S Saradha1 , R Abitha2
, R Abitha2 , K Hari Prasath3
, K Hari Prasath3 , S Logithkumar4*
, S Logithkumar4* , R Vijayashree5
, R Vijayashree5 , T Sobita Devi 6
, T Sobita Devi 6 and P Indhra7
and P Indhra7
1Department of Pharmacology, Shri Sathya Sai Medical College and Research Institute (SSSMCRI), Sri Balaji Vidyapeeth (Deemed to be University), Ammapettai, Chengalpet district, Tamilnadu, India.
2Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpet district, Tamil Nadu, India.
3Department of Pathology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpet district, Tamil Nadu, India.
4Central animal house, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpet district, Tamil Nadu, India.
5Department of community medicine, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpet district, Tamil Nadu, India.
Corresponding Author E-mail: logithkumars2001@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2852
Abstract
Background and Objective: Carcinoma of the skin is the commonest cancer in the world. This study aims to assess the anti-cancer effect of the ethanolic pod and leaf extracts of Moringa Oleifera on 7,12 - dimethylbenz anthracene (DMBA) induced skin carcinoma in mice. Methodology: Animals were divided into 6 groups of 5 each. 7,12 - dimethylbenz anthracene (DMBA) was used topically for four weeks to induce tumour. Group 1 received placebo, Group 2 - standard drug 5- Fluorouracil, Groups 3, 4 received pod extract and Groups 5,6 received leaf extract of Moringa Oleifera of concentration 500 and 1000mg/kg respectively for 3 weeks. Hematological and biochemical parameters such as Hemoglobin, RBC, WBC and platelet counts, SGOT and SGPT, blood urea nitrogen and serum creatinine were done before cancer induction and at the end of 7 weeks. Histopathological examination of the skin, liver and kidney were done at the end of 7 weeks. Results: There was reduction in tumor size in the Moringa Oleifera pod and leaf extract treated groups. Histopathology revealed infiltration of the cells with scarring of epidermis in the extract treated groups indicating the healing of tissues more pronounced at higher concentration. Control group showed atypical squamous cells whereas the standard drug treated group showed infiltration and scarring. Conclusion: This study exhibits a dose dependent anticancer effect of Moringa oleifera pod and leaf extracts in mice which was comparable with the standard drug 5-Fluorouracil.
Keywords
Anti-cancer effect; DMBA, 5-Fluorouracil; Moringa Oleifera; Pod and Leaf extracts; Skin cancer
Download this article as:| Copy the following to cite this article: Saradha S, Abitha R, Prasath K. H, Logithkumar S, Vijayashree R, Devi T. S, Indhra P. Assessment of Anticancer Activity of Crude Ethanolic Extracts of Moringa Oleifera Pod and Leaves on 7,12 - Dimethylbenz Anthracene Induced Skin Cancer in Mice. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Saradha S, Abitha R, Prasath K. H, Logithkumar S, Vijayashree R, Devi T. S, Indhra P. Assessment of Anticancer Activity of Crude Ethanolic Extracts of Moringa Oleifera Pod and Leaves on 7,12 - Dimethylbenz Anthracene Induced Skin Cancer in Mice. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/4be7PuF |
Introduction
Cancer is one of the deadliest diseases and its treatment is always challenging.The incidence of Cancer is expected to rise by around 70%, 20 years from now. According to the Cancer facts and figures 2022, it is predicted that 1.9 million patients are to be newly diagnosed and 6,09,360 deaths due to cancer is likely to occur in the United States.1
Carcinoma of the skin is the commonest cancer in the world.2 1-2% of Cancers in India are skin cancers. There are various modalities of treatment available for the treatment of skin cancers such as surgery, chemotherapy, radiotherapy, immunotherapy but their cost and toxicity are the major concerns.
To overcome these difficulties researchers are constantly studying the anti-cancer effects of various natural plant sources.3Moringa Oleifera commonly called the Drumstick is traditionally used in ayurvedic medicine to treat several diseases. Moringa oleifera being an Indian medicinal plant easily cultivatable in the southern Asian continentis a great source of nutritional content with high economic importance.
Moringa oleifera has medicinal values in its every part like leaves, pods, flower, bark and root. Leaves serve as medicine for asthma, dyslipidemia and flowerscan be used in treatment of arthritis.4
Moringa oleifera has been found to have diverse medicinal activities and is studied in vitro and in vivo models of diabetes mellitus, hypertension, peptic ulcer, epilepsy, fever, inflammatory disorders, bacterial and fungal infections.5,6
This study is done to investigate the anti-cancer effect of Moringa oleifera pod and leaf extracts in 7,12 –diethylbenzanthracene (DMBA) induced skin cancer in mice.
Materials and Methods
Animals
30 Swiss Albino mice, 4-6 weeks old and weighing approximately 20-35 grams were obtained from the animal house of the Institution. The animals were quarantined for one week before the study. Animals were housed in clean polypropylene cages availing free access to standard pellet diet and water at appropriate temperature with natural day-light cycle. The study procedures were done as per the guidelines of CCSEA.
Chemicals
7,12 – dimethylbenz anthracene (DMBA), was purchased from Sigma-Aldrich Ltd., Bangalore. Acetone was used as a solvent for DMBA.5-Fluoro uracil (0.5%), was used as the standard anticancer drug. Ethanol was used for pod and leaf extraction. Normal saline was used as a placebo for mice in control group.
Ethics
The study was conducted after being approved by the Institutional animal ethics committee.(IAEC2/Proposal:62/A.Lr:45/Dt:10.08.2021)
Extract preparation
Moringa oleifera leaves and pods were gathered from nearby locality and washed thoroughly and shade dried for a week. Dried specimen was crushed in a mixer grinder and extracted with ethanol for 72 hours. Extracts after filtration was evaporated completely on water bath. After extraction the residue obtained was dissolved in 65% ethanol and centrifuged at -4 degrees at 4000rotations per minute for 10 minutes and supernatant was used.
 |
Figure 1: Preparation of Pod extract |
 |
Figure 2: Preparation of Leaf extract. |
Experimental design
The study had 6 groups with 5 animals in each.
Table 1: Experimental Design
|
Groups |
Induction + Treatment |
|
I |
DMBA + Normal saline (10ml/kg, p.o) for 21 days |
|
II |
DMBA + 0.5% 5-Fluoro uracil (topical application) for 21 days(7) |
|
III |
DMBA +Moringa Oleifera ethanolic pod extract (MOPE 500mg/kg p.o) for 21 days |
|
IV |
DMBA +Moringa Oleifera ethanolic pod extract (MOPE 1000mg/kg p.o) for 21 days |
|
V |
DMBA +Moringa Oleifera ethanolic leaf extract (MOLE 500 mg/kg p.o) for 21 days |
|
VI |
DMBA +Moringa Oleifera ethanolic leaf extract (MOLE 1000mg/kg p.o) for 21 days |
DMBA – 7, 12DimethylBenzanthracene.p.o – per oral. MOPE – Moringa Oleifera Pod extract. MOLE – Moringa Oleifera Leaf extract.
Dose of the extracts in groups III to VI were calculated based on previous study.8
Tumour induction
Tumour was induced as per the standard method suggested in previous literature.9 The dorsal aspect of each mouse was shaved for about 1 inch. 7,12 – dimethylbenz anthracene (DMBA)100µg, dissolved in 100µL acetone was used to induce tumour. The solution was painted topically over the shaved area twice weekly for 4 weeks to all animals. The appearance of palpable skin tumour was considered as tumour induction.
Following the 4th week, treatment was started to each group and continued for next 3 weeks. At the end of 7th week, the study was terminated. 0.3ml blood was collected under anaesthesia for estimation of haematological and biochemical parameters and all animals were euthanized and tumour tissue collected for histopathological examination.
 |
Figure 3: Mice with hair removed on dorsum. Mice with tumour. |
General parameters
Behaviour of the mice, movements, food and water intake was observed daily and body weight measured at weekly intervals.
Tumour assessment
Measurement of tumour size was done using the digital vernier calliper of 0.01mm precision with the mice held in hand. Tumour area is body surface area occupied by the tumour calculated by the formula π/4*length*width. Length of the tumour indicates the longest diameter along the vertical axis and width indicates the diameter along the horizontal axis.10 Measurements were taken at the end of 4th and 7th week of study.
Haematological and biochemical parameters
The Haemoglobin, RBC, WBC and platelet count, liver enzymes – SGOT, SGPT, blood urea and serum creatinine were evaluated for all animals before the start of the study(baseline) and at completion (at the end of 7th week).
Histopathological examination
After study completion, euthanasia of animals was done and the tissues (skin with tumour, liver and kidney) were sent for histopathological examination. The tissues were processed and sectioned in microtome and stained with haematoxylin and eosin. Examination of the sectioned slides were done under light microscopy.
Statistical Analysis
Statistical Package for Social Sciences v-26was used for statistical analysis. Descriptive statistics was done to obtain the basic characteristics of the data. For the intragroup comparison, parametric paired t test was used to find the p value. For weight, Graphical representation was used to find the difference among the groups.
Results and Discussion:
Tumour size
There was progressive increase in tumour size in the control group. There was a decrease in tumour size in the groups receiving MOLE and MOPE at different concentration (500mg/kg and 1000mg/kg) which was comparable with the standard treated group. Table 2.
Table 2: Tumour Size in mm
|
|
DMBA |
DMBA +5-FU |
DMBA+ MOPE (500 mg/kg) |
DMBA+ MOPE (1000 mg/kg) |
DMBA+ MOLE (500 mg/kg) |
DMBA+ MOLE (1000 mg/kg) |
|
4th week |
9 |
8.5 |
7.8 |
8.6 |
8.4 |
9 |
|
7th week |
13 |
0 |
2 |
0 |
1.5 |
0 |
Body Weight
The weight of mice measured over regular intervals during the study showed significant changes. The control group showed marked decline in their weight suggesting the effect of carcinogenesis. The standard treated group gained weight during the treatment after initial fall. The animals treated with ethanolic extracts of Moringa showed similar weight gain after treatment, preceded by loss after the induction.
 |
Figure 4: Body Weight in Grams. |
Haematological and Biochemical parameters
Haemoglobin (Hb), total erythrocyte count (RBC), total leukocyte (WBC) count, platelet count, liver enzymes – SGOT, SGPT, blood urea and serum creatinine were assessed before tumour induction and after the termination of the study. It was observed that there was a reduction in the Hb, RBC, WBC and platelet count and a rise in the liver enzymes and renal parameters in the control group when the baseline (before tumour induction) readings were compared with the week 7 values. The standard anti-cancer drug 5-FU and the MOPE and MOLE treated groups showed a rise in the haematological parameters and near normal biochemical parameters at the end of the study which were comparable with the baseline values.
Table 3: Hb and Platelet Count
|
|
Hb gm/dL |
Platelet Count 103/Cu.mm |
||||
|
|
Baseline |
Week 7 |
P value |
Baseline |
Week 7 |
P Value |
|
Control |
13.80±.556 |
11.14±.702 |
.004* |
274.88±35.60 |
138.80±28.83 |
.000* |
|
Standard(5-FU) |
11.00±.341 |
14.00±.353 |
.003* |
278.80±37.70 |
299.60±22.60 |
.010* |
|
MOPE-500 |
14.48±.334 |
13.40±.367 |
.000* |
264.20±75.85 |
272.40±55.50 |
.039* |
|
MOPE-1000 |
11.10±.514 |
13.66±.511 |
.018* |
272.00±79.90 |
296.60±66.22 |
.010* |
|
MOLE-500 |
14.58±.221 |
12.22±.485 |
.009* |
262.00±12.28 |
278.00±51.20 |
.015* |
|
MOLE-1000 |
13.50±.393 |
14.44±.415 |
.004* |
271.20±71.92 |
290.10±66.69 |
.013* |
* P value <0.05 indicating Statistical significance
Table 4: RBC Count and WBC Count
|
RBC Count million/Cu.mm |
WBC Count 103/Cu.mm |
|||||
|
Baseline |
Week 7 |
P Value |
Baseline |
Week 7 |
P Value |
|
|
Control |
8.28±.030 |
7.93±.035 |
.000* |
5.90±.578 |
3.66±.498 |
.006* |
|
Standard(5-FU) |
8.81±.087 |
8.94±.038 |
.039* |
6.16±.089 |
6.86±.378 |
.023* |
|
MOPE-500 |
8.76±.014 |
8.81±.032 |
.041* |
6.44±.181 |
6.56±.240 |
.051 |
|
MOPE-1000 |
8.82±.038 |
8.93±.043 |
.023* |
6.28±.286 |
6.64±.240 |
.036* |
|
MOLE-500 |
8.57±.043 |
8.64±.045 |
.051 |
6.65±.556 |
6.75±.129 |
.069 |
|
MOLE-1000 |
8.85±.034 |
8.96±.040 |
.032* |
6.54±.867 |
6.88±.311 |
.047* |
* P value <0.05 indicating Statistical significance
Table 5: Liver Enzymes: SGOT, SGPT
|
SGOT U/L |
SGPT U/L |
|||||
|
Baseline |
Week 7 |
P value |
Baseline |
Week 7 |
P value |
|
|
Control |
58.40±2.31 |
65.40±2.30 |
.000* |
36.80±4.65 |
40.00±4.74 |
.000* |
|
Standard |
59.61±2.22 |
60.80±4.32 |
.071 |
35.23±6.49 |
35.21±3.42 |
.063 |
|
MOPE-500 |
58.23±3.20 |
59.13±5.24 |
.062 |
37.81±6.64 |
38.82±4.49 |
.059 |
|
MOPE-1000 |
57.29±2.96 |
58.52±5.24 |
.061 |
35.44±4.72 |
35.71±4.49 |
.051 |
|
MOLE-500 |
59.25±1.88 |
60.72±6.05 |
.050* |
36.25±2.50 |
37.25±6.18 |
.052 |
|
MOLE-1000 |
58.43±2.20 |
59.60±5.41 |
.063 |
35.80±2.38 |
35.57±5.35 |
.049* |
* P value <0.05 indicating Statistical significance
Table 6: Renal Function Tests: BUN, Serum creatinine
|
BUN mg/dl |
Creatinine mg/dl |
|||||
|
Baseline |
Week 7 |
P value |
Baseline |
Week 7 |
P value |
|
|
Control |
15.04±.05 |
18.35±.03 |
.000* |
.60±.02 |
.93±.03 |
.000* |
|
Standard |
15.01±.60 |
15.10±.14 |
.251 |
.62±.03 |
.64±.04 |
.085 |
|
MOPE-500 |
16.51±.73 |
16.76±.01 |
.167 |
.61±.02 |
.62±.02 |
.293 |
|
MOPE-1000 |
15.66±.49 |
15.76±.35 |
.272 |
.65±.05 |
.67±.03 |
.138 |
|
MOLE-500 |
16.06±.11 |
16.17±.12 |
.136 |
.64±.03 |
.67±.06 |
.037* |
|
MOLE-1000 |
15.21±.03 |
15.30±.14 |
.121 |
.67±.06 |
.66±.05 |
.257 |
* P value <0.05 indicating Statistical significance
Histopathological examination
The skin section of the control group showed atypical squamous cells with keratin pearls on histopathological examination at the end of the study. There was also evidence of hyperkeratosis and chronic granulomatous tissues. The standard (5-FU) treated group exhibited signs of healing with significant scarring at the dermis. MOPE treated group showed infiltration of tissues with fibrosis signifying the process of healing from the inflammation. The MOLE treated group also showed fibrosis and signs of healing in skin tissues. The section taken from the kidney and liver from the control group showed marked congestion owing to the effect of DMBA. The sections of control kidney showed thickened glomerular basement membrane. The control hepatic cells exhibited hydropic degeneration associated with congestion. The standard and test groups had normal kidney and liver morphology in histopathological analysis. Based on the above findings it is suggestive that the carcinoma induced have produced marked changes in the skin, kidney and liver of mice in control group while the changes in the standard and test groups were less pronounced and showed signs suggestive of healing.
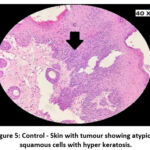 |
Figure 5: Control – Skin with tumour showing atypical squamous cells with hyper keratosis. |
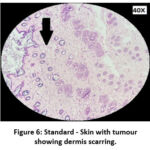 |
Figure 6: Standard – Skin with tumour showing dermis scarring. |
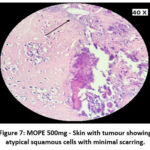 |
Figure 7: MOPE 500mg – Skin with tumour showing atypical squamous cells with minimal scarring. |
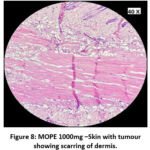 |
Figure 8: MOPE 1000mg –Skin with tumour showing scarring of dermis. |
 |
Figure 9: MOLE 500mg – Skin with tumour showing fibrosis of dermis with minimal infiltration. |
 |
Figure 10: MOLE 1000mg – Skin with tumour showing scarring of dermis. |
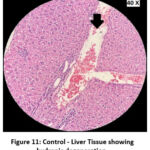 |
Figure 11: Control – Liver Tissue showing hydropic degeneration. |
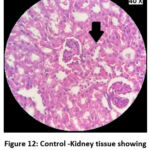 |
Figure 12: Control -Kidney tissue showing inflammatory infiltrates. |
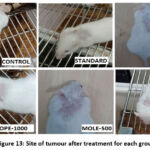 |
Figure 13: Site of tumour after treatment for each group. |
The invitro antitumor effects of Moringa oleifera leaf extract by Methyl Thiazolyl Tetrazolium bromide (MTT) assay have already been studied. It was observed that chloroform fraction showed higher anticancer activity. The fractions exhibited a dose dependent inhibition of cancer cells with maximum activity at 416 µg mL-1.11
It has earlier been observed that the polyphenolic fraction of Moringa Oleifera leaves had potent antioxidant effect and demonstrated the ability to scavenge free radicals, metal chelation properties and offered protection against DNA nicking. It was also evident that leaf extracts could prevent oxidative stress in rat tissues.12
The anticancer effects of Moringa Oleifera leaf and bark extracts in cancer cell lines of breast and colon have been explored and the extracts have found to have anticancer potential.13
The anti-tumour effect of Moringa oleifera leaf extract in immunodeficient nude mice has been assessed against human hepatocellular carcinoma (Hep G2) cells using a hollow fibre assay. Hollow fibres containing HepG2 cells were implanted subcutaneously in mice and leaf extract was given orally for four days. The recovered fibres were analyzed by trypan blue exclusion assay and it was observed that HepG2 cells decreased by 60% at a dose of 200mg/kg of moringa leaf extract.14
The cytotoxic effect of ethyl acetate leaf extract in lymphoma ascites model of Dalton has been evaluated. Lymphoma was induced in mice by intraperitoneal injection of DLA (Dalton’s Lymphoma ascites) and moringa ethyl acetate extract treatment given orally for ten days. It was observed that the treated mice had significantly longer survival rate when compared with the untreated mice.15
The active substances such as phenolic acid, flavonoids and niazimicin in M.oleifera prevent the progress of cancer by down regulation of genes controlling tumour progression and induction of apoptosis.5
Moringa oleifera flower is found to have trypsin inhibitors that has shown potent antitumor and antiangiogenic effects on sarcoma cells.16 Probably this could have been the cause of antitumor effect of Moringa oleifera pod and leaf, though in the current study the mechanisms of anti-tumour effect were not studied.
It is evident from our study that ethanolic extracts of Moringa Oleifera pod and leaf have anticancer effect against 7,12-DMBA induced skin cancer in mice. The tumour size reduced to zero, the body weight showed an improvement after the initial fall due to cancer induction. The haematological and biochemical parameters exhibited a response similar to the standard drug when treated with the pod and leaf extracts of Moringa Oleifera. The histopathological examination of the skin tissue after treatment revealed that the pod and leaf extracts had shown a complete tumour regression.
Moringa Oleifera pod and leaf extracts have shown reduction in skin tumour size and improvement in haematological, biochemical parameters and histopathological examination in the current study.
Conclusion
Skin carcinoma is one of the most common tumours in humans. Our study exhibits a dose dependent anticancer effect of Moringa oleifera pod and leaf extracts in mice which were comparable with the standard drug 5-Fluorouracil. Hence Moringa Oleifera pod and leaf can be considered as an adjuvant therapy for skin cancers along with the standard treatment if further studies are conducted on human subjects.
Acknowledgement
All the authors are thankful to Chettinad Academy of Research and Education for providing the facilities for the conduct of this research.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
This research was not funded by any external agency.
References
- Cancer Facts and Figures 2022.
- Helmy AH, Al-Nabulsi Z, Chambers M, Fernandez-Diaz S. Skin Cancer Excision Analysis in a Single Rural Center in Scotland’s Highlands. Cureus. 2022;14(2):e21954. Published 2022 Feb 6. doi:10.7759/cureus.21954
CrossRef - Barhoi D, Upadhaya P, Barbhuiya SN, Giri A, Giri S. Aqueous Extract of Moringa oleifera Exhibit Potential Anticancer Activity and can be Used as a Possible Cancer Therapeutic Agent: A Study Involving In Vitro and In Vivo Approach.J Am Coll Nutr.2021;40(1):70-85. doi:10.1080/07315724.2020.1735572
CrossRef - Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness. 2016;5(2):49-56. doi:https://doi.org/10.1016/j.fshw.2016.04.001
CrossRef - Brilhante RSN, Sales JA, Pereira VS, et al. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac J Trop Med.2017;10(7):621-630. doi:10.1016/j.apjtm.2017.07.002
CrossRef - Abdull Razis AF, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac J Cancer Prev.2014;15(20):8571-8576. doi:10.7314/apjcp.2014.15.20.8571
CrossRef - Voiculescu VM, Lisievici CV, Lupu M, et al. Mediators of Inflammation in Topical Therapy of Skin Cancers. Mediators Inflamm. 2019;2019:8369690. Published 2019 Jan 10. doi:10.1155/2019/8369690
CrossRef - Purwal L, Pathak AK, Jain UK.In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline. 2010; 1: 655-665.
- Gopalakrishnan T, Ganapathy S, Veeran V, Namasivayam N. Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed Pharmacother. 2019;111:178-187. doi:10.1016/j.biopha.2018.12.071
CrossRef - Bazin M, Purohit NK, Shah GM. Comprehensive measurement of UVB-induced non-melanoma skin cancer burden in mice using photographic images as a substitute for the caliper method [published correction appears in PLoS One. 2017 Mar 6;12 (3):e0173718]. PLoS One. 2017;12(2):e0171875. Published 2017 Feb 10. doi:10.1371/journal.pone.0171875
CrossRef - Mumtaz MZ, Kausar F, Hassan M, Javaid S, Malik A. Anticancer activities of phenolic compounds from Moringa oleifera leaves: in vitro and in silico mechanistic study. Beni-Suef University Journal of Basic and Applied Sciences. 2021;10(1). doi:https://doi.org/10.1186/s43088-021-00101-2
CrossRef - Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47(9):2196-2201. doi:10.1016/j.fct.2009.06.005
CrossRef - Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS One. 2015;10(8):e0135814. Published 2015 Aug 19. doi:10.1371/journal.pone.0135814
CrossRef - Jung IL, Lee JH, Kang SC. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol Lett.2015;10(3):1597-1604. doi:10.3892/ol.2015.3482
CrossRef - Krishnamurthy PT, Vardarajalu A, Wadhwani A, Patel V. Identification and characterization of a potent anticancer fraction from the leaf extracts of Moringa oleifera L. Indian J Exp Biol. 2015;53(2):98-103.
- Patriota LLS, Ramos DBM, Dos Santos ACLA, et al. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem Toxicol.2020;145:111691. doi:10.1016/j.fct.2020.111691
CrossRef








