Manuscript accepted on :13-10-2023
Published online on: 02-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Dito Anurog
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. Patorn Piromchai
Asmaa Moafa1,2 , Sara A. Aldossary1
, Sara A. Aldossary1 , Mohammed Al mohaini3
, Mohammed Al mohaini3 and Abdulkhaliq J. Alsalman.4
and Abdulkhaliq J. Alsalman.4
1Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, 31982 Alahsa, Saudi Arabia.
2Pharmacy Services Department, Johns Hopkins Aramco Healthcare (JHAH), Saudi Arabia.
3Basic Sciences Department, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Alahsa 31982, Saudi Arabia
4 Department of Clinical Pharmacy, Faculty of Pharmacy, Northern Border University, Rafha 91911, Saudi Arabia.
Corresponding Author E-mail: saldossary@kfu.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/2805
Abstract
Gentamicin is an extensively used antibiotic with potent antimicrobial exertion, but its clinical mileage is limited by its eventuality to induce hepatotoxicity. This study aimed to probe the defensive effect of aspirin against gentamicin-convinced hepatotoxicity in a rat model. Adult manly Wistar rats were divided into four groups control, aspirin, gentamicin, and aspirin- gentamicin. The creatures were treated for 15 successive days, and colorful biochemical parameters were estimated. Pre-treatment with aspirin significantly downgraded the adverse goods of gentamicin on liver weight. It also eased the elevation of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) situations, indicating the preservation of liver function. Aspirin treatment suppressed hepatic lipid peroxidation, as substantiated by a reduction in malondialdehyde (MDA) situations. likewise, it averted the reduction of glutathione (GSH) situations and catalase exertion convinced by gentamicin administration. These findings suggest that aspirin exerts a hepatoprotective effect by reducing oxidative stress and enhancing antioxidant defense mechanisms. The protective mechanisms of aspirin may involve its anti-inflammatory properties, as well as its antioxidant effects. Aspirin has the potential to inhibit inflammation-induced liver injury and modulate signaling pathways involved in cell survival and apoptosis. However, further investigations are needed to elucidate the precise molecular mechanisms underlying its protective effects. Overall, pre-treatment with aspirin demonstrated a protective effect against gentamicin-induced hepatotoxicity in this rat model. It mitigated liver damage, preserved liver function, and enhanced antioxidant defense mechanisms. These findings suggest that aspirin could be a potential therapeutic agent for the prevention and management of drug-induced liver injury. Further studies, including clinical trials, are necessary to determine the optimal dosage, duration, and safety profile of aspirin in humans.
Keywords
Aspirin; Gentamicin; Hepatotoxicity; Rats
Download this article as:| Copy the following to cite this article: Moafa A, Aldossary S. A, Al mohaini M, Alsalman A. J. Protective Effect of Aspirin Against Gentamicin-Induced Hepatotoxicity in Rats Model. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Moafa A, Aldossary S. A, Al mohaini M, Alsalman A. J. Protective Effect of Aspirin Against Gentamicin-Induced Hepatotoxicity in Rats Model. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/4aGts6z |
Introduction
The use of certain medications, while beneficial for treating various conditions, can sometimes lead to adverse effects on the liver. Gentamicin, a widely used antibiotic, has been associated with hepatotoxicity, which refers to liver damage caused by toxic substances. In recent years, research has focused on exploring potential protective agents that can mitigate the hepatotoxic effects of gentamicin. One such agent of interest is aspirin, a commonly used medication with known anti-inflammatory and antioxidant properties1.
Gentamicin-induced hepatotoxicity is characterized by liver dysfunction, increased liver weight, and an increase in liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in the serum. Hepatic lipid peroxidation, reduction in the level of antioxidants, and impairment of cellular functions contribute to the development of gentamicin-induced liver injury. Aspirin, also known as acetylsalicylic acid, has been investigated for its potential hepatoprotective properties. Studies have suggested that aspirin possesses antioxidant and anti-inflammatory properties, which may help alleviate liver damage induced by gentamicin2-4. The mechanism underlying the protective effect of aspirin is believed to involve the inhibition of oxidative stress and the modulation of inflammatory pathways.
The goal of this study was to examine the effect of aspirin on gentamicin-induced hepatotoxicity in an animal model. Specifically, we examined the impact of aspirin on liver weight, serum liver enzymes (ALT and AST), as well as oxidative stress markers, such as malondialdehyde (MDA), glutathione (GSH), nitric oxide (NO), and catalase activity. Male Wistar rats were randomly grouped into four: control, aspirin group, gentamicin group, and aspirin-gentamicin group5. The animals were treated according to the designated groups, and liver weight, serum liver enzymes, and oxidative stress markers were assessed during the experimental time. The doses of gentamicin and aspirin were chosen to depend on previous studies6-7.
Material and Method
Drugs
Gentamicin (CAS- No 1405410, 100 pure) and aspirin (CAS- No 502658 chastity 98) were purchased from Sigma- Aldrich Chemical Company (St.Louis, Missouri, USA). Other reagents were of logical grade.
Animals
Wistar rats (70 – 80 days old) importing were selected (Faculty of Pharmacy, King Faisal University,), housed in clean polypropylene coops, and maintained on a 12- hour light/ dark cycle at a temperature of 20 – 25C with ad libitum access to standard food and water.7 days pre-experimental rats were handled daily for minimize their physiological responses to handling for posterior protocols. All treatment described was performed according to the Research Ethics Committee of King Faisal University.
Experimental protocol
Animals were sectioned into four groups consisting of six rats each. Group I(control) entered the vehicle. Group II (aspirin group) entered aspirin 10mg/ ml (200mg/ kg/ day intragastrical). Group III (gentamicin group) was treated with gentamicin (100 mg/ kg/ day by intraperitoneal) in a single injection. Group IV (aspirin- gentamicin group) was treated with aspirin and gentamicin by the same schedule. Groups II and IV entered aspirin from day 1 until day 15(the total period of the trial). Groups III and IV entered gentamicin for 10 successive days, starting from the sixth day of the trial until day 15. The selected doses of gentamicin and aspirin were chosen based on previous studies7.
Sample medication and biochemical tests
animals were killed by stunning and cervical dislocation under Schedule 1 according to the United Kingdom Animals (Scientific Procedures) Act 1986 a day after the last dose of gentamicin. blood samples were taken and left for 1 hour to clot. Centrifuge blood sample for 10 twinkles at 5000 rpm to get a pure serum that was also stored at a 20-degree temperature. To estimate serum aspartate aminotransferase as well as alanine aminotransferase kit (Randox Laboratories Ltd. UK, recommending, colorimetric accouterments) were used.
liver tissue washed with ice-cold saline and stored at 80-degree temperature. exercising cold potassium phosphate buffer, the liver was homogenized. Homogenates were separated at 5000 rpm for 10 twinkles at 4 ℃ and the supernatant was used for determining malondialdehyde and minimizing glutathione degree and exertion of catalase exercising colorimetric accoutrements of the assay as framed by the manufacturer’s instructions forbio-diagnostic (Bodaghi- Namilehet.al. 2018). Depending on the manufacturer, Cayman Chemical Co, USA, instructions, the degree of nitric oxide (NO) level was determined by exercising a tackle of the colorimetric assay.
Statistical analysis
To assay the collected data, all the data were expressed as mean ±S.E.M (pars of the repeated trials). A one-way analysis of friction (ANOVA) was done, followed by the Tukey test for the multiple comparisons. SPSS interpretation 21 was used. The differences were determined at the significance position of p<0.05.
Results
Effects of aspirin on the measured biochemical parameters
Treatment rats with gentamicin significantly increased liver weight compared to control rats (figure 1) whereases treatment rats with aspirin were suitable to reverse the effect of gentamicin on liver weight. While in Figure 2, dramatic increase in serum alanine aminotransferase and aspartate aminotransferase situations was witnessed in the animals that were subjected to gentamicin compared to the control group. Our results demonstrated that the treatment of aspirin, after gentamicin administration urged a significant reduction in the serum situations of the aminotransferases. Further, the treatment with aspirin significantly suppressed hepatic lipid peroxidation and averted reductions in GSH position and catalase exertion because of gentamicin administration.
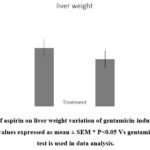 |
Figure 1: Effect of aspirin on liver weight variation of gentamicin-induced hepatotoxicity in rats.
|
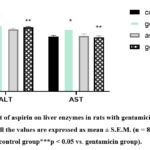 |
Figure 2: Factors Influencing the Knowledge, Attitude and Use of CAM Modalities.
|
Table 1: Effect of aspirin on antioxidant levels in gentamicin-induced hepatotoxicity in animals. All the values are expressed as mean ± S.E.M., n = 8 in each group. a p < 0.05 vs. control group.b p < 0.05 vs. gentamicin.
|
GSH (mg/g tissue) |
NO (nmol/100mg tissue) |
MDA |
CATALASE (u/g tissue) |
Dose (mg/kg) |
Treatment |
|
115.3±1.2 |
90.3±0.6 |
25.50±0.36 |
5.5±0.47 |
Control |
|
|
105±4.3 a |
102.3±1.55 a |
51.71±1.03 a |
3.4±0.32 a |
100mg/kg |
gentamicin |
|
134.4±3.3 |
132.7±1.3 |
36.8±0.47 |
4.7±0.56 |
200mg/kg |
aspirin |
|
116.4±1.8 b |
109.7±1.02 b |
40.26±0.82 b |
5.3±0.54 b |
|
Aspirin+ gentamicin |
*MDA: malondialdehyde, NO: nitric oxide, GSH: glutathione.
Discussion
The current study aimed to examine the effect of aspirin against gentamicin-induced hepatotoxicity in a rat model. The results demonstrated that pre-treatment with aspirin significantly mitigated the adverse effects of gentamicin on liver weight, serum liver enzymes, and oxidative stress markers.
Gentamicin administration resulted in a dramatically elevation in liver weight, which is indicative of liver damage. This elevated liver weight may be caused by edema, congestion, and cellular infiltration caused by the toxic effects of gentamicin on hepatocytes8. However, pre-treatment with aspirin reversed this effect, suggesting a protective role against gentamicin-induced hepatotoxicity 9-11. The ability of aspirin to counteract the increase in liver weight may be attributed to its anti-inflammatory properties, as inflammation is known to contribute to liver injury. Aspirin’s anti-inflammatory effects have been well documented and may involve the inhibition of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and the modulation of various signaling pathways involved in the inflammatory response 12.
Elevation in liver enzymes is usually known as the prediction of hepatocellular damage. In this study, gentamicin administration led to a significant elevation in serum ALT and AST levels, indicating liver injury 13. However, pre-treatment with aspirin significantly reduced the serum levels of these liver enzymes. This finding suggests that aspirin exerted a protective effect on liver cells and preserved their structural and functional integrity 14-16. The mechanisms underlying the hepatoprotective effects of aspirin may involve the inhibition of inflammation-induced hepatocyte apoptosis and the modulation of oxidative stress pathways 17. Aspirin has been shown to inhibit the release of pro-inflammatory mediators and to promote the expression of anti-apoptotic proteins, thereby preventing hepatocyte damage and promoting cell survival.
Oxidative stress plays a crucial role in gentamicin-induced hepatotoxicity. Increased release of reactive oxygen species (ROS) and impaired antioxidant defense mechanisms contribute to liver cell damage 18. In the present study, aspirin treatment significantly suppressed hepatic lipid peroxidation, as evidenced by the reduction in malondialdehyde (MDA) levels 16. This indicates that aspirin acted as an antioxidant and attenuated lipid peroxidation, thereby protecting liver cells from oxidative damage. Aspirin’s antioxidant properties may be attributed to its capability to kick free radicals out of the body, inhibit ROS production, and enhance the activity of endogenous antioxidant enzymes 18-22.
Moreover, aspirin treatment reduced the depletion of glutathione (GSH) levels and catalase activity induced by gentamicin application. GSH is an important endogenous antioxidant that helps neutralize ROS and maintain redox balance in cells 14-19. Catalase is an enzyme involved in the detoxification of hydrogen peroxide, a reactive oxygen species. The preservation of GSH levels and catalase activity by aspirin suggests its ability to enhance the antioxidant defense mechanisms in the liver and protect against oxidative stress-induced liver injury14-20. Aspirin may exert its effects on GSH and catalase through the modulation of transcription factors and signaling pathways involved in antioxidant gene expression and activity.
The mechanism underlying the protective effect of aspirin against gentamicin-induced hepatotoxicity is likely multifactorial. Aspirin possesses anti-inflammatory properties, which can attenuate inflammation-induced liver injury. It also acts as an antioxidant, reducing oxidative stress and preventing oxidative damage to liver cells. Additionally, aspirin may modulate signaling pathways involved in cell survival and apoptosis, contributing to its hepatoprotective effects.
The findings of this study support previous research indicating the potential hepatoprotective properties of aspirin. However, further investigations are warranted to elucidate the exact molecular mechanisms underlying its protective effects and to determine the optimal dosage and duration of aspirin treatment for maximum efficacy. Additionally, future studies should explore the long-term effects of aspirin treatment, as well as its potential interactions with other medications commonly co-administered with gentamicin15.
Conclusion
In conclusion, this study demonstrates that pre-treatment with aspirin attenuates gentamicin-induced hepatotoxicity in a rat model. Aspirin mitigated liver damage, preserved liver function, and enhanced antioxidant defense mechanisms. These findings highlight the potential of aspirin as a protective agent against drug-induced liver injury. Further studies are needed to fully understand the mechanisms involved and to explore its clinical applications in humans. The use of animal models provides valuable insights into the potential benefits of aspirin in the prevention and management of hepatotoxicity, but further clinical trials are necessary, especially in high-risk patients treated with gentamicin to establish its efficacy and safety in human patients13.
To conclude, the findings of this work provide evidence for the protective effect of aspirin against gentamicin-induced hepatotoxicity in a rat model. Pre-treatment with aspirin demonstrated beneficial effects on liver weight, serum liver enzymes, and oxidative stress markers. These findings suggest that aspirin possesses hepatoprotective properties and may be a potential therapeutic option for the prevention and management of drug-induced liver injury.
Acknowledgment
Thanks to deanship of scientific research king faisal university for supporting this work (grant 5181).
Conflict of Interest
The authors declare no conflict of interest.
Funding source
There is no funding source.
References
- Aboubakr, Mohamed, and Abdelazem Mohamed Abdelazem. (2016)”Hepatoprotective effect of aqueous extract cardamom against gentamicin induced hepatic damage in rats.” Int J Basic Appl Sci 5, no. 1: 1.
CrossRef - Elderbi, M. A., Mohamed, A. W. H., Hadi, A. H. A., &Dabobash, M. D. (2014). Potential protective effect of gum Arabic against doxorubicin-induced Cardiotoxicity in Wistar albino rats. International Journal of Pharmaceutical Sciences and Research, 5(3), 1023.
- Bejeshk, M. A., Aminizadeh, A. H., Rajizadeh, M. A., Khaksari, M., Lashkarizadeh, M., Shahrokhi, N., … & Azimi, M. (2022). The effect of combining basil seeds and gum Arabic on the healing process of experimental acetic acid-induced ulcerative colitis in rats. Journal of Traditional and Complementary Medicine, 12(6), 599-607
CrossRef - Yadav, N., Sharma, S., Sharma, S., & Sharma, K. (2017). Critical Analysis of protective role of plants against gentamicin induced nephrotoxicity. Indian J Environ Sci, 21(1), 1-34.
- Sha, S. H., Qiu, J. H., & Schacht, J. (2006). Aspirin to prevent gentamicin-induced hearing loss. New England Journal of Medicine, 354(17), 1856-1857.
CrossRef - Alrashedi, M. G. (2018). The protective role of thymoquinone against drugs toxicity: a review. Journal of Pharmaceutical Research International, 24(3), 1-11.
CrossRef - Bektur, N. E., Sahin, E., Baycu, C., & Unver, G. (2016). Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicology and Industrial Health, 32(4), 589-600.
CrossRef - Mangunsong, S., Putra, M. A., &Taswin, M. (2021, December). The Protective Effects of Betacarotene from Carrot (Daucus carota L.) on Paracetamol Induced nephrotoxicity in Male Laboratory Rats. In International Conference on Nutrition (Vol. 1, No. 1, pp. 134-140).
- Abd-Elhakim, Y. M., Moselhy, A. A., Aldhahrani, A., Beheiry, R. R., Mohamed, W. A., Soliman, M. M., … & M. El Deib, M. (2021). Protective effect of curcumin against sodium salicylate-induced oxidative kidney damage, nuclear factor-kappa dysregulation, and apoptotic consequences in rats. Antioxidants, 10(6), 826.
CrossRef - Ul Hassan, Dr. H. (2022). Protective effect of selenium against methotrexate induced hepatotoxicity. International Journal of Current Science Research and Review, 05(04). https://doi.org/10.47191/ijcsrr/v5-i4-09
CrossRef - Salman, H., Daoud, A., & Dabbagh, M. (2020). Evaluation of a Protective Effect of the Water Extract of Thymus Leaves Against Diclofenac Sodium-Induced Renal Toxicity in the Syrian Hamster. Evaluation, 7(2), 151-157.
- Nale, L. P., More, P. R., More, B. K., Ghumare, B. C., Shendre, S. B., & Mote, C. S. (2012). Protective effect of Carica papaya L. seed extract in gentamicin induced hepatotoxicity and nephrotoxicity in rats. Int J Pharm Bio Sci, 3(3), 508-515.
- Jafaripour, L., Naserzadeh, R., Ahmadvand, H., Hadipour Moradi, F., Ghobadi, K., Alizamani, E., & Nouryazdan, N. (2019). Effects of L-glutamine on oxidative stress in gentamicin induced hepatotoxicity rats. Journal of Kerman University of Medical Sciences, 26(1), 36-42.
- Yildirim, B. A., Kordali, S., Kapakin, K. A. T., Yildirim, F., Senocak, E. A., & Altun, S. (2017). Effect of Helichrysum plicatum DC. subsp. plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. Journal of Zhejiang University. Science. B, 18(6), 501.
CrossRef - Chen, Y., Huang, W. G., Zha, D. J., Qiu, J. H., Wang, J. L., Sha, S. H., & Schacht, J. (2007). Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hearing research, 226(1-2), 178-182.
CrossRef - Kyle, M. E., Wang, J. C., & Shin, J. J. (2015). Ubiquitous aspirin: a systematic review of its impact on sensorineural hearing loss. Otolaryngology–Head and Neck Surgery, 152(1), 23-41.
CrossRef - El Sayed, E., Mohamed, R., Mohamed, S., Rahman, A., Ezz El Din H, H., Khaled, N., … & Kholy, E. (1997). Studies on nephrotoxicity aspirin and gentamicin in combination in rats.
- Kharkheli, E., Kevanishvili, Z., Maglakelidze, T., Davitashvili, O., & Schacht, J. (2007). Does vitamin E prevent gentamicin-induced ototoxicity. Georgian Med News, 146, 14-17.
- Podhaisky, H.P., Abate, A., Polte, T., Oberle, S. and Schröder, H., 1997. Aspirin protects endothelial cells from oxidative stress–possible synergism with vitamin E. FEBS letters, 417(3), pp.349-351.
CrossRef - Grosser, N. and Schröder, H., 2003. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway.
CrossRef Arteriosclerosis, thrombosis, and vascular biology, 23(8), pp.1345-1351.
CrossRef - Lu, G., Tong, Z., Ding, Y., Liu, J., Pan, Y., Gao, L., Tu, J., Wang, Y., Liu, G. and Li, W., 2016. Aspirin protects against acinar cells necrosis in severe acute pancreatitis in mice. BioMed Research International, 2016.
CrossRef - Chang, P.Y., Chen, Y.J., Chang, F.H., Lu, J., Huang, W.H., Yang, T.C., Lee, Y.T., Chang, S.F., Lu, S.C. and Chen, C.H., 2013. Aspirin protects human coronary artery endothelial cells against atherogenic electronegative LDL via an epigenetic mechanism: a novel cytoprotective role of aspirin in acute myocardial infarction. Cardiovascular research, 99(1), pp.137-145.
CrossRef







