Mais A. Abood* , Nabaa M. Ibrahem
, Nabaa M. Ibrahem and Ali Rahman Jasim
and Ali Rahman Jasim
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail: mailto:may.abood@copharm.uobaghdad.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2831
Abstract
Historically, medicinal herbs have been utilized as an important origin of chemicals with particular therapeutic potentials, and they continue to be a great place to find new medication candidates. Parthenocissus quinquefolia L. is a member of the grape-growing family Vitaceae. It is indigenous to Central and North America. It is widely dispersed in Iraqi gardens and plant houses from north to south. Traditionally, it has many uses, like relieving constipation, treating jaundice, expectorant, emetic, and others. At the same time, its proven activities include antioxidant activity, antimicrobial, anti-diabetic, thrombin inhibitor effect, and medicine for treating eyelid eczema. Parthenocissus quinquefolia contains valuable phytochemicals like alkaloids, saponins, steroids, terpenoids, polyphenolic compounds (flavonoids, phenolic acids, and tannins), anthraquinones, cardiac glycosides, coumarins, and reducing sugars that make it responsible for its critical pharmacological effects. The current review discussed the pharmacognosy, phytochemistry, and pharmacological activity of Parthenocissus quinquefolia (L.).
Keywords
Creeper; Parthenocissus; Pharmacognosy; Phytochemistry; Quinquefolia; Virginia
Download this article as:| Copy the following to cite this article: Abood M. A, Ibrahem N. M, Jasim A. R. A Summary of the Pharmacological Activity, Phytochemistry, and Pharmacognosy of Parthenocissus quinquefolia (L.): Review Article. Biomed Pharmacol J 2024;17(1). |
| Copy the following to cite this URL: Abood M. A, Ibrahem N. M, Jasim A. R. A Summary of the Pharmacological Activity, Phytochemistry, and Pharmacognosy of Parthenocissus quinquefolia (L.): Review Article. Biomed Pharmacol J 2024;17(1). Available from: https://bit.ly/49OElCH |
Introduction
Parthenocissus quinquefolia (L.) is a significant member of the Vitaceae family of medicinal herbs. Due to their reputation as the origin of wine, raisin, and grape cultivation, this family is highly significant economically 1. Additionally, this family is well-known for its pharmacological activity, which includes its ability to prevent cancer 2, relieve sore throats, treat rheumatism, arthritis, gastrointestinal tract issues, heal fractures, protect bone coming from postmenopausal bone loss, and have anti-parasitic, anti-diabetic, anti-dysentery, anti-diarrhea, diuretic, anti-inflammatory, anti-convulsant, anxiolytic, and anti-cholesterol formation activity 3.
Of the approximately twelve species of the Parthenocissus genus, nine are in Asia, three are in North America, and just one is in Central America and the Caribbean 4. Greek terms parthenos (meaning “virgin”) and kissos (meaning “ivy,” which was Latinized to form Cissus) are the sources of the Parthenocissus name. This naming is related to the ability of these creepers to form seeds without pollination 5. Most of this genus plants are used as ornamental creepers for decorative requirements, as they appear most obviously in the spring with their new bright green leaves. At the same time, in the autumn, they shine in dramatic changes in leaf color that may vary from yellow, orange-brown, and red to violet 6. It has become naturalized in southern Africa, Australia, tropical and temperate Asia, and Europe. Although P. quinquefolia is widely recognized to grow in household gardens from north to south and to germinate in numerous plant houses, its distribution in Iraq has yet to be formally documented 7.
Taxonomic classification
Plantae or plant is the Kingdom, Viridiplantae is the Subkingdom, Streptophyta is the Infrakingdom, Embryophyta is the Superphylum, Tracheophyta is the Phylum, and Spermatophytina is the Subphylum.
Magnoliopsida is the class; Rosanae is the superorder; Vitales is the order; Vitaceae is the family; Parthenocissus Planch is the genus; and Parthenocissus quinquefolia is the species8, 9.
Common names of Parthenocissus quinquefolia
Parthenocissus quinquefolia is also called woodbine, Virginia creeper, American ivy, and five-leaved ivy. In Iraq, it’s known as MAKHALEB AL-KETT 7. The plant synonyms are Ampelopsis hederacea, Ampelopsis quinquefolia, and Hedera quinquefolia L. 8, 9.
Plant description
Quinquefolia is derived from the Greek word meaning “five leaves.” Quinque signifies five, whereas folia refers to leaves or foliage 10.
Parthenocissus quinquefolia (Fig. 1) shows climbing characteristics that may reach up to 30 meters in height; its five leaflet leaves, which have enlarged or jointed nodes, emerge sporadically on shoots. These may produce little, forked tendrils with flower clusters; the greenish clusters develop in late spring and ripen into small, hard, purplish-black berries in early fall, opposing the leaves. The tendrils are capped with small, intensely sticky pads. The stamens develop opposing the petals, and most of the tiny flowers’ components appear in groups of four or five 11.
 |
Figure 1: Parthenocissus quinquefolia. A- Climbing feature at the Iraqi gardens. B- Red-colored leaves and berry clusters. |
Traditional uses of Parthenocissus quinquefolia
The bark and fresh young stem are traditionally used to relieve constipation, besides their usage as emetic, expectorant, and tonic 12. A soft and moist material prepared by a hot plant decoction can be applied to the body to reduce edema 13. While a tea prepared from the plant is used to treat jaundice, a tea prepared from the roots is used to treat diarrhea and gonorrhea. A tea made from the leaves is an astringent, aperient, and diuretic 13, 14. Fruit aids with fever treatment 15. Also, the plant was used as a natural source of pink dye obtained from the fruit 13. As an Ayurvedic ethno-medicinal plant, P. quinquefolia is used as an antihyperglycemic agent by the Indian population 16.
Native Americans (Cherokee people) have a rich ethnobotanical heritage; they prepared P. quinquefolia as an infusion for jaundice 17. Parthenocissus quinquefolia berries are rich in oxalic acid, and this gives the expectation of being poisonous upon eating 18. Since the plant was indigenous to Central and North America, most of the traditional uses were in the different tribes of the Native Americans, which are summarized in tab.1.
Table 1: Traditional uses of Parthenocissus quinquefolia in populations/Tribes.
|
Traditional uses |
Different populations/Tribes |
|
Infusion used for jaundice. |
Native American /The Cherokee and Iroquois. |
|
Herbal remedy for diarrhea, swelling, and as a urinary aid. |
Native American /The Cherokee and Iroquois. |
|
Decoction of the root for diarrhea treatment. |
Native American /Mesquakie. |
|
Pink dye from the fruit for the skin |
Northern American Indian /Kiowa. |
|
Antihyperglycemic agent. |
Indian population. |
Phytochemical constituents of Parthenocissus quinquefolia
Phytochemical investigation performed on the bark, stem, leaves, and fruit extracts of P. quinquefolia indicated the presence of significant secondary metabolites, including terpenoids, alkaloids, saponins, steroids, polyphenolic compounds (flavonoids, phenolic acids, and tannins), anthraquinones, cardiac glucosides, coumarins, and reducing sugars. The essential secondary metabolites identified and isolated from P. quinquefolia include stilbenes, polyphenolic compounds, terpenes 7, fatty acids, and others 19-21.
Stilbenes compounds
The name “stilbenes” comes from the Greek word “stilbos,” which means “shining”; these plants exhibit fluorescence 22. Stilbenes are phytoalexins that plants create as a defense mechanism or a means of fending off illness when they are harmed, stressed, or exposed to other environmental factors. Stilbenes exist in two stereoisomer configurations: the crowdy Cis (Z) isomer and the relieved Trans (E) isomer, and have a skeleton composed of C6-C2-C6 carbon atoms 23. Many types of stilbenes were detected and isolated from P. quinquefolia, including two monomeric stilbenes (trans-resveratrol, piceatannol), one glycoside stilbene (resveratrol 2-O-β- glucopyranoside), two resveratrol oligomer (parthenocissins A, B), three resveratrol dimers (resveratrol trans-dehydrodimer named ɣ-viniferin, ϵ-viniferin, and pallidol), one oligostilbene (parthenocissins N), two oligostilbene resveratrol dimer (cyphostemmin A, cyphostemmin B), and one resveratrol trimer (Miyabenol C)24-26. Some Stilbenes compounds’ chemical structures of P. quinquefolia have been demonstrated in Fig.2.
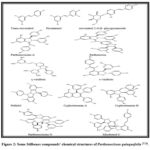 |
Figure 2: Some Stilbenes compounds’ chemical structures of Parthenocissus quinquefolia 27-29. |
Polyphenolic compounds
A- Flavonoids are essential secondary metabolites extracted from different medicinal plants and known for their use in nutraceutical, pharmaceutical, therapeutic, and cosmetic applications 30. In terms of chemistry, flavonoids are composed of a 15-carbon skeleton that is arranged in the C6-C3-C6 structure, in which both of the C6 are benzene rings named A and B, while the C3 acts as a bridge link between the A and B rings. This later bridge can again be cyclized by oxygen and produce a new ring called the C ring (oxygen-containing pyrene ring31).
This compound’s ability to modify important cellular enzyme activities results in several health advantages, including anti-inflammatory, anti-mutagenic, anti-carcinogenic, and antioxidant properties. Furthermore, flavonoids are valuable against many diseases, including cancer, Alzheimer’s, atherosclerosis, and others. This is due to their potent inhibition of numerous enzymes like phosphoinositide 3 kinase, cyclo-oxygenase, xanthine oxidase, and lipoxygenase30, 32. Numerous flavonoids, including quercetine-3O-α-L-rhamnoside, myricetine-3-O-α-L-rhamnoside, quercitrin (quercetin O-glycoside), and rutin (quercetin 3-O-rutinoside), have been found as glycosides (Fig. 3) 24. On the other hand, flavonoids can exist as free aglycones (Fig. 4), like quercetin, kaempferol, isorhamnetin, and luteolin 7.
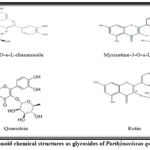 |
Figure 3: Flavonoid chemical structures as glycosides of Parthenocissus quinquefolia 33, 34. |
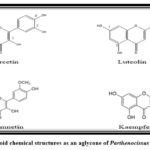 |
Figure 4: Flavonoid chemical structures as an aglycone of Parthenocissus quinquefolia 34, 35. |
Also, other types of flavonoids, which are anthocyanins(Fig. 5), were isolated and identified from the fruit (berries) of P. quinquefolia and considered natural colorants like delphinidin (blue pigment), petunidin (dark-red or purple pigment), cyaniding (reddish-purple pigment), malvidin (reddish blue pigment), peonidin (purplish-red pigment), and pelargonidin (orang pigment)36.
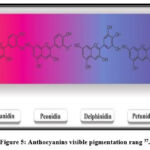 |
Figure 5: Anthocyanins visible pigmentation rang 37. |
B- Phenolic acids are one of the simple and essential secondary metabolites with phenol units in their structure 38. Some essential phenolic acids (Fig. 6) have been demonstrated in the whole plant of P. quinquefolia, like coumaric acid, caffeic acid, and chlorogenic acid, which is derived from cinnamic acid. It also contains gallic acid, a benzoic acid derivative 39, 7.
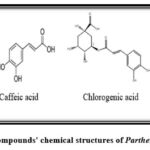 |
Figure 6: Phenolic acid compounds’ chemical structures of Parthenocissus quinquefolia 40, 34. |
Terpenes and Terpenoids
Among phytochemicals, triterpenes are the most representative class, with approximately 30,000 known constituents representing a portion of all plants’ lipid substances 41. Only one triterpenic compound was identified from P. quinquefolia, which is β- Amyrine (β-Amyrylhexadecanoate hexadecanoate), and its structure is shown in Fig. 7. This compound was extracted from dried leaves using dichloromethane for 24 h in a Soxhlet apparatus, then eluted with a mixture of solvents of increasing polarity using column chromatography and evaluated for antithrombotic effect 42.
 |
Figure 7: β- Amyrin chemical structure isolated from Parthenocissus quinquefolia. |
B- Phytosterols are cholesterol-like molecules found in higher plants 43. Upon TLC and HPLC examination of the petroleum ether fraction of the whole plant demonstrated the presence of stigmasterol and beta-sitosterol, and the quantities of both compounds in the petroleum ether fraction were calculated and found to be 2.33 μg for stigmasterol and 4.4 μg for beta-sitosterol 7.
Fatty acids
Aliphatic monocarboxylic acids are known as fatty acids and typically consist of a 4–28 carbon chain that is even-numbered, unbranched, and perhaps saturated or unsaturated 44, 45. Plants synthesize a wide variety of fatty acids, although only a few are major and standard constituents46. So, the most abundant and significant fatty acids of P. quinquefolia seeds were palmitic, oleic, and linoleic acids, which evaluated the plant’s seed extract for antioxidant capacity. 20, 47. The most abundant and significant fatty acids of P. quinquefolia are demonstrated in Fig. 8.
 |
Figure 8: Chemical structure of the most abundant and significant fatty acids of P. quinquefolia 48. |
Others
Other constituents of P. quinquefolia have been reported, including tropane alkaloid in glycosidic form, which was isolated from the chloroform fraction of the whole plant, and the proposed structure has been identified using liquid chromatography-mass spectrometry and Fourier transform infrared analysis. Butanol fraction of the whole plant could contain sennoside C, Asiatic acid or its analogue, Madecassic acid, and triglyceride 1, 2-dipalmitoyl-3-oleoyl-glycerol, which were identified based on the NIST library and multiple analytical tests7.
Pharmacological activity of Parthenocissus quinquefolia
Antidiabetic activity
Diabetes mellitus (DM), is a chronic metabolic illness, a significant global health issue, and one of the leading causes of morbidity in the world, with a significant burden in developing countries 16,49. The number of people with diabetes was 171 million in 2000; by 2030, it is predicted to reach 366 million 50, 51.
Exercise and diet are crucial for managing hyperglycemia, yet 90% of patients with type 2 diabetes struggle to maintain long-term glucose control and need antihyperglycemic medications to achieve this 52.
An Indian Ayurvedic herb was tested for its antidiabetic properties in a Zucker diabetic rat model using blood insulin levels and an oral glucose tolerance test. To ascertain the plant’s mode of action, 250 mg/kg body weight of lyophilized 70% ethanolic extract was used. Consequently, a potent antidiabetic impact was observed, wherein blood glucose levels decreased in direct proportion to insulin levels. This suggests that P. quinquefolia contributes to improving insulin release53.
Anti-inflammatory activity
Inflammation is a body response and physiological defense mechanism against foreign invaders or exposure to tissue injury, oxidative stress, or pre-existing disease 54, 55. Inflammation is of two types, acute and chronic, in which the accumulation of plasma proteins in tissues occurs, leading to increased fluid perfusion that results in swelling. This is followed by leukocyte release, which moves toward the affected area and subsequently releases cytokines, which are pro-inflammatory mediators and cause immune cell activation 56-58. These cytokines include tumor necrosis factor-alpha and different types of interleukins (IL-1, 6, 8, and 10) 47,59.
Three groups of six albino rats of each gender, weighing between 150 and 170 g, were used to affirm the anti-inflammatory properties of P. quinquefolia. The first group received a vehicle, which is dimethyl sulfoxide, and was designed as a positive control. The second group received oral treatment with an n-hexane fraction of P. quinquefolia (250 mg/kg 53) by gastric gavage for seven consecutive days. Ten milligrams of diclofenac sodium were administered to the third group 60. This group is used as an indicator to determine the level of anti-inflammatory activity.
The plant’s n-hexane fraction, which was analyzed using the GC/Mass technique, revealed that it contains unsaturated fatty acids, mainly oleic and palmitoleic acid. Through nuclear factor suppression, these fatty acids block the expression of pro-inflammatory cytokines such TNFα and IL-6, which contribute significantly to their reduction. DNA transcription, cytokine synthesis, and cell proliferation are all facilitated by the protein complex known as kappa-light-chain enhancer of activated B cell (NF)-κB signaling. When NF-kB translocates into the nucleus, two genes associated to inflammation, TNF-α and IL-6, are elevated 61, 62.
Furthermore, in the visceral adipose tissue, they inhibit NF-κB activation and diminish messenger ribonucleic acid mRNA expression for IL6 and TNF-α. Also, Siritin-1 (SIRT-1) is an enzyme in the cell nucleus and has a regulatory role in the cell 63. Similarly, polyphenols block the development of COX-2 enzymes, which convert arachidonic acid to prostaglandin, which is involved in swelling, discomfort, and redness and is released during inflammation 64. Thus, by reducing the percentage of exudate and granuloma in rat models of inflammation, P. quinquefolia demonstrated its anti-inflammatory properties. Moreover, it markedly decreased serum levels of IL-6 and TNF-α 65.
Antimicrobial activity
The ethanolic extract of P. quinquefolia roots functions as an antifungal agent against different fungal species like yeast candida and Aspergillus niger. Furthermore, it is used as an antibacterial agent against gram-positive bacteria like Staphylococcus aureus, Streptococcus pyogenes, and Bacillus subtilis, and also against gram-negative bacteria like Salmonella typhi, Pseudomonas fluorescence, and Klebsiella pneumonia 21. Aeromonas hydrophila and Aeromonas caviae were significantly suppressed by P. quinquefolia aqueous extract, with 59.68% and 55.90% inhibition, respectively, in a study on antibacterial activity 66. Therefore, P. quinquefolia evolves broad-spectrum activity against bacteria; again, this supports the traditional use of the plant.
Antioxidant activity
The ethanolic extract of P. quinquefolia bark and stem demonstrated a robust protective effect against free radicals, including reactive oxygen species produced in humans by both endogenous and external sources. The main factor stimulating many diseases is oxidative stress, such as degenerative and chronic diseases like diabetes mellitus, cardiac conditions, atherosclerosis, cancer, and immunosuppression 67, 68. As a result, antioxidants are the most effective at eradicating free radicals 69. On the other hand, the chloroformic extract of the leaves and berries revealed such high free radical-scavenging activity. These findings support the plant’s traditional usage in treating dangerous human ailments 19.
Another hexane extract study demonstrated the strong antiradical activity of P. quinquefolia seed oil. The fatty acid content of the plant seeds, with oleic acid (21.96%), palmitic acid (20.61%), and linoleic acid (48.23%) as the main constituents, exhibits this activity 20.
Medicine for treating eyelid eczema
Virginia creeper (P. quinquefolia) is formulated in combination with other plant materials (Artemisia rupestris, saffron, Rubiaceae borreria stricta, C. sativus, Tea begonia, and others) to produce a medicine for eyelid eczema. That effect is exerted by prompting blood circulation, clearing toxins and heat, removing dampness, and moisturizing dryness 70.
Thrombin inhibitor effect
Serious coronary disorders such as myocardial infarction and stroke are highly affected by blood coagulation control, which may represent the critical point in the treatment of these cases. The dichloromethane extract from P. quinquefolia applies a new effective antithrombin natural agent identified as amyrylhexadecanoate-amyrin 42.
Conclusion
Parthenocissus quinquefolia L. is a vibrant plant that produces a variety of valuable secondary metabolites such as stilbenes (trans-resveratrol, piceatannol, resveratrol 2-O-β- glucopyranoside, parthenocissins A, ɣ-viniferin, pallidol, cyphostemmin A, and miyabenol C), polyphenolic compounds (phenolic acids like gallic acid, caffeic acid, chlorogenic acid coumaric acid, and flavonoids like quercetine-3O-α-L-rhamnoside, myricetine-3-O-α-L-rhamnoside, quercitrin, rutin, quercetin, kaempferol, isorhamnetin, and luteolin), triterpene (β-Amyrine), fatty acids (palmitic acid, oleic acid, and linoleic acids), steroids (stigmasterol and β- sitosterol), an alkaloid (tropane alkaloidal compound in a glycosidic form), and others (sennoside C, Asiatic acid or its analogue Madecassic acid, and the triglyceride 1, 2-dipalmitoyl-3-oleoyl-glycerol). These important secondary metabolites support the pharmacological activity of P. quinquefolia, known for its antioxidant effect, antimicrobial, anti-diabetic, thrombin inhibitor effect, medicine for treating eyelid eczema, and anti-inflammatory effect.
Acknowledgments
The authors are deeply grateful to the College of Pharmacy, University of Baghdad, for giving us the support to accomplish this work.
Conflicts of Interest
There are no conflicts of interest.
Funding Source
There is no funding source.
References
- Wen J, Lu LM, Nie ZL, Liu XQ, Zhang N, Ickert‐Bond S, et al. A new phylogenetic tribal classification of the grape family (Vitaceae). J. Syst. Evol., 2018; 56(4):262-72. Available from: http://doi.org/10.1111/jse.12427.
CrossRef - Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science., 1997; 275(5297):218-20. Available from: http://doi.org/ 10.1126/science.275.5297.218.
CrossRef - Fernandes G, Banu J. Medicinal properties of plants from the genus Cissus: A review. J. Med. Plants Res., 2012 Apr 30; 6(16):3080-6. Available from: http://doi.org/ 10.5897/JMPR11.1637.
CrossRef - Soejima A, Wen J. Phylogenetic analysis of the grape family (Vitaceae) based on three chloroplast markers. American Journal of Botany, 2006 Feb; 93(2):278-87. Available from: http://doi.org/ 10.3732/ajb.93.2.278.
CrossRef - Fralish JS, Franklin SB. Taxonomy and ecology of woody plants in North American forests: ( Excluding Mexico and Subtropical Florida). New York: John Wiley & Sons; 2002 Feb 8. 612 pp. Available from: https://doi.org/10.1017/S146604660426015X.
CrossRef - Végh B, Schmidt G, Diószegi M. Characteristics of invasive taxa of Parthenocissus in the Buda Arboretum, Hungary. Scientific Papers. Series B, Horticulture. 2015 Jan 1; Vol. LIX: 427-34.
- Ismail NR, Kadhim E J. Phytochemical Screening and Isolation of New Compounds. IJDDT., 2021; 11(3): 1033-1039. Available from: http://doi.org/10.25258/ijddt.11.3.66.
- Thornhill MR, Krings A, Lindbo D, Stucky J. Guide to the vascular flora of the savannas and flatwoods of Shaken Creek Preserve and vicinity (Pender & Onslow Counties, North Carolina, USA). Biodiversity Data J., 2014 May; 16(2): 1-422. Available from: http://doi.org/ 10.3897/BDJ.2.e1099.
- Weakley AS. Flora of the southern and mid-Atlantic states. North Carolina Botanical Garden: UNC Herbarium; 2012. 1225 pp.
- Gledhill D. The names of plants. 4th edition. Cambridge University Press; 2008. Available from:
https://doi.org/10.1017/CBO9780511550898.
CrossRef - Jackson RS. Wine science: principles and applications. Fifth Edition. Academic press; 2020. Available from: https://doi.org/10.1016/C2017-0-04224-6.
CrossRef - Usher G. A dictionary of plants used by man. London: Constable and Company Ltd.; 1974. 619 pages.
- Moerman DE. Native American Ethnobotany. Portland, Oregon: Timber Press; 1998. 927pp.
- Foster S, Duke JA. A field guide to medicinal plants: eastern and central North America. Houghton Mifflin (T); 1990.
- Grieve M, Leyel C. A modern herbal. Penguin; 1980.
- Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. Journal of clinical biochemistry and nutrition, 2007; 40(3):163-73. Available from: http://doi.org/ 10.3164/jcbn.40.163.
CrossRef - Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines, 2018; 5(4):121. Available from: http://doi.org/10.3390/medicines5040121.
CrossRef - Facciola S, editor. Cornucopia: a source book of edible plants. Kampong publications; 1990. 677 pages.
- Zaheer-Ud-Din K, Summiya F, Anjum P, Sardar AA, Siddiqui SZ. Phytochemical properties and antioxidant activities of leaves and fruits extracts of Parthenocissus quinquefolia (L.) Planch. Bangladesh J. Bot., 2018; 47(1):33-8. Available from: URL:http://www.bdbotsociety.org/…/05.pdf.
- Zardi-Bergaouia A, Jouinia M, Znatia M, El Ayeb-Zakhamab A, Janneta HB. Physico-chemical properties, composition and antioxidant activity of seed oil from the Tunisian Virginia Creeper (Parthenocissus quinquefolia (L.) planch). Journal of the Tunisian Chemical Society, 2016; 18:89-95. Available from: URL: http://www.bdbotsociety.org/…/05.pdf.
- S. DO. Preliminary phytochemical screening and antibacterial activity of Parthenocissus quinquefolia (L.) Planch. Int J of Life Sciences., 2017.
- Rupasinghe HV. Applications of NMR Spectroscopy. Bentham Science Publishers; 2015.Chapter 1, Application of NMR spectroscopy in plant polyphenols associated with human health; p. 3-92. Available from: https://doi.org/10.1016/B978-1-60805-999-7.50001-X.
CrossRef - Morabito G, Miglio C, Peluso I, Serafini M. Polyphenols in human health and disease. Academic Press; 2014. Chapter 85, Fruit polyphenols and postprandial inflammatory stress; p. 1107-1126.
CrossRef - Yang J, Wang A, Ji T, Su Y. Chemical constituents from Parthenocissus quinquefolia. Zhongguo Zhong Yao Za Zhi, 2010 Jun 1; 35(12):1573-6. Available from: http://doi.org/ 10.4268/cjcmm20101215.
CrossRef - Tanaka T IM, Murata H. Stilbene derivatives in the stem of Parthenocissus quinquefolia. Phytochemistry, 24 July 1998; 48(6):1045-9. Available from: https://doi.org/10.1016/S0031-9422(98)00071-5.
CrossRef - Yang JB WA, Ji TF, Su YL. Two new oligostilbenes from the stem of Parthenocissus quinquefolia. J Asian Nat Prod Res., 2014; 16(3):275-80. Available from: http://doi.org/ 10.1080/10286020.2013.877451.
CrossRef - Pezet R PC, Jean-Denis JB, Tabacchi R, Gindro K, Viret O. δ-Viniferin, a resveratrol dehydrodimer: one of the major stilbenes synthesized by stressed grapevine leaves. Journal of agricultural and Food Chemistry, 2003; 51(18): 5488-92. Available from: http://doi.org/10.1021/jf030227o.
CrossRef - Mohammed ZY, Al-Jumaily EF, and Yaseen N. In Vitro Cytotoxic Study for Purified Resveratrol Extracted from Grape Skin Fruit Vitis vinifera. Iraqi J Pharm Sci., 2009; 18 (suppl.): 19-25. Available from: https://doi.org/10.31351/vol18issSuppl.pp19-25.
CrossRef - Ducrot PH KA, Bala AE, Majira A, Kerhoas L, Delorme R, Einhorn J. Cyphostemmins AB. two new antifungal oligostilbenes from Cyphostemma crotalarioides (Vitaceae). Tetrahedron letters, 1998; 39(52): 9655-8. Available from: https://doi.org/10.1016/S0040-4039(98)02207-2.
CrossRef - Panche A, Diwan A, Chandra S. Flavonoids: an overview. J Nutr Sci., 2016; 5:1-15. Available from: http://doi.org/ 10.1017/jns.2016.41.
CrossRef - Symonowicz M, Kolanek M. Flavonoids and their properties to form chelate complexes. Biotechnology and Food Science, 2012 Dec 31; 76(1):35-41. Available from: https://doi.org/10.34658/bfs.2012.76.1.35-41.
- Hassan ZY, Hassan TY, and Abu- Raghif AR. Evaluation the Effectiveness of Phenolic Compound of Salvia frigida on Induced Atopic Dermatitis in Experimental Mice. Iraqi J Pharm Sci., 2022; 31(1): 154-166. Available from: https://doi.org/10.31351/vol31iss1pp154-166.
CrossRef - Abdul-jalil, TZ. Screening of Rutin From Seeds and Leaves Extracts of Dill, Coriander and Fennel Cultivated in Iraq. Pharmacie Globale (IJCP)., 2013; 4(3): 1-6.
- Ibrahim RM. A review on Active Constituents and Pharmacological Effects of Eriobotrya Japonica Lindl. (Loquat). Iraqi J Pharm Sci., 2021; 30(1): 41-55. Available from: https://doi.org/10.31351/vol30iss1pp41-55.
CrossRef - Jaafar NS, Jaafar IS, and Noori ZS. Cressa cretica Pharmacognosy, and Pharmacology (A review). Iraqi J Pharm Sci., 2021; 30(2): 31-40. Available from: https://doi.org/10.31351/vol30iss2pp31-40.
CrossRef - Ticha MB, Meksi N, Attia HE, Haddar W, Guesmi A, Jannet HB, et al. Ultrasonic extraction of Parthenocissus quinquefolia colorants: Extract identification by HPLC-MS analysis and cleaner application on the phytodyeing of natural fibres. Dyes and Pigments, 2017; 141:103-11. Available from: https://doi.org/10.1016/j.dyepig.2017.02.002.
CrossRef - Ananga A, Georgiev V, Ochieng J, Phills B, Tsolova V. Production of anthocyanins in grape cell cultures: a potential source of raw material for pharmaceutical, food, and cosmetic industries. The Mediterranean genetic code-grapevine and olive, 2013; 247-87. Available from: http://doi.org/ 10.5772/54592.
CrossRef - Jaafar NS, Hamad MN, Abdulkhalik ZM, Noori ZS, Mohammad MH. Phytochemical investigation And high performance thin layer chromatography (HPTLC) identification of flavonoids and phenolic acids in Euphorbia cyathophora (Family: Euphorbiaceae) cultivated in Iraq. Ann Trop Med Public Heal., 2020; 23(19): Available from: http://doi.org/10.36295/ASRO.2020.232124.
CrossRef - Vincente AR, Manganaris GA, Ortiz CM, Sozzi GO, Crisosto CH. Postharvest Handling: A Systems Approach. Third Edition. Academic Press; 2014. Chapter 5, Nutritional Quality of Fruits and Vegetables; p. 69–122. Available from: https://doi.org/10.1016/B978-0-12-408137-6.00005-3.
CrossRef - Al-Shammaa DA, Saour KY, and Abdul-Khalik ZM. Phytochemical Investigation for the Main Active Constituents in Arctium lappa L. Cultivated in Iraq. Iraqi J Pharm Sci., 2013; 22(1): 18-24. Available from: https://doi.org/10.31351/vol22iss1pp18-24.
CrossRef - Blundell R, Azzopardi J, Briffa J, Rasul A, Vargas-de la Cruz C, Shah MA. Analysis of pentaterpenoids. Recent Advances in Natural Products Analysis. Elsevier; 2020. p. 457-75.
CrossRef - Chistokhodova N, Zhiviriga I, Nguen C, Miles G, Uzhegova N, Solodnikov SY. β-Amyrylhexadecanoate from Parthenocissus quinquefolia as a thrombin inhibitor. Pharmaceutical Chemistry Journal, 2002; 36(5):245-7. Available from: http://doi.org/10.1023/A:1020517412615.
CrossRef - Fahmi ZM, Khadeem EJ, Hasan HF, Luaibi OK. The Antibacterial Effect of Phytosterols Isolated from Echinops heterophyllus in Comparison with MEBO ® and Standard Antimicrobial Agents. Ajps., 2014; 14(2):6–8. Available from: https://doi.org/10.32947/ajps.v14i2.154.
CrossRef - AD. M. Compendium of chemical terminology. Oxford: Blackwell Science. 1997:68.
- Brondz I. Fatty Acids. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. 2016.
CrossRef - Fatiha AI. Advances in lipid metabolism. London, United Kingdom: IntechOpen; 2020.3, Plant lipid metabolism. 1-6.
- WA. J-g. GC-MS Analysis on Fatty Acids in Parthenocissus quinquefolia Seed. Journal of Anhui Agricultural Sciences, 2010.
- Ibrahim RM, Alshammaa DA. Pharmacological Aspects of Borago officinalis (Borage): A Review. Iraqi J Pharm Sci., 2023; 32(1):1-13. Available from: https://doi.org/10.31351/vol32iss1pp1-13.
CrossRef - Khamees AH, Fawzi HA, Sahib HB. Phytochemical investigation and assessment of the hypoglycemic activity of two herbal extracts from selected Iraqi medicinal plants in alloxan-stimulated diabetic rats: a comparative study. F1000Research, 2020; 9:247. Available from: http://doi.org/ 10.12688/f1000research.22788.1.
CrossRef - Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care, 2004; 27(5):1047-53. Available from: http://doi.org/ 10.2337/diacare.27.5.1047.
CrossRef - Abdulsaid RT, Jabbar AS,and Akar TK. Impact of Type 2 Diabetes Mellitus on Pulmonary Function Tests. Iraqi J Pharm Sci., 2023; 32(2): 25-32. Available from: https://doi.org/10.31351/vol32iss2pp25-32.
CrossRef - Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care, 2000; 23(5):583-8. Available from: http://doi.org/10.2337/diacare.23.5.583.
CrossRef - Kumar S, Kunaparaju N, Zito SW, Barletta MA. Effect of Wrightia tinctoria and Parthenocissus quinquefolia on blood glucose and insulin levels in the Zucker diabetic rat model. Journal of complementary and integrative medicine, 2011; 8(1):1-12. Available from: https://doi.org/10.2202/1553-3840.1538.
CrossRef - Kareem AA, Aziz TA, Ahmed ZA, Othman HH, and Hussain SA. Anti-Inflammatory Activity of Gingko biloba Extract in Cotton Pellet-Induced Granuloma in Rats: A comparative Study with Prednisolone and Dexamethasone. Iraqi J Pharm Sci., 2022; 31(1):184-193. Available from: https://doi.org/10.31351/vol31iss1pp184-193.
CrossRef - Khaleel FM, Ghudhaib KK, Ali FE. The Influence of Obesity and IL-6 on Infertile Iraqi Women with COVID-19 Complications. Baghdad Sci. J., 2023; 20 (4 Special Issue): 1532-1539. Available from: https://doi.org/10.21123/bsj.2023.9081.
CrossRef - Nordqvist C. Everything you need to know about inflammation. Med News Today. Medilexicon, 2017 Nov.
- Abduljabbar HH, and Ibrahim MA. Study of the Anti-Inflammatory Effect of Tamsulosin in Rat by Evaluating IL-4, IL-6 and TNF-α: An airway Model. Iraqi J Pharm Sci., 2022; 32(1): 283-289. Available from: https://doi.org/10.31351/vol32iss1pp283-289.
CrossRef - Oleiwi AR, Zgair AK. Estimation levels of CTHRC1and some cytokines in Iraqi patients with Rheumatoid Arthritis. Baghdad Sci. J., 2023; 20(3 Suppl.): 928-936. Available from: https://doi.org/10.21123/bsj.2023.8036.
CrossRef - Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine & growth factor reviews, 2015; 26(5):475-87. Available from: http://doi.org/10.1016/j.cytogfr.2015.07.004.
CrossRef - Shakya A K, Kaur A, Al-Najjar B O and Naik R R. Molecular modeling, synthesis, characterization and pharmacological evaluation of benzo oxazole derivatives as non-steroidal antiinflammatory agents. Saudi Pharm J., 2016 Sep; 24(5):616-624. Available from: http://doi.org/ 10.1016/j.jsps.2015.03.018.
CrossRef - Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology, 2011; 29:415-45. Available from: http://doi.org/ 10.1146/annurev-immunol-031210-101322.
CrossRef - Lian J-J, Cheng B-F, Gao Y-X, Xue H, Wang L, Wang M, et al. Protective effect of kaempferol, a flavonoid widely present in varieties of edible plants, on IL-1β-induced inflammatory response via inhibiting MAPK, Akt, and NF-κB signalling in SW982 cells. Journal of Functional Foods, 2016; 27:214-22. Available from: https://doi.org/10.1016/j.jff.2016.09.003.
CrossRef - Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell metab., 2013; 18(4):533-45. Available from: http://doi.org 10.1016/j.cmet.2013.09.004
CrossRef - Martín AR, Villegas I, Sánchez‐Hidalgo M, De La Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol., 2006; 147(8):873-85. Available from: http://doi.org/10.1038/sj.bjp.0706469.
CrossRef - Ahmed H. Jwaid. Anti-inflammatory effect of Iraqi Parthenocissus quinquefolia L.hexane extract. Biochem. Cell. Arch., 2022; 22(1): 1221-1225.
- Rattanata N, Daduang S, Phaetchanla S, Bunyatratchata W, Promraksa B, Tavichakorntrakool R, et al. Antioxidant and antibacterial properties of selected Thai weed extracts. Asian Pacific Journal of Tropical Biomedicine, 2014; 4(11):890-5. Available from: https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0422.
CrossRef - Yempada P, Marrisetti AL, Battu GR, and Vedula GS. In silico, In vitro studies of Anti-Oxidant and Anthelminthic Abilities of Phytoconstituents from Rhynchosia cana (Wild.) DC. Iraqi J Pharm Sci., 2022; 31(1): 251-269. Available from: https://doi.org/10.31351/vol31iss1pp251-269.
CrossRef - Ibrahim NM, Kadhim EJ. Phytochemical Investigation and Antioxidant Activity of Iraqi Tribulus terrestris. Iraqi J Pharm Sci., 2015; 24(1): 68-73. Available from: https://doi.org/10.31351/vol24iss1pp68-73.
CrossRef - Shaheen S, Manzoor A. Phytochemical screening and antioxidant potential of Parthenocissus quinquefolia (L.) planch extracts of bark and stem. Pak J Pharm Sci., 2018; 31(5):1813-6.
- Mohajeri SA, Hedayati N, Bemani-Naeini M. Available saffron formulations and product patents. Woodhead Publishing; 2020. Chapter 33, Saffron; P. 493-515.
CrossRef








