G. Mallika 1, 2 and K. Shailaja1*
and K. Shailaja1*
1Department of Botany, UCS, Osmania University, Hyderabad Telangana, India.
2Departmne of Botany, Govt. Degree College for Women, Sangaredd, Telangana State, India.
Corresponding Author E-mail: gootymallika@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2813
Abstract
Objective: The objective of the present investigation is to study the phytochemical composition, antioxidant and antibacterial activity of petroleum ether, chloroform, ethanol, methanol and water extracts of the leaf part of Celtis timorensis Spr. The preliminary phytochemical studies were conducted according to standard procedures. Total phenolic content was estimated using the FCA reagent method. The antioxidant efficiency of extracts was evaluated by using molybdate and DPPH methods. The antibacterial potency of leaf extracts was studied using the disc diffusion method against eight human pathogenic bacterial strains. Results: The results of preliminary phytochemical study revealed the presence of alkaloids, phytosterols, phenolic components, tannins, flavonoids, terpenoids, glycosides and saponins. The total phenolic content of the tested extracts exhibited a range between 8.82 to 68.32 mg GAE/g dwt. The highest total phenolic content was observed in the methanol extract (68.32±1.03 mg GAE/g dwt.) and the highest total antioxidant capacity was observed in the methanol extract of leaf part (700.0±0.71 mg ASE/g dwt.).Regarding DPPH scavenging activity the highest DPPH-reducing activity (>90%) was observed by methanol, ethanol and water extracts of the leaf part. Ethanol and water extracts of leaf samples strongly inhibited the gram-negative bacterial species Pseudomonas aeruginosa and Salmonella enterica (13 mm for each species) respectively. While gram-positive species i.e. Bacillus megatherium Artherobacter protophormiae and P. aeruginosa were moderately inhibited by chloroform, ethanol and water extracts (12 mm for each) respectively. Conclusion: In conclusion, the selected medicinal plant C. timorensis extracts exhibited good antioxidant activity, strong antibacterial activity and rich bioactive components. It required further studies on the isolation, and characterization of active principle to evaluate its pharmacological properties.
Keywords
Antibacterial activity; Celtis timorensis; DPPH; total phenolic content
Download this article as:| Copy the following to cite this article: Mallika G. Shailaja K. Phytochemical Composition, Antioxidant and Antibacterial Studies on Celtis Timorensis Leaf Extract. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Mallika G. Shailaja K. Phytochemical Composition, Antioxidant and Antibacterial Studies on Celtis Timorensis Leaf Extract. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/40dNyAC |
Introduction
Infectious diseases caused by bacterial species are a major health issue for global population. The major human infections causing bacterial species are Pseudomonas aeruginosa, E. coli, Proteus vulgaris, Staphylococcus aureus and Bacillus subtilis1,2.Medicinal plants used in traditional medicinal systems are a major source for the treatment of infectious diseases caused by microorganisms. These plants are rich in a wide variety of chemical constituents such as alkaloids, terpenoids, flavonoids and tannins and essentials have been found as potent antimicrobial agents3,4. Scientific studies on the antibacterial activity of medicinal plants have reported that traditional medicinal plant extracts/ phytoconstituents have potent antimicrobial activity 5, 6, 7.
Celtis timorensis Spr. is a medium-sized tree of flowering plant belongs to the family Ulmaceae, distributed in India, Nepal, Thailand, Vietnam, and Malaysia. Different parts i.e. leaf, stem, root, fruit, etc. of this plant have been used by the tribes of Indian states to cure various human diseases such as dysentery, jaundice, memory enhancement, toothache, urinary tract infection and food8, 9, 10, 11. The review on phytochemical composition, antibacterial, antioxidant and pharmacological studies on Celtis timorensis indicate that very few and sporadic attempts are noticed on preliminary phytochemical 12, DPPH13 antibacterial 14, antidepressant15, acute and sub-acute toxicity12 and wound healing activity16of leaf extracts of Celtis timorensis. But no previous report was noticed on phytochemical analysis, molybdate dependant antioxidant, DPPH and antibacterial activity of Celtis timorensisleaf part. Hence, we selected Celtis timorensis leaf extracts to evaluate the phytochemical analysis and antioxidant and antibacterial studies of five extracts.
Materials and methods
Plant material
Celtis timorensis leaves were collected from Tirumala hills of Andhra Pradesh. The plant specimen was identified (Voucher #1379) by Dr K. Madhava Chetty, Assistant Professor (Rtd.), Department of Botany, S.V. University, Tirupati.
Preparation of plant extracts
The collected plant sample was washed with distilled water and dried at room temperature. Fifty grams of plant material was pounded and successively extracted with Petroleum ether (PE), chloroform (CE), ethanol (EE) and methanol (ME) using Soxhlet apparatus for 6-8 hours. The extracts were filtered and concentrated under reduced pressure to dryness and the extracts were used for the assay. The yield of each extract was depicted (Table 1).
Table 1: Yield of Celtis timorensis leaf extracts
|
S. No. |
Extract Type |
Yield (%) |
|
Leaf Extracts |
||
|
1 |
Petroleum ether |
4.28% |
|
2 |
Chloroform |
2.86% |
|
3 |
Ethanol |
14.32% |
|
4 |
Methanol |
14.56% |
|
5 |
Water |
5.0% |
Water extract preparation
One gram of plant material after the successive extraction was taken and soaked in 50 ml distilled water for 24 h and filtered. The filtrate was concentrated in a water bath at 400C, and subjected to phytochemical, antioxidant and antibacterial studies.
Preliminary phytochemical screening
Qualitative phytochemical tests for alkaloids, saponins, phytosterols17, starch, glycosides, phenolic components, gums and tannins18were determined using standard methods.
Total phenolic content of Celtis timorensis leaf extracts
The total phenolic quantity of Celtis timorensis leaf extract was estimated using methods as described 19.
Total antioxidant capacity of Celtis timorensis leaf extracts
Ammonium molybdate dependant antioxidant capacity of Celtis timorensis leaf extracts was determined using methods as described20.
DPPH reducing activity
DPPH scavenging capacity of Celtis timorensis leaf extracts was determined using methods as described 20.
Antibacterial studies
Microorganisms used
The microorganisms used in the present study are Micrococcus luteus (MTCC 9341), Arthrobacter protopharmiae (MTCC 688), Alkaligenes faecalis (MTCC 10757), Enterococcus faecalis (MTCC 439), Bacillus megatherium (MTCC 5981), Lactobacillus acidophilus (MTCC 10307), Salmonella enterica (MTCC 3858) and Pseudomonas aeruginosa (MTCC 1688) to test the extracts. The organisms were purchased from the MTCC center, IMTECH, Chandigarh, India.
Antimicrobial activity
The antibacterial efficacy of C. timorensis leaf petroleum ether (PE), chloroform (CE), ethanol (EE) methanol (ME) and water extracts were studied using the disc diffusion method21. Paper discs (4 mm) impregnated with distinct concentrations of plant extracts were placed on the petri-plates, containing 20ml of Mueller Hinton Agar (MHA) media (Peptone 17.5 g, meat extract 2 g, starch 1.5 g and agar-agar 17 g/L) seeded with 0.1ml of overnight grown microbial suspension. The discs saturated with methanol ethyl acetate and petroleum ether served as negative controls. Bacterial-free zones present around the discs were treated as positive results. The zones were measured after 24 hours and tabulated.
Results& Discussion
Preliminary phytochemical screening of medicinal plant extracts is essential to know the phytochemical components present in them. Qualitative phytochemical analysis of petroleum ether, chloroform, ethanol, methanol and water extracts C. timorensis leaf showed the presence of alkaloids, phytosterols, phenolic components, tannins, flavonoids, terpenoids, glycosides and saponins (Table 2).Prasanth Kumar et al., (2014)12 reported the preliminary phytochemical composition of C. timorensis leaf ethanol extract, revealing the presence of tannins, flavonoids, alkaloids, triterpenoids, saponins, glycosides and carbohydrates.
The total phenolic content of petroleum ether, chloroform, ethanol, methanol and water extracts of C. timorensis leaf was estimated using Folin Ciocalteau reagent method. Gallic acid was used as the standard component and the amount of total phenolic components were expressed in milligrams Gallic acid equivalents per gram dry weight (mg GAE/g dwt.). The results revealed that the total phenolic content of the tested extracts exhibited a range between 8.82 to 68.32 mg GAE/g dwt. (Figure 1). The highest total phenolic content was observed in methanol extract (68.32±1.03 mg GAE/g dwt.). This may be due to the solubility of phenolic components in methanol. Higher amounts of the total phenolic content of methanol extract of different medicinal plant parts were reported and stated that methanol solvent is efficient for extraction of a good amount of phenolic components 22, 23, 24.
|
Type of component |
PE |
CE |
EE |
ME |
WE |
|
Alkaloids |
NR |
NR |
NR |
+ |
NR |
|
Saponins |
NR |
NR |
NR |
NR |
+ |
|
Phytosterols |
+ |
NR |
+ |
+ |
+ |
|
Phenolic components |
NR |
NR |
+ |
+ |
+ |
|
Tannins |
NR |
+ |
+ |
+ |
NR |
|
Flavonoids |
NR |
+ |
NR |
NR |
NR |
|
Terpenoids |
+ |
+ |
+ |
+ |
NR |
|
Glycosides |
+ |
+ |
+ |
+ |
NR |
|
Gums and mucilages |
NR |
NR |
NR |
NR |
NR |
PE: Petroleum ether extract; CE: Chloroform extract; EE: Ethanol extract; ME: Methanol extract; WE: Water extract; NR: No Reaction
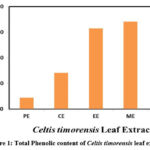 |
Figure 1: Total Phenolic content of Celtis timorensis leaf extracts |
Figure 1 Description: Total phenolic content of C. timorensis leaf extracts was estimated using FCA reagent method. The total phenolic content was expressed in Gallic acid equivalents milligrams/ gram dry weight (GAE mg/g dwt.).
The total phenolic content of leaf water extract of C. timorensis was reported as 9.7 mg 25. In the present study, we found a higher amount of total phenolic content at 31.99 mg GAE/g dwt. The variation in the chemical composition of medicinal plants may be influenced by environmental factors and geographical conditions of the plants growing, collection of plant samples, drying and the part that is used26, 27. In addition to the total phenolic content of water extract, we found the total phenolic content in petroleum ether, chloroform, ethanol and methanol extracts of C. timorensis leaf sample.
The results on the ammonium molybdate-dependant antioxidant capacity of C. timorensis leaf extracts ranged from 174.75±0.29 to 700.0±0.71 mg ASE/g dwt. (Figure 2). The highest total antioxidant capacity was observed in the methanol extract of the leaf part (700.0±0.71 mg ASE/g dwt.). The strong antioxidant capacity of methanol extract may be the presence of a higher amount of phenolic components. The strong antioxidant capacity of methanol extract from different medicinal plants was well reported 28, 29, 30.The results were correlated with the phenolic content of leaf extracts. A similar type of correlation of total phenolic content with total antioxidant activity was reported by several researchers 31, 32, 33.
 |
Figure 2: Total antioxidant capacity of Celtis timorensis leaf extracts |
Figure 2 Description: Total antioxidant capacity of C. timorensis leaf extracts was estimated using ammonium molybdate reagent method. The total antioxidant capacity was expressed in terms of ascorbic acid equivalents milligams/ gram dry weight (AAE mg/g dwt.).
DPPH scavenging activity of petroleum ether, chloroform, ethanol, methanol and water extracts of C. timorensis leaf part showed concentration dependant DPPH scavenging activity. The highest DPPH-reducing activity (>90%) was observed by methanol, ethanol and water extracts of the leaf part (Table 3).While petroleum ether extract failed to reduce DPPH purple colour. Ethanol and methanol extracts exhibited the lowest IC50 values (50µg/ml). The strong DPPH-reducing activity of ethanol and methanol extracts of the leaf part may be due to the presence of a higher amount of total phenolic content. Several researchers have reported strong DPPH quenching capacity of methanol or ethanol extracts of medicinal plants 34, 35, 36, 37. Rajaneekar et al., (2013a)13 reported the DPPH scavenging activity of methanolic extract of leaf sample. It showed maximum inhibition as more than 100% DPPH scavenging activity at 60 µl/ml (concentration not mentioned). In the present study, the methanol extract of C. timorensis showed 92% as maximum DPPH scavenging activity at 250µg/ml respectively.
The antibacterial activity of petroleum ether, chloroform, ethanol, methanol and water extracts of the leaf part of C. timorensis revealed that all the tested pathogens exhibited concentration dependant antibacterial activity (Tables 4 to 8). Ethanol and water extracts of the leaf sample strongly inhibited the gram-negative bacterial species Pseudomonas aeruginosa and Salmonella enterica (13 mm for each species) respectively (Tables 6 & 8). While gram-positive species i.e. Bacillus megatherium Artherobacter protophormiae and P. aeruginosa were moderately inhibited by chloroform, ethanol and water extracts (12 mm for each) respectively (Tables 5, 6, 8). Reaming tested organisms expressed very feeble antibacterial activity by all the tested extracts. The strong antibacterial activity of ethanol, chloroform and water extracts of the leaf part of C. timorensis may be due to the presence of a good amount of phenolic components. The phenolic components exhibit their antibacterial action in many ways. It can cause morphological changes in bacterial cells i.e. shapes, wrinkles on cell membrane and damage cell membrane in both outer and inner membranes. The mechanism of antibacterial activity of polyphenols is closely related to the chemical nature, and position of hydroxyl and methyl groups38, 39, 40.
Table 3: DPPH scavenging capacity of leaf extracts of Celtis timorensis
|
(%) DPPH Scavenging capacity |
|||||||
|
Ethanol Extract (EE) |
Methanol Extract (ME) |
Water Extract (WE) |
Gallic Acid |
||||
|
Concentration (µg/ml) |
% DPPH inhibition |
Concentration (µg/ml) |
% DPPH inhibition |
Concentration (µg/ml) |
% DPPH inhibition |
Concentration (µg/ml) |
% DPPH inhibition |
|
12.5 |
9.86±1.56 |
12.5 |
11.22±1.02 |
100 |
11.22±1.02 |
1 |
16.21±0.02 |
|
25 |
19.39±1.02 |
25 |
23.47±3.68 |
200 |
27.21±3.12 |
2 |
28.02±0.12 |
|
*50 |
50.16±1.56 |
*50 |
49.32±1.18 |
*300 |
51.70±1.02 |
4 |
48.11±1.21 |
|
75 |
68.71±2.57 |
75 |
68.03±2.57 |
400 |
75.51±1.02 |
6 |
60.04±1.02 |
|
100 |
80.13±4.68 |
100 |
78.23±2.12 |
500 |
91.50±0.59 |
8 |
72.00±1.00 |
|
125 |
86.73±1.56 |
125 |
84.35±3.86 |
100 |
11.22±1.02 |
10 |
84.00±1.1 |
|
250 |
91.84±1.02 |
250 |
92.18±0.59 |
200 |
27.21±3.12 |
12 |
92.01±1.12 |
Table 4: Antibacterial activity of Celtis timorensis leaf Petroleum ether extract
|
Microorganism |
Petroleum Ether extract Concentration (µg/Disc*) Zone of inhibition (mm) |
|||
|
50* |
100* |
150* |
300* |
|
|
Micrococcus luteus (MTCC 9341), |
5.33±0.57 |
6.00±1.0 |
6.33±0.57 |
6.66±0.57 |
|
Arthrobacter protopharmiae (MTCC 688), |
5.67±0.76 |
6.50±0.5 |
NA |
6.83±0.76 |
|
Alkaligenes faecalis (MTCC 10757) |
6.00±0.5 |
6.30±1.08 |
6.67±0.28 |
7.00±0.5 |
|
Enterococcus faecalis (MTCC 439), |
7.17±0.28 |
6.30±1.08 |
6.50±0.5 |
5.83±0.76 |
|
Bacillus megatherium (MTCC 5981) |
6.50±0.86 |
8.00±0.5 |
8.17±0.17 |
9.00±0.5 |
|
Lactobacillus acidophilus (MTCC 10307) |
NA |
7.66±0.76 |
8.17±0.57 |
9.17±0.28 |
|
Salmonella enterica (MTCC 3858) |
5.50±0.5 |
6.67±0.28 |
8.17±0.57 |
9.33±0.28 |
|
Pseudomonas aeruginosa (MTCC 1688) |
6.17±0.28 |
5.67±0.28 |
7.67±0.76 |
9.33±0.28 |
- The concentration of extract µg/disc, NA: No Activity
Table 5: Antibacterial activity of Celtis timorensis leaf Chloroform extract.
|
Microorganism |
Chloroform Extract Concentration (µg/Disc*) Zone of inhibition (mm) |
|||
|
50* |
100* |
150* |
300* |
|
|
Micrococcus luteus (MTCC 9341), |
7.50±0.5 |
6.50±1.0 |
6.67±0.76 |
8.17±0.57 |
|
Arthrobacter protopharmiae (MTCC 688), |
5.67±0.76 |
7.16±0.57 |
6.67±0.76 |
7.83±0.57 |
|
Alkaligenes faecalis (MTCC 10757) |
6.33±0.28 |
6.83±0.57 |
7.17±0.57 |
9.17±0.28 |
|
Enterococcus faecalis (MTCC 439), |
6.30±1.08 |
6.83±0.57 |
7.67±0.76 |
9.33±0.28 |
|
Bacillus megatherium (MTCC 5981) |
7.33±0.28 |
8.33±0.28 |
9.17±0.28 |
12.00±0.5 |
|
Lactobacillus acidophilus (MTCC 10307) |
6.33±0.28 |
8.17±0.57 |
8.33±0.28 |
9.33±0.28 |
|
Salmonella enterica (MTCC 3858) |
6.50±1.0 |
7.50±0.5 |
8.17±0.57 |
8.33±0.28 |
|
Pseudomonas aeruginosa (MTCC 1688) |
6.00±0.5 |
6.83±0.57 |
7.17±0.57 |
9.00±0.5 |
*Concentration of extract µg/disc.
Table 6: Antibacterial activity of Celtis timorensis leaf Ethanol extract
|
Microorganism |
Ethanol Extract Concentration (µg/Disc*) Zone of inhibition (mm) |
|||
|
50* |
100* |
150* |
300* |
|
|
Micrococcus luteus (MTCC 9341), |
6.50±1.0 |
6.67±0.76 |
7.17±0.57 |
9.17±0.28 |
|
Arthrobacter protopharmiae (MTCC 688), |
5.67±0.76 |
7.50±0.5 |
8.17±0.57 |
12.33±0.28 |
|
Alkaligenes faecalis (MTCC 10757) |
5.83±0.57 |
6.50±1.0 |
9.17±0.28 |
11.05±0.5 |
|
Enterococcus faecalis (MTCC 439), |
6.67±0.76 |
7.17±0.57 |
9.17±0.28 |
10.67±0.28 |
|
Bacillus megatherium (MTCC 5981) |
5.83±0.57 |
6.50±1.0 |
8.17±0.57 |
11.05±0.5 |
|
Lactobacillus acidophilus (MTCC 10307) |
6.50±1.0 |
7.17±0.57 |
8.33±0.28 |
10.67±0.28 |
|
Salmonella enterica (MTCC 3858) |
5.83±0.57 |
7.50±0.5 |
9.33±0.28 |
11.33±0.28 |
|
Pseudomonas aeruginosa (MTCC 1688) |
6.50±0.5 |
8.50±0.5 |
10.67±0.28 |
13.17±0.28 |
*Concentration of extract µg/disc.
Table 7: Antibacterial activity of Celtis timorensis leaf methanol extract
|
Microorganism |
Methanol extract Concentration (µg/Disc) Zone of Inhibition (mm) |
|||
|
50* |
100* |
150* |
300* |
|
|
Micrococcus luteus (MTCC 9341), |
6.50±1.0 |
7.17±0.57 |
6.67±0.76 |
NA |
|
Arthrobacter protopharmiae (MTCC 688), |
5.67±0.76 |
6.50±1.0 |
6.83±0.57 |
7.50±0.5 |
|
Alkaligenes faecalis (MTCC 10757) |
5.83±0.57 |
NA |
6.67±0.76 |
8.33±0.28 |
|
Enterococcus faecalis (MTCC 439), |
5.33±0.57 |
5.83±0.57 |
NA |
7.50±0.5 |
|
Bacillus megatherium (MTCC 5981) |
5.50±1.0 |
6.50±1.0 |
6.83±0.57 |
8.17±0.57 |
|
Lactobacillus acidophilus (MTCC 10307) |
5.67±0.76 |
6.83±0.57 |
8.33±0.28 |
10.67±0.28 |
|
Salmonella enterica (MTCC 3858) |
5.33±0.57 |
6.50±1.0 |
7.17±0.57 |
6.83±0.57 |
|
Pseudomonas aeruginosa (MTCC 1688) |
5.33±0.57 |
5.83±0.57 |
6.67±0.76 |
8.17±0.57 |
*Concentration of extract µg/disc; NA: No Activity
Table 8: Antibacterial activity of Celtis timorensis leaf water extract
|
Microorganism |
Water extract Concentration (µg/Disc) Zone of Inhibition (mm) |
|||
|
50* |
100* |
150* |
300* |
|
|
Micrococcus luteus (MTCC 9341), |
5.33±0.57 |
5.67±0.76 |
6.50±1.0 |
7.17±0.57 |
|
Arthrobacter protopharmiae (MTCC 688), |
NA |
5.67±0.76 |
6.50±1.0 |
7.17±0.57 |
|
Alkaligenes faecalis (MTCC 10757) |
5.33±0.57 |
6.50±1.0 |
6.83±0.57 |
9.33±0.28 |
|
Enterococcus faecalis (MTCC 439), |
5.50±1.0 |
6.50±1.0 |
7.50±0.5 |
9.17±0.28 |
|
Bacillus megatherium (MTCC 5981) |
5.33±0.57 |
6.50±1.0 |
6.83±0.57 |
9.17±0.28 |
|
Lactobacillus acidophilus (MTCC 10307) |
5.50±1.0 |
6.50±1.0 |
6.83±0.57 |
7.50±0.5 |
|
Salmonella enterica (MTCC 3858) |
6.83±0.57 |
7.50±0.5 |
11.33±0.28 |
13.17±0.28 |
|
Pseudomonas aeruginosa (MTCC 1688) |
7.17±0.57 |
9.33±0.28 |
10.67±0.28 |
12.33±0.28 |
*Concentration of extract µg/disc; NA: No Activity
Conclusion
The present study results state that Celtis timorensis leaf extracts were rich in different groups of secondary metabolites. Of the tested five extracts, methanol extract showed a higher amount of total phenolic content and higher antioxidant capacity. Ethanol and methanol extract strongly inhibited DPPH radical in a concentration dependant manner. Ethanol and water extracts of leaf samples strongly inhibited the gram-negative bacterial species Pseudomonas aeruginosa and Salmonella enterica respectively. While gram positive species i.e. Bacillus megatherium Artherobacter protophormiae and P. aeruginosa were moderately inhibited by chloroform, ethanol and water extracts respectively. The strong antibacterial activity of ethanol, chloroform and water extracts of the leaf part of C. timorensismay be due to the presence of a good amount of phenolic components. In conclusion, the selected medicinal plant C. timorensisextractsexhibited good antioxidant activity, strong antibacterial activity and rich bioactive components. It required further studies on the isolation, and characterization of active principle to evaluate its pharmacological properties.
Conflict of interest
No conflict of interest
Funding source
No funding source
References
- Cheesbrough M. Medical Laboratory Manual for Tropical Countries. Oxford, United Kingdom: Oxford, Publishers, Microscopic examination of specimens. 1984; pp. 32–33.
- Peirano G. Multi resistant enterobacteriaceae new threat to an old probe; expect a review of anti-infective therapy. Expert Rev Anti Infect Ther. 2008; 6: 657–669.
CrossRef - Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J ApplMicrobiol. 2000; 88(2): 308–316.
CrossRef - Talib WH, Mahasneh AM. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules. 2010; 15(3):1811–1824.
CrossRef - Chassagne François, Samarakoon Tharanga, Porras Gina, Lyles James T, Dettweiler Micah, Marquez Lewis, Salam Akram M., Shabih Sarah, Farrokhi Darya Raschid, Quave Cassandra L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Frontiers in Pharmacology.2021; 11: Article 586548.
CrossRef - Gonelimali, Faraja D, Lin Jiheng, Miao Wenhua, Xuan Jinghu, Charles Fedrick, Chen Meiling, Hatab, Shaimaa R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Frontiers in Microbiology. 2018; 9.10.3389/fmicb.2018.01639.
CrossRef - Khameneh B, Iranshahy M, Soheili V, Bibi Sedigheh Fazly Bazzaz. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019; 8: 118.
CrossRef - Pullaiah T. Encyclopedia of world medicinal plants. 2006; 4: p.2019.
- Singh HB, Singh RS, Sandhu JS Ed. Herbal medicine of Manipur, a colour encyclopaedia. 2003; p. 67.
- Gouda, S, Sethy J, Chauhan NS. Ethno-medicinal use of plants by indigenous communities in and around Dampa Tiger Reserve, Mizoram, India. J. Biodiver. Conser.2017; 1(1): 16-17.
- Ahmed SI, Hayat MQ, Tahir M, Mansoor Q, Ismail M, Keck K, Bates RB. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med.2016; 16: 460.
CrossRef - Prasanth Kumar M, Suba V, Ramireddy B, Srinivas Babu P. Evaluation of antidiarrhoeal activity of ethanolic extract of Celtis timorensis leaves in experimental rats. Asian J Pharm Clin Res. 2014;7 (2): 185-188.
- Rajaneekar D, Sathyavati D, Sampath Kumar B, Jayachandra Reddy P, Abbulu K. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Celtis timorensis and Vanda spathulata. Asian J. Pharmaceu. Clini. Res.2013a; 6 (2): 153-155.
- Isurika SC, Wanninayake WMTA , Silva ARN, Bandara AWMKK, Ratnasooriya WD. Evaluation of in vitro Antibacterial Activity of Aqueous Extract of Celtis timorensis Span Leaves. Abstract presented in Allied Health Sciences, 12th International Conference, Organized by General Sir John Kotelawala Defence University, Sri Lanka, 2019; 257.
- Rajaneekar D, Anusha K, Sathyavathi D, Jayachandra Reddy P. Evaluation of methanolic extract of Celtis timorensis for its Antidepressant activity. Indo-American Journal of Pharmaceu. Res.2013b; 3 (3): 2533 – 2538.
- Prasanth Kumar M, Suba V, Ramireddy B. Wound healing activity of Celtistimorensis (Cannabaceae) leaf extract in Wistar albino rats. Indian Journal of Experimental Biology, 2017;55: 688 – 693.
- Auwal MS, Saka S, Mairiga IA, Sanda K.A, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum. 2014; 5(2): 95-100. PMID: 25568701; PMCID: PMC4279630.
- Shaikh J R, Patil MK. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020; 8. 603-608. 10.22271/chemi.2020.v8.i2i.8834.
CrossRef - Venkata Ratnam K, Bhakshu MD L, Venkata Raju R.R. Studies on antimicrobial and antioxidant properties of leaf extracts of Syzygium alternifolium (Wt.) Walp. Inter. J. Pharma. Pharmaceu. Sci.2015; 7 (2): 139-143.
- Vishnu Mohan Reddy P, Venkata Ratnam K, Venkata Raju RR. In Vitro antioxidant properties of Alangium salvifolium seed extracts. Res. J. Pharma. Technol. 2019; 12 (10): 4714 – 4718.
CrossRef - Cruickshank R, Duguid JP, Marmion BP, Swain RHA. Medical microbiology. London: Churchill Livingstone; 1975.
- Boeing JS, Barizão ÉO, e Silva BC. Montanher PE, Almeida VC, Visentainer JV. Evaluation of the solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chemistry Central Journal. 2014;8, 48..
CrossRef - Santas J, Carbó R, Gordon MH, Almajano MP: Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008; 107: 1210-1216.
CrossRef - Dieu-Hien Truong, Dinh Hieu Nguyen, Nhat Thuy Anh Ta, Anh Vo Bui, Tuong Ha Do, Hoang Chinh Nguyen, “Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia“, Journal of Food Quality, 2019; vol. 2019, Article ID 8178294, 9 pages.
CrossRef - Rajat Buragohain. Tree Foliages Fed to Dairy Animals in Mizoram: Traditional Medicinal Uses, Screening and Quantification of Phytochemicals. Int. J. Curr. Microbiol. App. Sci,2017; 6(10): 61-71.
CrossRef - Gobbo-Neto, L., Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quimica Nova, 2007; 30 (2): 374-381.
CrossRef - Petropoulos S, Fernandes A, Barros L, Ciric A, Sokovic M, Ferreira ICFR. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chemistry. 2018; 245 (2018): 7-12.
CrossRef - Shabbir M, Khan MR, Saeed N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement Altern Med. 2013; 22 (13):143.
CrossRef - Wilton Mbinda, Colleta Musangi. Antioxidant activity, total phenolic and total flavonoid contents of stem back and root methanolic extracts of Calotropis procera. The J. Phytopharmacol. 2019; 8(4): 161-166.
CrossRef - Kumaran A, Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India, LWT – Food Science and Technology.2007; 40 (2): 344-352.
CrossRef - Yu M, Gouvinhas I, Rocha J, Ana I, Barros RNA Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11, 10041 (2021).
CrossRef - Reddy ARK, Grace JR In Vitro Evaluation of Antioxidant Activity of Methanolic Extracts of Selected Mangrove Plants. Medicinal & Aromatic Plant,2016;5 (3): 1-5.
- Neethu Joy, Mahesh Mohan. Evaluation of In vitro Antioxidant and Cytotoxic Effect of Methanol Extract from Aerial parts of Myristica beddomei King ssp. ustulata W.J. de Wilde. Inter. J. Pharmaceu. Sci. & Drug Res.2020; 12(2): 115-121.
CrossRef - Nandhakumar E, Indumathi P. In vitro Antioxidant Activities of Methanol and Aqueous Extract of Annona squamosa (L.) Fruit Pulp. J. Acupun..& Meridi. Stu.2013; 6 (3): 142-148.
CrossRef - Khan MA, Rahman AA, Islam S, Khandokhar P, Parvin S, Islam Md. B, Hossain M, Rashid M, Sadik G, Nasrin S, Nurul Haque Mollah M, Khurshid Alam AHM A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res Notes. 2013;6, 24.
CrossRef - Adebiyi OE, Olayemi FO, Tan Ning-Hua, Zeng Guang-Zhi. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia, Beni-Suef University J. Basic and App. Sci. 2017; 6 (1): 10-14.
CrossRef - Ravishankar K, Indira N. Antioxidant Activity of Ethanolic Extract of Ricinus communis Leaf. Biomed Pharmacol J, 2012; 5(1): 179-183.
CrossRef - Efenberger-Szmechtyk, M., Agnieszka Nowak, Agata Czyzowska: Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products, Critical Reviews in Food Science and Nutrition, 2020; 1-31. DOI: 10.1080/10408398.2020.1722060.
CrossRef - Hayriye Cetin-Karaca. “Evaluation of natural antimicrobial phenolic compounds against foodborne pathogens”. University of Kentucky Master’s Thesis. 2011; 652.
CrossRef - Natalia Vaou, Elisavet Stavropoulou, Chrysa Voidarou , Christina Tsigalou, Eugenia Bezirtzoglou. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms.2021; 9, 2041.
CrossRef








