polyphenols in plasma when applied the dry extract obtained from theA. rugosa aerial part with 50 % ethanol. This technique offered the precise, accurate, and repeatable method for analyzing A. rugosa biocompounds in human plasma samples and detecting the analytes at very low concentrations (the lower limit of quantitation for both tilianin and rosmarinic acid was 0.5 ng/mL and 0.1 ng/mL for acacetin). The anti-inflammatory effects of flavones and two phenylpropanoid glucosides isolated from the A. rugosa aerial part were demonstrated in the in vitro studies using macrophages33.
Conclusion
The contents of hydroxycinnamic acids and flavonoids under the influence of triple extraction of the A. foeniculum herb were revealed using HPLC analysis. The main polyphenolic compounds in the A. foeniculum raw material during all stages of extraction were rosmarinic acid, apigenin-7-O-glucoside and apigenin. It was concluded that the third extraction was inefficient in terms of low content of polyphenols as well as excessive analysis time and solvent costs compared to the first and second extractions.
Acknowledgement
None to declare
Conflict of Interests
There were no commercial or financial links that may be deemed a potential conflict of interest during the research.
Funding Sources
The author(s) received no financial support for the research.
References
- Aćimović M, Šovljanski O, Pezo L, Travičić V, Tomić A, Zheljazkov VD, Zheljazkov VD, Ćetković G, Švarc-Gajić J, Brezo-Borjan T, Sofrenić I. Variability in biological activities of Satureja montana subsp. montana and subsp. variegata based on different extraction methods. Antibiotics. 2022; 11(9): 1235.
CrossRef - Zhang M, Bao X, Huang X, Li H, Li Y, Bao M. Optimization of the extraction strategy for polyphenols from Pieris japonica and evaluation of its antioxidant activity. Studies in Health Technology and Informatics. 2023; 23, 308: 11-19.
CrossRef - Stefan D-S, Popescu M, Luntraru C-M, Suciu A, Belcu M, Ionescu L-E, Popescu M, Iancu P, Stefan M. Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction.Molecules. 2022; 27: 7737.
CrossRef - Awad AM, Kumar P, Ismail-Fitry MR, Jusoh S, Ab Aziz MF, Sazili AQ. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants. 2021; 10(9): 1465.
CrossRef - Zhu F, Asada T, Sato A, Koi Y, Nishiwaki H, Tamura H. Rosmarinic acid extract for antioxidant, anti-allergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. Journal of Agricultural and Food Chemistry. 2014; 62: 885–892.
CrossRef - Gorchakova N., Heimuller E., Galkin A. Current safety data of the complex herbal medicine with sedative and cardioprotective actions. Innovative Biosystems and Bioengineering. 2018; 2(3): 163–174.
CrossRef - Nechita M-A, Toiu A, Benedec D, Hanganu D, Ielciu I, Oniga O, Nechita V-I, Oniga I. Agastache Species: A Comprehensive Review on Phytochemical Composition and Therapeutic Properties. Plants. 2023; 12: 2937.
CrossRef - Zielinska S, Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochemistry Reviews. 2014; 13: 391–416.
CrossRef - Quiñonez-Bastidas GN, Navarrete A. Mexican Plants and Derivates Compounds as Alternative for Inflammatory and Neuropathic Pain Treatment — A Review. Plants. 2021; 10: 865.
CrossRef - Sourestani MM, Malekzadeh M, Tava M. Influence of drying, storage and distillation times on essential oil yield and composition of anise hyssop (Agastache foeniculum (Pursh.) Kuntze). Journal of Essential Oil Research. 2014; 26: 177–184.
CrossRef - Park CH, Yeo HJ, Baskar TB, Park YE, Park JS, Lee SY, Park SU. In vitro antioxidant and antimicrobial properties of flower, leaf, and stem extracts of korean mint. Antioxidants. 2019; 8: 75.
CrossRef - Cao P, Xie P, Wang X, Wang J, Wei J, Kang WY. Chemical constituents and coagulation activity of Agastache rugosa. BMC Complementary Medicine and Therapies. 2017; 17(1): 93.
CrossRef - Shanaida M, Pryshlyak A, Golembiovska O. Determination of triterpenoids in some Lamiaceae species. Research Journal of Pharmacy and Technology. 2018; 7: 3113–3118.
CrossRef - Bielecka M, Zielińska S, Pencakowski B, Stafiniak M, Ślusarczyk S, Prescha A, Matkowski A. Age-related variation of polyphenol content and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. Industrial Crops and Products. 2019; 141: 111743.
CrossRef - Kim JW, Hong J-H. Physicochemical properties and physiological activities of Agastache rugosa extracts. Korean Journal of Food Preservation. 2021; 28 (1): 88–98.
CrossRef - Phenolic compounds of herbal infusions obtained from some species of the Lamiaceae family. Current Issues in Pharmacy and Medical Sciences. 2018; 31 (4): 194–199.[MF4]
CrossRef - Golembiovska O. Simultaneous determination of flavonoids and phenolic acids in different parts of Prunella vulgaris L. by High-Performance Liquid Chromatography with Photodiode Array Detection. International Journal of Pharmacognosy and Phytochemistry. 2014; 29 (1): 1248–1255.
- Rahbardar MG, Amin B, Mehri S, Mirnajafi-Zadeh SJ, Hosseinzadeh H. Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine. 2018; 40: 59–67.
CrossRef - Luo C, Zou L, Sun H, PengJ, Gao C, Bao L, Ji R, Jin Y, Sun S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020: 28.
CrossRef - Ijaz S, Iqbal J, Abbasi BA, Ullah Z, Yaseen T, Kanwal S, Mahmood T, Sydykbayeva S, Ydyrys A, Almarhoon ZM, Sharifi-Rad J, Hano C, Calina D, Cho WC. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomedicine and Pharmacotherapy. 2023; 162: 114687.
CrossRef - Borges G, Fong RY, Ensunsa JL, Kimball J, Medici V, Ottaviani JI, Crozier A. Absorption, distribution, metabolism and excretion of apigenin and its glycosides in healthy male adults. Free Radical Biology and Medicine. 2022; 185: 90–96.
CrossRef - Rahimi A, Alimohammadi M, Faramarzi F, Alizadeh-Navaei R, Rafiei A. The effects of apigenin administration on the inhibition of inflammatory responses and oxidative stress in the lung injury models: a systematic review and meta-analysis of preclinical evidence. Inflammopharmacology. 2022; 30 (4): 1259–1276.
CrossRef - Hou HD, Wu CY, Zhou J, Xu JD, Long F, Zhu JH, Zhou SS, Zhang W, Mao Q, Shen H, Shi ZQ, Wei YJ, Li SL. Holistic quality evaluation of commercial Agastache rugosa by multiple chromatographic and chemometric analysis. Journal of Pharmaceutical and Biomedical Analysis. 2022: 20, 210.
CrossRef - Bălănescu F, Botezatu AV, Marques F, Busuioc A, Marinca SO, Vînătoru C, Cârâc G, Furdui B, Dinica RM. Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil. International Journal of Molecular Sciences. 2023; 24: 828.
CrossRef - Kozłowska W, Piątczak E, Kolniak-Ostek J, Kochan E, Pencakowski B, Stafiniak M, Bielecka M, Płachno BJ, Strzemski M, Matkowski A. Upscaling biomass production of rosmarinic acid-rich hairy root cultures of Agastache rugosa (Fisch. & C.A.Mey.) Kuntze. Plant Cell, Tissue and Organ Culture. 2024; 156(2) : 41.
CrossRef - Duda SC, Mărghitaş LA, Dezmirean D, Duda M, Mărgăoan R, Bobiş O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Industrial Crops and Products. 2015;77: 499–507.
CrossRef - Benedec D, Hanganu I, Oniga I, Tiperciuc B, Olah NK, Raita O, Bischin C, Silaghi-Dumitrescu R, Vlase L. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pakistan Journal of Pharmaceutical Sciences. 2015; 28 (6): 2297–2303.
- Sas I, Grytsyk A, Koliadzhyn T, Koshovyi O. Comparative study of phenolic compounds of the herb of Betonica L. genus species of flora of Ukraine. ScienceRise: Pharmaceutical Science. 2021; 29 (1): 66–75.
CrossRef - Shanaida M, Jasicka-Misiak I, Makowicz E, Stanek N, Shanaida V, Wieczorek PP. Development of the HPTLC method for identifications of phenolic compounds and quantification of rosmarinic acid content in some Lamiaceae Martinov species. Journal of Pharmacy and Bioallied Sciences. 2020; 12: 139–145.
CrossRef - Saeb K, Gholamrezaee S, Asadi M. Variation of Antioxidant Activity of Melissa officinalis Leaves Extracts During the Different Stages of Plant Growth. Biomedical and Pharmacology Journal. 2011; 4(2): 237-243.
CrossRef - Lee YE, Lee E, Rinik UR, Kim JY, Jung BH, Kwon O. Bioavailability of Korean mint (Agastache rugosa) polyphenols in humans and a Caco-2 cell model: a preliminary study exploring the efficacy. Food & Function. 2023; 14 (19): 8933–8941.
CrossRef - Rinik UR, Kim JE, Lee E, Kwon O, Jung BH. Development of simultaneous quantitative analytical method for three active components of Korean mint (Agastache rugosa (Fisch. & C.A.Mey.) Kuntze) extract in human plasma using ultra-high-performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography B. 2024; 1; 1232: 123957.
CrossRef - Seo YH, Kang SY, Shin JS, Ryu SM, Lee AY, Choi G, Moon BC, Jang DS, Shim SH, Lee D, Lee KT, Lee J. Chemical Constituents from the Aerial Parts of Agastache rugosa and Their Inhibitory Activities on Prostaglandin E2 Production in Lipopolysaccharide-Treated RAW 264.7 Macrophages. Journal of Natural Products. 2019; 82(12): 3379-3385.
CrossRef







