Manuscript accepted on :03-10-2023
Published online on: 01-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Sonam Bhutia
Second Review by: Dr. Takkella Nagamma
Final Approval by: Dr. Anton R Kiselev
Aravinda Pai1*, Pooja Sharma P. C2, Venkatesh Kamath3, Chandrashekhar K. S4, Vasudev Pai5 and Muddukrishna B. S6
1Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India.
3Department of Pharmaceutical Biotechnology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India.
4Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India.
6Department of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India
Corresponding Author E-mail: aravind.pai@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2777
Abstract
In the present study, a congeneric series of novel substituted coumarin pyrazole carbaldehydes were synthesized. The compounds were characterized by various physical and spectroscopic methods. Preliminary cytotoxicity of the analogues was carried out using the MTT assay method on A-549 Lung cancer cell lines. The synthesized compounds possessed appreciable cytotoxicity against lung cancer cell lines. Out of the 8 synthesized compounds, the compound P-03 showed marked cytotoxicity of 13.5 mmol compared to standard doxorubicin which showed cytotoxicity value of 3.5 mmol. The compound P-03 was further investigated for its ability to induce apoptosis and its effect on cell cycle analysis. The compound P-03 was found to be an early apoptotic agent. After performing a cell cycle investigation, it was discovered that the compound P-03 effectively inhibited the G2/M phase of the cell cycle.
Keywords
Anticancer; Apoptosis; Coumarin; Cell Cycle; Hybrid; Mechanistic; Pyrazole
Download this article as:| Copy the following to cite this article: Pai A, Sharma P. C. P, Kamath V, Chandrashekhar K. S, Pai V, Muddukrishna B. S. Synthesis, Characterization and Mechanistic Anticancer Evaluation of Novel Analogues of Pyrazoles Derived from Substituted 3-Acetyl Coumarins. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Pai A, Sharma P. C. P, Kamath V, Chandrashekhar K. S, Pai V, Muddukrishna B. S. Synthesis, Characterization and Mechanistic Anticancer Evaluation of Novel Analogues of Pyrazoles Derived from Substituted 3-Acetyl Coumarins. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/48Dghla |
Introduction
Various illnesses with the potential to infiltrate or spread to different body parts include cancer, which is a category of diseases characterized by abnormal cell proliferation. About 90-95% of cancers are due to genetic mutations, which transform normal cell into malignant cells.1Cancer cells acquire a degree of autonomy from mutations of tumor suppressor gene, resulting in uncontrolled cell growth and its proliferation.
According to the WHO study 2020, cancer cases found to be 18 million and 10 million mortalities worldwide in 2018 and by 2040, the global prevalence is projected to double, to 29-37 million new cancer cases2. It is also a big health concern that needs to be tackled. The development of new anticancer therapeutics is one of medicinal chemistry’s top priorities because cancer accounts for roughly 70% of all fatalities. Because of the high demand for anticancer drugs, medicinal chemistry researchers have focused their efforts on the chemistry and biology of new anticancer agents3.
In recent years anticancer agents derived from natural products have gained the attention of researchers due to their wide range of therapeutic activity4. Coumarin, a versatile phenolic nucleus consisting of α -pyrone ring fused to a benzene ring that occurs naturally. Vogel first isolated it from tonka beans in 1820. Since tonka beans are rich in coumarins, the name came from the French word Coumarou. Coumarin-based compounds (Figure 1), both natural and synthetic, have anti-inflammatory, antibacterial, antiviral, antioxidant, and anticancer effects5.
 |
Figure 1: Basic ring structure of Coumarin |
Along with coumarin, pyrazole analogs are found to possess anticancer antioxidant and anticancer activity6. In the last decade pyrazole gained much interest of researchers since the its structure is frequently found as active ingredient in commercial drugs.
Because of its wide variety of biological activities, Pyrazole and its derivatives have attracted a lot of interest in recent decades7. Molecular modeling is used by thousands of researchers to design novel pyrazole analogues that can target cancer-related receptors such as protein kinase, tyrosine kinase, and vascular endothelial growth factor (VEGF)8.
Pyrazole, also called as 1,2 diazoles, contains three carbon atom and two nitrogen atoms present adjacent to each other which was denoted by the molecular formula C3H4N2. The pKb value for pyrazole is 11.5 which explains the weak basic nature of the ring and pKa of conjugated acid of pyrazole was found to be 2.49 at 25°C. Ludwig Knorr invented the word pyrazole in 1883. The Pyrazole ring itself possesses therapeutic anticancer, analgesic, anti-inflammatory, antioxidant, antimicrobial, anticonvulsant, antiviral etc. activities (Figure-2)9.
 |
Figure 2: Therapeutic properties of pyrazole nucleus. |
Ruxolitinib (blood cancer), Axitinib (renal cancer), Crizotinib (lung cancer), and other authorized anticancer medications all include the pyrazole ring as their fundamental core structure10 .
With this background, it was believed to synthesis some novel pyrazole analogues from substituted 3-Acetyl coumarin and test for antioxidant and antitumor activity.
Material and methods
Chemicals and analytical instruments
All the chemicals and reagents used in this investigation were from TCI and Sigma Aldrich. Using CDCl3, DMSO as the solvent, tetramethyl silane as an internal standard, and a Bruker Avance II spectrophotometer, the spectra for 1H NMR and 13C NMR were obtained. The units used to report chemical changes were parts per million (ppm). Waters LCMS equipment were used to obtain all mass spectra. Melting points were also measured using an Electro Thermal 9100 tool without any post-processing.
The infrared spectra were captured using a Shimadzu spectrometer, and the absorptions were measured using a wave number (cm-1) scale that spanned from 400 to 4000 cm-1.The synthesis plan for the target compounds is shown in Figure 3.
 |
Figure 3: General scheme for Coumarinyl pyrazole carbaldehyde synthesis. |
General procedure
Step 1 (Preparation of 3- acetyl coumarin):
Drop by drop, with constant stirring for five minutes, several salicylaldehyde derivatives each containing one equivalent (0.08 mole) of ethyl acetoacetate were added to the cool solution. About 5-6 drops of piperidine, the catalytic quantity, were added dropwise to the reaction mixture. Stirring continued for 6 to 48 hours. TLC was used to monitor the reaction’s completion while utilising a 3:2 n-hexane:ethyl acetate solvent solution. The methanol-based solvent was extinguished when the reaction was finished, and the reaction mixture was then poured over crushed ice. Purification was achieved by recrystallizing the obtained precipitates in toluene after filtering them out to obtain the crude product.
Step 2 (Preparation of Coumarinyl hydrazones).
One equivalent of the 3-acetyl coumarin derivatives (Product I) was put to a round-bottomed flask and dissolved in 10 ml of glacial acetic acid. With constant stirring, one equivalent of methanol-dissolved phenyl hydrazine derivatives was added to the solution. Using the solvent system n-hexane: ethyl acetate 3:2, stirring was continued for 1-2 hours until the reaction was complete. Orange-colored precipitates were seen after the reaction mixture was placed onto crushed ice. To achieve a pure product, the precipitates were filtered and washed with methanol.
Step 3 (Preparation of Coumarinyl pyrazole carbaldehyde’s)11
POCl3 (5ml) was added dropwise while stirring to a 25ml cold solution of DMF to create the Vilsmeier reagent. 30 minutes later, 5 mmo1 of Coumarinyl pyrazoles were added, portion by portion, and stirred continuously for 24 hours. After the reaction was finished, the liquid was poured over crushed ice, where yellow solid precipitates were seen. The mixture was then neutralised with a strong solution of NaOH. Precipitates were obtained, filtered to yield crude product, and refined using column chromatography with a ratio of 7:3 n-hexane to ethyl acetate.
Characterization
The synthesized compounds were examined using physical and spectroscopic techniques. Spectroscopic characterization was performed using UV, IR, MASS and NMR spectroscopic methods12. UV spectroscopy helps in identifying conjugation in molecules as conjugation is directly linked to UV absorption. The infrared spectroscopy helps in identifying the various functional groups present in molecules with specific bending or stretching vibrations.
The mass spectrum identifies the molecular ion peak that further helps in identifying the molecular weight of any sample molecules. NMR spectrum identifies the various type of magnetically equivalent protons present in the given structure. Through the spectral assignment, one can deduce the structure of a given organic molecule.
Anticancer activity using MTT assay method using A-549(Lung cancer cell lines)
According to the established protocol described in the literature, the MTT assay was carried out13. The cells were seeded on the 96 well plates along with media and test solution. Cell cultures’ media should be discarded. Aspirate the media slowly to check for adhering cells. For suspension cells, spin the 96-well plate at 1,000 x g at 4 °C for 5 minutes in a centrifuge that is compatible with microplates, then carefully aspirate the media. Each well should include 50 mL of serum-free medium and 50 mL of MTT solution. The plate should be incubated for three hours at 37 °C. After incubation, pour 150µL of MTT solvent into each well. The plate should be covered with foil and shook for 15 minutes using an orbital shaker. To completely dissolve the MTT formazan, the liquid might occasionally need to be pipetted. Read the absorbance at OD=590 nm.
PI Annexin V-FITC labelling for A549 cell apoptosis detection by flow cytometry.
According to the established protocol described in the literature, the apoptosis detection was carried out14.
Stepwise procedure
On the day before apoptosis was induced, 1 X106 cells per well for a 6-well plate were seeded using medium containing 10% FBS and 1% Pen Strep, respectively. These cells were then incubated overnight at 37°C with 5% CO2.
Test solutions in medium containing 10% FBS were substituted for the original media.
Under standard culture conditions, the treated cells were incubated for 24 hours.
After the cells were removed from the wells, the entire contents were transferred to the sterile FACS tubes.
The supernatant from the centrifugation of the cell contents at 2000 rpm for 5 minutes was discarded.
After centrifugation, rinsed the cells twice with cold PBS before resuspending them in 1 mL of 1X Binding Buffer at a concentration of approximately 1 x 106 cells/mL.
Transfer 500 mL (5 x 105 cells) of the cell suspension to a fresh FACS tube.
The tubes were filled with 8. 5 l Annexin V and 10 LPI, and the cells were then gently mixed before being incubated for 20 minutes at RT in the dark.
As quickly as feasible (within an hour), flow cytometry was used to analyze the cells.
Cell Cycle studies using A549 cells.
According to the established protocol described in the literature, the cell cycle analysis was carried out15.
Procedure
In a 6-well plate with 2 ml of medium, 1 x 106 cells were planted and grown for 24 hours. Following that, cells were given the appropriate concentrations of the specified samples, prepared in medium, and cultured for an additional 24 hours. The cells were then collected, centrifuged for 5 minutes at room temperature at 2000 rpm, and the supernatant carefully discarded while still holding the cell pellet. After resuspending the cell pellet in 2mL of 1XPBS, it was cleaned. Another time, the washing was done under the same circumstances. The particulate was kept in the supernatant, which was discarded. After resuspending the cells in 300 l of Sheath fluid, 1 mL of cold 70% EtOH was added drop by drop while being continuously gently shaken, and a final 1 mL of chilled 70% EtOH was added slowly all at once. The cells were then kept at 4 °C either overnight or for 30 minutes. The cells were centrifuged at 2000 rpm for 5 minutes after fixation. 2 ml of cold 1XPBS was used to wash the cell pellet twice. After that, the cell pellet was resuspended in 500 l of sheath fluid that included 0.05 mg/ml of PI and 0.05 mg/ml of RNaseA, and it was left to work for 20 minutes in the dark. Using FACS Calibre (BD Biosciences, San Jose, CA), it was possible to compare populations treated and untreated with drugs to assess the proportion of cells at different stages of the cell cycle.
Results and Discussion
Synthesis
The target compounds were synthesized in 3 steps via formation of variety of intermediates. In the step 1, 3-acetyl coumarin was synthesized from the reaction of various substituted salicyl aldehydes and ethyl acetoacetate via Knoevenagel condensation. In the step 2, Coumarinyl hydrazones were synthesized by the nucleophilic addition of substituted phenyl hydrazine to 3-acetyl coumarin. In the final cyclization step, Vilsmeier Haack reaction was utilized for the preparation of various substituted Coumarinyl pyrazole derivatives. The structures and important physical properties for the synthesized compounds were presented in the table 1.
 |
Table 1: Physico-chemical properties of synthesized test compounds |
Characterization
The synthesized test compounds were characterized by various spectroscopic methods including UV, IR, MASS, and NMR spectroscopic methods16.
In organic chemistry, the presence of free electrons or double (pi) bonds within a molecule can be determined using the UV/Visible spectroscopy approach. The term “Lamda-max” refers to the wavelength that a molecule absorbs most of, therefore it is possible to compare several compounds using this value17.
The lambda max of all the 8 synthesized coumarin-pyrazole carbaldehydes were presented in Table 2.
The UV spectra of one of the representative compound P-01 was presented in the Figure 4a.
Table 2: λ-max of synthesized compounds
|
Code |
λmax(nm) |
|
P-01 |
201.50 |
|
P-02 |
203.50 |
|
P-03 |
206.50 |
|
P-04 |
209.50 |
|
P-06 |
202 |
|
P-07 |
219 |
|
P-08 |
219 |
|
P-09 |
215 |
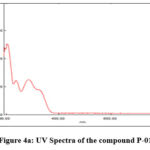 |
Figure 4a: UV Spectra of the compound P-01 |
IR spectroscopic analysis
IR spectra is useful to determine the functional groups present in the sample. Fingerprint region16 of IR spectra is unique for each compound since different compounds have different natural frequencies of vibrations, no two organic compounds will produce a similar spectrum.
The IR spectra for the representative compound P-01 is given in Figure 4b, the IR functional group values are provided in Table 3.
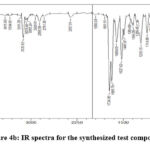 |
Figure 4b: IR spectra for the synthesized test compounds |
Table 3: IR functional group frequency values for the synthesized compounds
|
Functional groups |
IR values (cm-1) |
|
C=O |
1724.36 |
|
Aldehydic C=O |
1685.79 |
|
C=N |
1608.63 and 2357.01 |
|
Aldehydic CH |
2781.35 and 2856.58 |
|
C=C |
1527.62 |
|
Aromatic CH |
3055.24 |
MASS Spectrometric analysis
The LC-MS technique makes use of HPLC to isolate individual components from the mixture, followed by ionization and according to mass/charge ration ions are separated, directed into detector which recognizes and quantifies each ion18,19.
The popular Atmospheric Pressure Chemical ionization is the ion source used in LC-MS to generate ions from intact molecules. Since the LC-MS technique is precise, specific, sensitive the analysis is made at molecular level, it is easy to figure out structural details of the injected analyte.
The LCMS spectra for the representative compound P-01 is represented in Figure 5.
LC-MS (ITMS + cAPCI corona Full ms): Calculated for C19H12N2O3 [M+H]+ 317.32, found 317.11
 |
Figure 5: Mass spectrum of the representative compound. |
NMR spectroscopic analysis
NMR helps us by providing the information about different magnetically distinct atoms of the provided be it hydrogen or carbon. Also, it gives brief idea about the nature of immediate environment of each proton. So, it helps in determining the structure of the compound.
In the 1H NMR of the representative compound P-01 given below the characteristic peaks20. corresponding to protons were as follows
1H NMR (400MHz, CDCl3, δ in ppm): 10.061(1H, s, CHO), 8.525(1H, s, CH-pyrazole), 8.223(1H, s, CH of pyrone ring), 7.26-7.77 (9H, m, Ar-H)
The 1H NMR spectrum for the representative compound P-01 is presented in Figure 6.
 |
Figure 6: 1H NMR spectrum for the synthesized compound P-01 |
Anticancer activity
The cytotoxicity studies of the synthesized test compounds were performed on A549 lung cancer cell lines. All the synthesized compounds possessed appreciable anticancer activity against lung cancer cell lines. Out of the 8 compounds tested, the compound P-03 showed the most prominent anticancer activity with an IC50 of 13.5 mmol in comparison to the standard doxorubicin that showed an IC50 value of 3.63 mmol. Results of anticancer studies were presented in Table 4.
Table 4: Cytotoxicity of the synthesized test compounds against A549 lung cancer cell lines.
|
Compound |
IC50 mmol |
|
P-03 |
13.5 |
|
P-04 |
25 |
|
P-01 |
18 |
|
P-09 |
31.3 |
|
P-07 |
37.7 |
|
P-02 |
29.23 |
|
P-06 |
32 |
|
P-08 |
21.67 |
|
Doxorubicin |
3.63 |
To further substantiate their potential as anticancer agents, mechanistic studies including cell cycle analysis and apoptosis studies were performed.
Cell cycle analysis
The treatment of A549 cells at the concentrations of 12.5µM and 25µM with sample P03 has shown S phase and G2M phase arrest of 13.48%, 24.64% and 12.74%, 29.42% respectively.
Standard Colchicine at 25µM showed a G2M arrest of 36.55% in A549 cells as shown in the Figure 7.
 |
Figure 7: Cell cycle analysis of Compound P-03 with control and standard doxorubicin. |
The cell cycle analysis suggested the potential of the synthesized compound to inhibit the cell cycle at G2/M phase. The cell cycle analysis data was presented in Table 5 and Figure 8.
Table 5: Flow cytometry analysis of cell cycle arrest in A549 cell
|
FACS analysis of Cell cycle arrest in A549 cells |
||||
|
Samples |
SUBG0 |
G0/G1 |
S |
G2M |
|
Control |
1.30 |
86.08 |
8.10 |
4.88 |
|
P03_12.5µM |
1.32 |
73.14 |
13.48 |
12.74 |
|
P03_25µM |
0.44 |
46.38 |
24.64 |
29.42 |
|
Colchicine_25µM |
0.12 |
55.96 |
5.85 |
36.55 |
 |
Figure 8: Flow cytometry analysis of cell cycle arrest in A549 cells |
Apoptosis detection
The sample P03 treated at 12.5µM and 25µM has induced 5.89%, 29.82% early apoptosis and 13.42%, 11.22% late apoptosis in A549 respectively. Standard Doxorubicin at 25µM has shown total apoptosis of 46.93% in A549 cellsas shown in the Figure 9.
 |
Figure 9: Apoptosis detection with compound P-03 against A549 lung cancer cell lines with control and standard doxorubicin. |
The apoptosis studies suggested the potential of the synthesized compound in inducing late apoptosis. The FACS apoptosis data was presented in Table 6 and Figure 10.
Table 6: Flow cytometry analysis of Apoptosis detection in A549 cell lines
|
FACS analysis of Apoptosis detection in A549 cells |
||||
|
Sample |
Viable cells |
Early Apoptotic |
Late Apoptotic |
Necrotic cells |
|
Control |
98.58 |
0.01 |
0.17 |
1.24 |
|
P03_12.5µM |
77.14 |
5.89 |
13.42 |
3.55 |
|
P03_25µM |
55.42 |
29.82 |
11.22 |
3.54 |
|
Doxorubicin_25µM |
51.98 |
31.97 |
14.96 |
1.10 |
 |
Figure 10: Flow cytometry analysis of Apoptosis detection in A549 cell lines |
Conclusion
A series of novel substituted coumarin pyrazole hybrids were synthesized utilizing the concept of hybridization as a lead optimization approach. The synthesized compounds were characterized by physical as well as spectroscopic techniques. Anti cancer activity of the compunds was performed on A-549 lung cancer cell lines against standard doxorubicin. Out of the 8 compounds synthesized, the compound P-03 emerged as a potent antiproliferative agent with an IC50 value of 13.5 mmol in comparison to standard doxorubicin that possessed an IC50 value of 3.63 mmol. The presence of bromine attached to the coumarin moeity significantly enhanced its anticancer potential. To substantiate the results, mechanistic anticancer studies including cell cycle analysis and apoptosis detection studies were performed. Cell cycle analysis further confirmed its ability to inhibit cell cycle at G2/M phase. Apoptosis studies confirmed its role as a late apoptotic agent.
In future studies, an attempt will be made to study their anticancer potential in animal models to reconfirm its ability against lung cancer models.
Acknowledgement
The authors acknowledge the Manipal Academy of Higher Education for the support provided to carry out the present research.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
The current research is not funded by any government of private funding agencies.
References
- Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021 Feb 23;6(1):75.
- Rodriguez-Arrastia M, Martinez-Ortigosa A, Rueda-Ruzafa L, Folch Ayora A, Ropero-Padilla C. Probiotic supplements on oncology patients’ treatment-related side effects: a systematic review of randomized controlled trials. nt. J. Environ. Health Res. 2021 Apr 17;18(8):4265.
- Emami S, Dadashpour S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem.2015 Sep 18; 102:611-30.
- Bhanot A, Sharma R, Noolvi MN. Natural sources as potential anti-cancer agents: A review. Int. J. Phytomedicine . 2011 Jan 1;3(1):09.
- Patel M, Pandey N, Timaniya J, Parikh P, Chauhan A, Jain N, Patel K. Coumarin–carbazole based functionalized pyrazolines: Synthesis, characterization, anticancer investigation and molecular docking. RSC Adv. 2021;11(44):27627-44.
- Detsi A, Kontogiorgis C, Hadjipavlou-Litina D. Coumarin derivatives: an updated patent review (2015-2016). Expert opinion on therapeutic patents. 2017 Nov 2;27(11):1201-26.
- Akhtar J, Khan AA, Ali Z, Haider R, Yar MS. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem.2017 Jan 5;125:143-89.
- Ling Y, Liu J, Qian J, Meng C, Guo J, Gao W, Xiong B, Ling C, Zhang Y. Recent advances in multi-target drugs targeting protein kinases and histone deacetylases in cancer therapy. Curr. Med. Chem,2020 Dec 1;27(42):7264-88.
- Matada BS, Pattanashettar R, Yernale NG. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem . 2021 Feb 15;32:115973.
- Khatun B, Kamath V, Muddukrishna BS, Pai A. Extra Precision Docking and ADME Simulation Studies on Novel Analogues of Pyrazoles as Anticancer Leads.MJPS. 2020;6(1):7.
- Srikrishna D, Dubey PK. Facile, stepwise and diversity-oriented synthesis of 3-(2-Oxo-2H-Chromen-3-yl)-1-Phenyl-1H-Pyrazole-4-carbaldehydes. J Chem Pharm Res. 2017;9(11):99-108.
- Karthikeyan N, Prince JJ, Ramalingam S, Periandy S. Electronic [UV–Visible] and vibrational [FT-IR, FT-Raman] investigation and NMR–mass spectroscopic analysis of terephthalic acid using quantum Gaussian calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015 Mar 15;139:229-42.
- Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012 Mar 1;5(3):822-6.
- Khaghanzadeh N, Mojtahedi Z, Ramezani M, Erfani N, Ghaderi A. Umbelliprenin is cytotoxic against QU-DB large cell lung cancer cell line but anti-proliferative against A549 adenocarcinoma cells. DARU J. Pharm . 2012 Dec;20:1-6.
- Lu XX, Cao LY, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res. Int . 2016 Oct 16;2016.
- Solomons TW. Spectroscopic methods of structure determination. Organic Chemistry. 6th ed. University of South Florida. John Wiley and Sons, Inc. Canada. 1996.
- Akash MS, Rehman K, Akash MS, Rehman K. Ultraviolet-visible (UV-VIS) spectroscopy. Essentials of pharmaceutical analysis. 2020:29-56.
- Koleva BB, Kolev T, Seidel RW, Tsanev T, Mayer-Figge H, Spiteller M, Sheldrick WS. Spectroscopic and structural elucidation of 4-dimethylaminopyridine and its hydrogensquarate. Spectrochimica, Acta A Mol. Biomol. Spectrosc . 2008 Nov 15;71(2):695-702.
- Beck JL, Colgrave ML, Ralph SF, Sheil MM. Electrospray ionization mass spectrometry of oligonucleotide complexes with drugs, metals, and proteins. Mass Spectrom. Rev. 2001;20(2):61-87.
- Holzgrabe U, Diehl BW, Wawer I. NMR spectroscopy in pharmacy. J. Pharm. Biomed. Anal , 1998 Aug 1;17(4-5):557-616.







