Kumar MR Bhat1 , Takkella Nagamma2
, Takkella Nagamma2 and Anjaneyulu Konuri3*
and Anjaneyulu Konuri3*
1Department of Anatomy, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2Department of Biochemistry, Manipal Tata Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3Department of Anatomy, Manipal Tata Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Corresponding Author E-mail: Anjaneyulu.konuri@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2768
Abstract
Trigonella foenum-graecum known as fenugreek, is a widely used spice and an abundant source of phytoestrogens. Fenugreek has a wide range of pharmacological effects, such as hypoglycemic, hypolipidemic, obesity, antioxidant activity, osteoporosis, anti-inflammatory, and antirheumatic effects. Phytoestrogens are one of the constituents of fenugreek. These are nonsteroidal compounds that resemble the structure of 17β -estradiol and can bind to the ERα and ERβ estrogen receptors. It acts as an estrogen receptor modulator, producing antiestrogenic and/or estrogenic effects. The protective effect was also apparent in the cellular architecture of the kidney, liver, and common carotid artery. Several beneficial effects are ascribed to the phytoestrogens present in fenugreek. In the present review, we focused on the medicinal values of phytoestrogens in fenugreek
Keywords
Bone health; Menopausal symptoms; Phytoestrogens; Trigonella foenum-graecum; Tumor cells
Download this article as:| Copy the following to cite this article: Bhat K. M. R, Nagamma T, Konuri A. Medicinal value of Phytoestrogens in Trigonella foenum-graecum L (Fenugreek) - Review. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Bhat K. M. R, Nagamma T, Konuri A. Medicinal value of Phytoestrogens in Trigonella foenum-graecum L (Fenugreek) - Review. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3R5pKuf |
Introduction
The fenugreek (Trigonella foenum-graecum L.) belongs to the family Fabaceae. It is one of the most significant commercial spice crops. Its fresh leaves, shoots (tender), and seeds are widely used in food, medicine, and cosmetics1. Fenugreek showed many pharmacological effects, such as hypoglycemic effect2, hypocholesterolemic effect3, antioxidant action4, gastroprotective activity5, stimulator of appetite6, antirheumatic effect7, for reducing the adipose tissue weight in the body8. It has also been shown that flavonoids in fenugreek act as an estrogen-mimicking agent.
Phytochemical analysis shows that the Fenugreek seed contains carbohydrates (45-60%), including mucilaginous fiber; proteins (20-30%), mostly tryptophan and lysine; lipids (5-10%); and pyridine-type alkaloids, mostly choline, trigonelline (0.5%), carpaine and gentianine. The flavonoids such as orientin, apigenin, vitexin, and quercetin are also found in them. Amino acids (4-hydroxy isoleucine (0.09%); arginine (Arg), histidine (His), and lysine (Lys)) are also detected in seeds. Saponins (0.6-1.7%); glycosides generating sapogenins on hydrolysis (cholesterol and vitamins A, B1, C), and 0.015% volatile oils (sesquiterpenes) similarly identified in fenugreek seeds9. Fenugreek is also found to contain yamogenin, gitogenin, tigogenin, and trigoneoside, choline, saponin, and nicotinamide10. It has been found that unhydrolyzed fenugreek seeds contain phytoestrogens such as isoflavones (daidzein, genistein), lignans (secoisolariceresinol), and coumestrol in hydrolyzed fenugreek seeds11.
Fenugreek seeds contain diosgenin12, which is recognized to be a significant precursor for the production of testosterone and estrogens13. Even the highest dose of 1000 mg/kg body weight, fenugreek seeds (FenuSMART®; FHE) administered to animals in a toxicological evaluation did not cause appreciable toxicological changes in feed consumption, body weight, biochemical parameters, behavioral observations, ophthalmic examinations, and urinalysis compared to normal animals.
Materials and Methods
The literature search was conducted in Scopus, PubMed, ScienceDirect, and Google Scholar to identify all published articles on Trigonella foenum graceum (Fenugreek) between June 2021 and September 2022. The search criteria was based on the phytoestrogen role of fenugreek in postmenopause, pregnancy, offspring, sexual functions, bone and skin health, milk production, ovary, vagina, cardiovascular system, eye, tumor cells, pancreas, and kidney. Studies with full text in the English language were included in this review. Non-English studies were excluded from the search. In each manuscript, the search outcomes were reviewed by all the authors.
Phytoestrogen
Phytoestrogens are one of the constituents of fenugreek, and these are nonsteroidal compounds that resemble the structure of 17β -estradiol and can bind to the ERα and ERβ estrogen receptors. It acts as an estrogen receptor modulator and produces antiestrogenic and/or estrogenic effects depending on the blood estrogen levels14. Several health benefits are due to phytoestrogens, such as lowering the hot flashes and risk of cardiovascular disease (CVD), metabolic syndrome, brain function disorders, osteoporosis, and other malignancies (breast, prostate, and bowel). On the contrary, it has also been shown that phytoestrogens may act as anti-estrogenic agents and adversely disrupt endocrine functions15. The main functions of phytoestrogens are depicted in Figure 1.
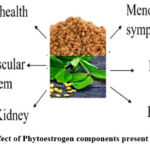 |
Figure 1: Effect of Phytoestrogen components present in fenugreek. |
In addition to phytoestrogen activity via estrogen receptors and their downstream gene activity, they also can act by altering the specific gene epigenetics, by the initiation of AMP-activated protein kinase pathway, by kinase inhibition activity, by activating the PPAR (NRC1C) family, or by the initiation of antioxidant or electrophile response element (EpRE)-mediated gene expression by stimulation of nuclear factor erythroid 2-related factor 2-Keap1 signaling16. Furthermore, Sirotkin and Abdel have extensively explored the potential issues with understanding and using phytoestrogens since they have many targets and various estrogen receptor-dependent and -independent modes of action17. Because pS2 gene expression is used to test the estrogenicity of multiple drugs, fenugreek seeds were shown to promote pS2 transcription, which might impact the transcription of estrogen-sensitive genes, resulting in estrogenic effects18.
Use of Phytoestrogens of Fenugreek in Typical Menopausal Symptoms
Menopausal changes are known to be initiated by the decrease in estrogen level, which induces signs like osteoporosis, depression, hot flashes, anxiety, and dementia. As life expectancy increases with advances in medical sciences, women live 1/3 of their lives with disorders related to menopause. The current treatment for these complications is estrogen replacement therapy (ERT). However, prolonged ERT increases the frequency of uterine, ovarian, and breast cancer, limiting ERT in clinical use. Instead, phytoestrogen-rich plant products such as fenugreek are considered the alternative strategy to treat menopause-related ailments without many adverse side effects.
At a dosage of 1000 mg per day, fenugreek (Trigonella foenum-graecum) standardized extract (FenuSMARTTM) has been demonstrated to enhance plasma estradiol, decrease the frequency of hot flashes, and play a significant role in managing the lipid profile in menopausal women19. When treated with fenugreek seed extract, postmenopausal women were reported to show improvements in somatic, psychological, and urogenital issues (reduction in hot flashes, pain in leg muscles and joints, night sweats, less irritability, and vaginal dryness). In these women, a significant rise in estradiol and progesterone and a decline in follicular stimulating and luteinizing hormone levels were observed, indicating the achievement of hormonal balance20. Phytoestrogen-rich food such as fenugreek has effectively reduced the severity of premenopausal/menopausal symptoms such as forgetfulness, swelling, and weight fluctuation21.
Menopause and the associated loss of estrogen-related hormones cause inflammation, which results in the expression of circulating cytokines and other inflammatory responses. Menopause is associated with an increase in triglycerides (T.G.), low-density lipoproteins (LDL), increased tunica intima and media thickness, and an increased risk of cardiovascular diseases (CVD). Inflammation in menopause with dyslipidaemia is a source of TNF- α (increases the expression of leptin and metabolic syndrome), a contributing factor in the etiopathogenesis of atherosclerosis22.
Menopausal obesity is associated with decreased PPAR-γ and adiponectin expression. Few studies support fenugreek’s phytoestrogen action in postmenopausal women23,24. According to a meta-analysis, the cardiometabolic risk is decreased by fenugreek in humans25. The advantages of fenugreek in reducing cholesterol and metabolic disorders are discussed in another systematic review. This effect may be due to the existence of phytoestrogens26. Fenugreek therapy for 12 weeks lowered total cholesterol (TC), TG, AST, and ALT while increasing mRNA expression of PPAR and adiponectin. TNF-α and leptin levels returned to normal following supplementation with fenugreek seed extract. Furthermore, fenugreek seed extracts reduced renal glomerular hypertrophy, micro-steatosis in the liver, and thickness of tunica intima (T.I.) and media (T.M.) of the common carotid artery (CCA). The phytoestrogen components of fenugreek are responsible for the protective effect24, 27.
Menopausal cognitive impairment with estrogen insufficiency results in lower levels of brain-derived neurotrophic factor (BDNF). Declined BDNF levels influence the growth of neurons, dendritic spine density, and arborization in postmenopause. Ovariectomized rat menopausal model supplemented with fenugreek seed extract and choline-DHA suggestively elevated BDNF, dendritic branching points, and intersections, thus modulating the learning and memory problems caused by menopause through phytoestrogen properties28. Similarly, it was shown that ovariectomized rats displayed poor memory and learning abilities and neuronal atrophy and death in the hippocampal CA1 and CA3 regions. Fenugreek seed extract dramatically improved memory and learning while protecting hippocampus neuronal architecture and survival in these ovariectomized rats. These changes could be due to the phytoestrogen components in fenugreek, and they may be useful in addressing menopausal-induced cognition deficits29.
Use of Phytoestrogen in Fenugreek in Sexual Functions
Phytoestrogens present in the fenugreek are shown to have several effects on sexual function. Significant improvement in sexual interest was observed after supplementation with the fenugreek husk19. Crude fenugreek seed oil significantly affected spermatogenesis in young male patients with complaints of infertility due to natural oligospermia30, 31. An increase in sexual function, fertility, free testosterone levels, and sperm profile has been recorded in male individuals treated with the fenugreek extract32,33. Kassemet et al. stated that Trigonella foenum-graecum (TFG) administration has reduced plasma androgen levels and significantly decreased sperm count31. This effect could be due to saponins in fenugreek seeds, which can act like estrogen. The reduction in sperm count and plasma testosterone level may also be attributed to the fact that TFG can increase prolactin, and its high levels are known to obstruct the production of gonadotropin-releasing hormone (GnRH). This intern leads to a decline in FSH and LH and thus decreases spermatogenesis34. It has been shown that phytoestrogens on long-term exposure (or high-dose exposure) severely affect sperm count by reducing male reproductive function35 and increasing apoptosis of developing germ cells36.
Similarly, Sreeja and Anuj found that when fenugreek seeds are included in the diet of rabbits, the testis weight is reduced, seminiferous tubules and interstitial testis tissue sections are damaged, which is followed by a drop-in androgen hormone and sperm concentrations18. Several studies show that fenugreek induces a significant decrease in sperm motility and count, a rise in spermatozoa morphology and chromosomal abnormalities35, and a decreased concentration of testosterone and sperm mass and motility36. Male infertility and antiandrogenic activities of fenugreek are attributed to a decrease in cholesterol level, which is the primary precursor of the sex hormone2.
Effect on Pregnancy and Offspring
In a study of prenatal exposure to fenugreek seeds (aqueous extract), the progeny of exposed mice in the post-weaning period showed growth retardation, such as reduced body weight and reduced brain weight at birth. These pups (both male and female) also showed a significant decrease in neurobehavioral performances, such as locomotor activity, motor coordination, and a substantial decrease in successful impulsive alternations in the T-maze test. These changes may be attributed to phytoestrogens such as form ononetin in fenugreek37. Contrary to that, if consumed in moderation, fenugreek is only safe for pregnant women. Excessive intake may result in premature uterine contractions38. Consuming an excess of fenugreek in late pregnancy causes miscarriage and premature labor39.
Phytoestrogens in Fenugreek on Bone Health
A study showed that estrogen deficiency worsens the increases in bone turnover indicators, bone mineralization and mechanical properties of tibial metaphysis. Trigonelline (alkaloid), found in TFG seeds and coffee, has demonstrated phytoestrogen action and did not affect the bones of non-ovariectomized (with an average level of estrogen) rats, while in ovariectomized (with low estrogen level) rats phytoestrogens significantly lower the mineralization and mechanical characteristics of cancellous bone. Trigonelline’s detrimental effects on the skeletal system depend on estrogen, and it only affects cancellous bone in ovariectomized rats4. However, supplementation of TFG seed extract significantly increased the maximum flexor load, tibia dry weight, and preserved the trabecular and cortical bone thickness suggestively compared to ovariectomized rats41. The protective action of fenugreek on osteoporosis by enhancing bone mineral density and restoring bone physiology is via the estrogenic activity of trigonelline in them42. Fenugreek mucilage in arthritic rats significantly decreased the activity of inflammatory enzymes myeloperoxidase and lipoxygenase43. Diosgenin, another constituent in fenugreek seed, is also shown to increase the protein synthesis of bone matrix and Runx2 transcription in an osteoblastic cell line MC3T3-E144. Diosgenin is also known to inhibit osteoclastogenesis, invasion, and proliferation of osteoclast cells by downregulating the Akt, IkB kinase activator, and NFkB-regulated gene expressions45.
Skin Health
Oral or topical estradiol treatment in postmenopausal women is known to enhance skin thickness and collagen synthesis, and inhibition of the ERα and ERβ (estrogen receptors) by the antagonist decreases the secretion of these proteins. Because of the risks of breast and endometrial cancer associated with estradiol, topical use of phytoestrogens is an alternate therapy for age-related skin alterations in postmenopausal women. Research has been conducted to demonstrate the advantages of phytoestrogens as a substitute for HRT to improve or decrease the detrimental influence of menopause-induced aging of the skin46. Fenugreek supplementation enhances the secretion of COL1A1 (collagen type 1 alpha 1) and COL3A1 (and type 3 alpha 1) through estrogen receptor α, and estrogen receptor β, and is primarily arbitrated by ERβ in fibroblasts of menopausal women. Thus, the phytoestrogen constituent of the fenugreek may act as a ligand to ERα and ERβ47.
Breast Size and Milk Production
Nursing women also use dietary supplementation of fenugreek as a galactagogue to enhance insufficient breast milk production and improve the indications of breast milk sufficiency in newborns48-54. Similarly, breastfeeding women fed with fenugreek tea three times a day showed increased breast milk volume and an increase in prolactin levels in the early days of breastfeeding. However, such influence of fenugreek was found in the latter days of breastfeeding55. Further, it has also been shown that the consumption of fenugreek increases breast size in women 56.
Earlier studies explain the possible mechanisms by which the phytoestrogens act as galactagogues agents. They may act via binding to receptors in the mammary gland and induce the proliferation of alveolar cells. They may also bind to the ERβ through the pituitary membrane isoform (associated estrogen) and promote the expression of prolactin. These phytoestrogens act as agonists to dopamine receptor-activated pathways and increase prolactin secretion57.
Egg Production
Dietary supplementation of phytoestrogen plant products such as fenugreek seeds improved the egg mass and production, egg weight, albumen, and yolk content in aged laying hens. An increase in the crude fat and calcium in the yolk and a decrease in cholesterol in the egg yolk were observed in these hens. The supplementation of fenugreek seeds with flax seeds also positively affected plasma hormones (LH & FSH)58. Adding turmeric and fenugreek in the hen’s diet increases production, quality of eggs and improves the financial efficiency of hen foods (Sinai local) at the late phase of productive age (59 – 74 weeks)59. Another study showed that supplementing the mandarah hen diets with fenugreek delayed sexual maturity but increased ovarian weight, egg mass with increased egg yolk albumin, and reduced cholesterol level60.
Effect on Ovary
Fenugreek also influences the structure and functions of the ovary. Treating the mice with fenugreek seed extract significantly decreases the number of primaries, secondary, and griffin follicles61. It was also found that fenugreek directly affects ovarian growth factors or decreases the pituitary gonadotropin hormones (FSH and LH)62.
Swaroop et al. investigated the effectiveness of TFG seed extract on PCOS in women aged 18 to 45 years. Reduced levels of LH were observed after the first month of administration and reverted to baseline after two months in the individuals who received fenugreek seed extract. After three months of treatment, the LH levels have significantly increased. Similarly, FSH levels gradually increased. Further, the irregular cycle rate remained unaffected in women after one month. At two months, 33.33% of patients stated a regular cycle, but at three months (the conclusion of the trial period), 71.43% of patients reported a normal cycle63.
Estradiol 2 has a significant role in the pathophysiology of ovarian hyper-stimulation syndrome (OHSS) through VEGF. Prevention of OHSS requires a reduction in E2 levels. A recent study found that fenugreek extract effectively reduces blood Estradiol 2 (E2) levels in ovarian hypersensitivity syndrome animal models64. Fenugreek extract may also act through inhibition of aromatase, leading to reduced testosterone breakdown to estrogen65, which may be the reason for the reduction in serum E2 levels in these animals.
On the Uterus and Vagina
Research by Hilles et al. stated that fenugreek seeds, which contain phytoestrogens with estrogenic activity, increased uterine weight and proliferation of epithelial cells, which increased the uterine layers. The use of fenugreek seeds is also considered a safe contraceptive method66. Vaginal atrophy is one of the most prevalent postmenopausal complications. Consuming estrogen hormone to progress these symptoms is inevitable. Alternatively, Fenugreek vaginal cream, containing phytoestrogens, effectively reduced postmenopausal vaginal atrophy and endorsed using this cream instead of estrogen (synthetic) to decrease the complications67. However, it was also found that symptoms of atrophic vaginitis are diminished with fenugreek vaginal cream, although it is not as efficient as ultra-low-dose estrogen68.
Cardiovascular System
In various animal and clinical studies, fenugreek is claimed to be hypocholesterolemic69 owing to the presence of saponin (diosgenin) and other sapogenins70. In the digestive tract, the saponins are hydrolyzed partly into sapogenins. Numerous studies described the hypocholesterolemic effect of saponins and diosgenin71, 72 in fenugreek.
The hypocholesterolemic role of fenugreek was shown by increased fecal excretion of bile acids, sterols and reduced cholesterol in the liver73. Dietary fiber (galactomannans or beta-glucans) helps in the management of hypercholesterolemia. Fenugreek-derived galactomannans have abundant efficacy in decreasing plasma cholesterol. Additionally, soluble fiber elements lower LDL and increase HDL74. These beneficial changes in the lipid profile by dietary fenugreek protect against coronary heart disease and atherosclerosis. In a study of rats supplemented with 400 mg/kg/day of the fenugreek seed extract, HDL levels improved significantly, and total cholesterol, TG, and LDL decreased significantly. This effect might be attributed to the saponins found in fenugreek extract, which slow down cholesterol absorption and hepatic cholesterol formation by lowering the activity of the regulatory enzyme of cholesterol synthesis24, 27.
Diosgenin-derived phytoestrogen from fenugreek has been shown to decline plasma and hepatic TG levels75. Experimental studies have established the lipid-lowering potential of diosgenin. Diosgenin reduced the elevated cholesterol and increased HDL fractions in rats fed with cholesterol. Besides, diosgenin prevented cholesterol absorption and inhibited its uptake and accumulation in the liver76. Diosgenin-mediated modulation of cholesterol-metabolizing enzymes reduced hepatosteatosis and hypercholesterolemia77. Diosgenin also prevented angiotensin II-induced remodeling of cardiac fibroblasts in rats by suppressing the signaling growth factor β1 / Smad3 pathway. Subsequently, diosgenin could have therapeutic potential to treat cardiac fibrosis78.
Eye
A study confirmed that steroid hormones significantly influence ocular surface equilibrium and functions, and a reduction in these steroid hormones in conditions such as menopause can affect the eye’s surface. However, in postmenopausal women, phytoestrogen treatment significantly alleviated the signs and symptoms of dry eye syndrome79.
It has been reported that tear osmolality and levels of sex hormones are negatively correlated, and these sex hormones have a role in the pathogenesis of postmenopausal dysfunctional tear syndrome. Daily consumption of 200 mb of fenugreek seeds significantly improved signs and symptoms of postmenopausal women with severe evaporative dysfunctional tear syndrome without any significant side effects attributed to the phytoestrogens in fenugreek80.
Tumor Cells
Phytoestrogens from different sources are known to act by various mechanisms, providing several health benefits and protect/treat different cancers (oral, breast, colon, liver, lung, prostate) and many other carcinomas81. In MCF-7 breast cancer cell lines, fenugreek seeds (ethanol extract) lowered cell feasibility and triggered (early) apoptotic alterations, such as reversing phosphatidylserine activity and reducing the potential of the mitochondrial membrane. Furthermore, DNA fragmentation and cell cycle arrest during the G2/M phase have been described82. In vitro, experiments have shown that the fenugreek constituent diosgenin, steroidal saponins which can act as phytoestrogen by preventing bcl-2 and increasing the expression of caspase-3 protein, promotes apoptosis and decreasing cell proliferation in the cell line of HT-29 human colon cancer. Diosgenin, in the fenugreek, is shown to have antitumor activity in azoxymethane-induced colon cancer model of rats and cell lines of HT-29 human colon cancer by inhibiting the aberrant crypt foci formation and inhibition of proliferation of cancer cell line along with apoptosis induction83.
The diet containing fenugreek seeds inhibited colon cancer by significantly decreasing β-glucuronidase activity, thus preventing the hydrolysis of the carcinogen-glucuronide conjugate, which may release a carcinogenic substance that affects the colonocytes. It also declined the action of mucinous, thus reducing the hydrolysis of protective mucin in the colon and protecting the colonial lumen. These alterations are due to phytoestrogen saponins and other constituents of the fenugreek seeds84.
Pancreas
Fenugreek controls blood sugar levels mainly by the presence of saponins (phytoestrogen components) inhibiting digestion and absorption of carbohydrates and enhancing the insulin peripheral activity85. In a trial with streptozotocin-induced diabetic rats, 45 days of oral diosgenin (15, 30, and 60 mg/kg) administration at elevated doses have resulted in the restoration of blood glucose and enzymes involved in the carbohydrate metabolism, indicating the antidiabetic potential86. Saponin and an amino acid called 4-hydroxyleucine in fenugreek were attributed to increasing the insulin level in hyperglycemic rats and humans and showing antidiabetic effects87.
Kidney
TFG seed extract restored kidney function in diabetic rats by ameliorating ultra-morphologic abnormalities, such as irregular thickening of the base membrane of the glomerulus. These results indicate that TFG extract offered a defense against functional and morphological kidney injury88. Sayed et al. stated that renal histology of diabetic rats showed a mild diffuse mesangial expansion compared with normal glomeruli. In contrast, rats supplemented with 5% fenugreek seed powder mixed with a rat pellet diet showed a reduced score of mesangial development89.The renal damage caused by AlCl3 was confirmed by elevated plasma nephrotoxicity markers and altered histological features of the renal tissue. Aqueous fenugreek seed extract restored the function of diabetic rat kidneys through its phytoestrogenic antioxidant activity. On the other hand, flavonoids in the TFG seed powder have a beneficial effect on collagenous structure (filtration membrane and basement membrane)90. The male albino rats treated with 2.5% TFG showed preserved architecture, normal glomeruli, and tubules with normal cortical blood vessels. The medulla showed normal tubules and blood vessels of interstitial tissue91. Thebeneficial effects of Trigonella foenum-graecum on different organs were presented in Table 1.
Table 1: Beneficial effects of Trigonella foenum-graecum
|
S. No |
Different organs |
Beneficial effect |
|
1. |
Bone |
Lowers the mineralization and mechanical characteristics of cancellous bone4 and also increases the maximum flexor load, dry weight, preserves the trabecular and cortical bone thickness.41 |
|
2 |
Skin |
Improves the secretion of COL1A1 and COL3A1through ERα, and ERβ47.47 |
|
3 |
Ovary |
Affects ovarian growth factors or decreases the pituitary gonadotropin hormones.64 |
|
4 |
Uterus and vagina |
Increases uterine weight and proliferation of epithelial cells66, effectively reduces postmenopausal vaginal atrophy.67 |
|
5 |
Cardiovascular system
|
The hypocholesterolemic role is shown by increased faecal excretion of bile acids, sterols and reduced cholesterol.73 |
|
6 |
Eye |
Improves signs and symptoms of postmenopausal women with severe evaporative dysfunctional tear syndrome.80 |
|
7 |
Tumor cells |
In MCF-7 breast cancer cell lines- lowered cell feasibility and triggered early apoptotic alterations such as reversing phosphatidylserine activity and lowering the potential of mitochondrial membrane.82 |
|
8 |
Pancreas |
Increasing the insulin level in hyperglycemic rats and humans and showing antidiabetic effects.87 |
|
9 |
Kidney |
Preserved architecture, normal glomeruli, and tubules with normal cortical blood vessels.91 |
Conclusion
Several components present in the fenugreek act like estrogen-mimicking agents. Several health benefits are attributed to phytoestrogens, such as lowering the menopausal adverse symptoms like hot flushes, inflammation, menopausal obesity, lowering the risks of CVD, type 2 diabetes, menopausal cognitive impairment, sexual functions, osteoporosis, breast cancer, and prostate cancer. Other purposes include maintaining skin health, enhancing breast milk and egg production, increasing uterine weight, and maintaining ocular surface equilibrium. Based on the numerous health benefits highlighted in this study and several previously reported scientific findings, fenugreek may be advised and used as part of our regular diet. This natural herb is safe to consume in limited amounts and can provide a variety of health advantages.
Conflict of Interest
The authors declare no conflict of interest.
Founding Source
No external funding for this study.
References
- Malhotra SK. “Fenugreek (Trigonella foenumgraecum L.). In book: Genetic Resources, Chromosome Engineering and Crop Improvement. Ist Edition Chapter 4.” CRC Press. 2011; 801-46.
- Banarase NB, Khadabadi SS, Farooqui IA, Aswar PB, Bhopale NM. A novel effervescent tablet of fenugreek extract. Research J. Pharm. and Tech. 2008; 1(3):252-54.
- Ajabnoor MA, Tilmisany AK. Effect of Trigonella foenum graceum on blood glucose levels in normal and alloxan-diabetic mice. Journal of ethnopharmacology. 1988; 22(1):45-49.
CrossRef - Nisha, Rao PB. Trigonella foenum-graecum: A Common Indian Spice with Medicinal Properties. Research J. Pharm. and Tech. 2016; 9(2):173-76.
CrossRef - Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002; 81:393-97.
CrossRef - Max B. This and That: The essential pharmacology of herbs and spices. Trends. Pharma Sci. 1992; 3:15-20.
CrossRef - Suresh P, Kavitha CN, Babu SM, Reddy VP, Latha AK. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund’s adjuvant-induced arthritis in albino rats. Inflammation. 2012; 35(4):1314-21.
CrossRef - Handa T, Yamaguchi K, Sono Y, Yazawa K. Effects of fenugreek seed extract in obese mice fed a high-fat diet. Biosci. Biotechbol. Biochem. 2005; 69(6):1186-88.
CrossRef - Bahadur S, Roy A, Chanda R, Baghel P, Saha S, Choudhury A. Assessment of Some Phytochemcial and Physicochemical Properties of Fenugreek Seed Mucilage. Research J. Pharm. and Tech. 2016; 9(9):1321-24.
CrossRef - Agustini K, Sriningsih S, Effe J. Acute toxicity study of ethanolic extract of fenugreek seeds (Trigonella foenum-graecum L.) on white rats. J Indones Med Plant. 2015; 8(1):9-13.
- Joshi M, Nair S. HPLCanalysis of Trigonella Foenum-Graecum seeds to assess phytoestrogens. Int. J.food and nutritional sciences. 2014; 3(1):61-4.
- Taylor WG, Zulyniak HJ, Richards KW, Acharya SN, Bit-tman S. Variation in diosgenin levels among 10 accessions of fenugreek seeds produced in western Canada. Journal of agricultural and food chemistry. 2002; 50:5994-97.
CrossRef - Wankhede S, Mohan V, Thakurdesai P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: A randomized controlled pi-lot study. Journal of Sport and Health Science. 2016; 5:176-82.
CrossRef - Pilsakova L, Riecansky I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010; 59(5):651–64.
CrossRef - Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010; 31(4):400–19.
CrossRef - Rietjens IMC, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol. 2017; 174(11):1263-80.
CrossRef - Sirotkin AV, Harrath AH. Phytoestrogens and their effects. Eur J Pharmacol. 2014; 741:230-36.
CrossRef - Sreeja S, Anju VS, Sreeja S. In vitro estrogenic activities of fenugreek Trigonella foenum graecum seeds. Indian J Med Res. 2010; 131:814-19.
- Begum SS, Jayalakshmi HK, Vidyavathi HG, Gopakumar G, Abin I, Balu M. A Novel Extract of Fenugreek Husk (FenuSMART™) Alleviates Postmenopausal Symptoms and Helps to Establish the Hormonal Balance: A Randomized, Double-Blind, Placebo-Controlled Study. Phytother Res. 2016; 30(11):1775-84.
CrossRef - Thomas JV, Rao J, John F, Begum S, Maliakel B, Krishnakumar IM. Phytoestrogenic effect of fenugreek seed extract helps in ameliorating the leg pain and vasomotor symptoms in postmenopausal women: A randomized, double-blinded, placebo-controlled study. PharmaNutrition. 2020; 14:100209.
CrossRef - Joshi M, Nair S. Incorporation of Fenugreek and Sesame Seeds for Alleviation of Menopausal Symptoms. International Journal of Social Sciences. 2015; 4(4):263-66.
CrossRef - Stachowiak G, Pertyński T, Pertyńska-Marczewska M. Metabolic disorders in menopause. Przeglad Menopauzalny. 2015; 14:59–64.
CrossRef - Wu T, Yue R, He M, Xu C. Effect of Fenugreek on vasomotor symptoms in menopausal women: A protocol for systematic review and meta-analysis. Medicine. 2020; 99(23):e20526.
CrossRef - Nagamma T, Konuri A, Bhat KMR, Maheshwari R, Udupa P, Nayak Y. Modulation of inflammatory markers by petroleum ether fraction of Trigonella foenum‐graecum L. seed extract in ovariectomized rats. Journal of food biochemistry. 2021; 45(4):e13690.
CrossRef - Khodamoradi K, Khosropanah MH, Ayati Z, Chang D, Nasli-Esfahani E, Ayati MH, et al. The effects of fenugreek on cardiometabolic risk factors in adults: A systematic review and meta-analysis. Complementary Therapies in Medicine. 2020; 52(1):102416.
CrossRef - Askarpour M, Alami F, Campbell MS, Venkatakrishnan K, Hadi A, Ghaedi E. Effect of fenugreek supplementation on blood lipids and body weight: A systematic review and meta-analysis of randomized controlled trials. Journal of Ethnopharmacology. 2020; 253(10):112538.
CrossRef - Nagamma T, Konuri A, Nayak CD, Kamath SU, Udupa PE, Nayak Y. Dose-dependent effects of fenugreek seed extract on the biochemical and haematological parameters in high-fat diet-fed rats. Journal of Taibah University Medical Sciences. 2019; 14(4):383-89.
CrossRef - Konuri A, Bhat KMR, Rai KS, Gourishetti K, Phaneendra YS. Supplementation of fenugreek with choline-docosahexaenoic acid attenuates menopause-induced memory loss, BDNF and dendritic arborization in ovariectomized rats. Anat Sci Int. 2021; 96(2):197-211.
CrossRef - Konuri A, Bhat KMR, Rai KS, Nagamma T, Rajesh T, Sivakumar G. Dietary supplementation of fenugreek seed extract and choline-Docosahexaenoic cid on oxidative stress and neurodegeneration in ovariectomized rats with bilateral common carotic artery occulusion. Res J Pharm Technol. 2021; 14(4):2153-51.
CrossRef - Kamal R, Yadav R, Sharma J. Efficacy of the ste-roidal fraction of fenugreek seed extract on fertility of male albino rats. Phytotherapy research. 1993; 7:134-38.
CrossRef - Kassem A, Al-AghbariA, AL-Habori MT, Al-Mamary M. Evaluation of the potential antifertility effect of fenugreek seeds in male and female rabbits. Contraception. 2006; 73:301–6.
CrossRef - Al-Yahya AA. Reproductive, cytological and bio-chemical toxicity of fenugreek in male Swiss albino mice. African Journal of Pharmacy and Pharmacology. 2013; 7:2072-80.
CrossRef - Mohammed HA, Kamil DQ, Ahmed SA. Effect of fenugreek (Trigonella foenum-graecum) seed aqueous extract on testes tissue of anabolic steroid treated adult mice. World Journal of Agricultural Research. 2015; 4:35-39.
- Helal EGE, Fadal HAE, Ikechukwu GC, Abd-El-Aziz MA, Ahmed SS. Phytoestrogens Soymilk and Fenugreek Oil Perturb Lipid and Hormonal Profile and Other Physiological Parameters of Male Albino Rats. The Egyptian Journal of Hospital Medicine. 2019; 76(2):3494-99.
CrossRef - Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Molecular and Cellular Endocrinology. 2010; 321(2):152-60.
CrossRef - Assinder S, Davis R, Fenwick M, Glover A. Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction. 2007; 133:11-19.
CrossRef - Khalki L, M’hamed SB, Bennis M, Chait A, Sokar Z. Evaluation of the developmental toxicity of the aqueous extract from Trigonella foenum-graecum (L.) in mice. J Ethnopharmacol. 2010; 131(2):321-25.
CrossRef - John LJ, Shantakumari N. Herbal Medicines Use During Pregnancy: A Review from the Middle East. Oman Med J. 2015; 30(4):229-36.
CrossRef - Kilshaw S, Omar N, Major S, Mohsen M, El Taher F, Al Tamimi H. Causal explanations of miscarriage amongst Qataris. BMC Pregnancy and Childbirth. 2017; 17(1):1-2.
CrossRef - Folwarczna J, Zych M, Nowińska B, Pytlik M, Janas A. Unfavorable effect of trigonelline, an alkaloid present in coffee and fenugreek, on bone mechanical properties in estrogen-deficient rats. Mol Nutr Food Res. 2014; 58(7):1457-64.
CrossRef - Konuri A, Bhat KMR, Srinivasa SR, Devkar RA, Henry T. Beneficial Role of Hydro-alcoholic Seed Extract of Trigonella foenum graecum on Bone Structure and Strength in Menopause Induced Osteopenia. Ethiop J Health Sci. 2018; 28(6):787-94.
- Rathi A, Ishaq M, Najmi AK, Akhtar M. Trigonelline Demonstrated Ameliorative Effects in Dexamethasone Induced Osteoporotic Rats. Drug Res (Stuttg). 2020; 70(6):257-64.
- Sindhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant-induced arthritic rats. International immunopharmacology. 2012; 12(1):205–11.
CrossRef - Alcantra EH, Shin MY, Sohn HY, Park YM, Kim T, Lim JH. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synt sis and bone specific transcription factor Runx2 in osteoblastic MC3T3 -E1cells. J Nutr Biochem. 2011; 22:1055-63.
CrossRef - Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferati on through the down regulation of Akt, IκB kinase activation and NFκB regulated gene expression. Oncogene. 2006; 25:1463-73.
CrossRef - Chen Y, Tang YM, Yu SL, Han YW, Kou JP, Liu BL. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015; 13(8):578-87.
CrossRef - Yusharyahya SN, Bramono K, Hestiantoro A, Edwar SQ, Kusuma I. Fenugreek (Trigonella foenum-graceum) increases postmenopausal fibroblast-associated COL1A1 and COL3A1 production dominantly through its binding to estrogen receptor beta. Journal of Applied Pharmaceutical Science. 2020; 10(4):022-027.
CrossRef - Fleiss P. Herbal remedies for the breastfeeding mother. Mothering. 1988; Summer:68-71.
- Tiran D. The use of fenugreek for breast feeding women. Complement Therap Nursing Midwifery. 2003; 9:155-56.
CrossRef - Ghasemi V, Kheirkhah M, Mohsen Vahedi M. The Effect of Herbal Tea Containing Fenugreek Seed on the Signs of Breast Milk Sufficiency in Iranian Girl Infants. Iran Red Crescent Med J. 2015; 17(8):e21848.
CrossRef - Turkyilmaz C, Onal E, Hirfanoglu IM, Turan O, Koc E, Ergenekon E. The effect of galactagogue herbal tea on breast milk production and short-term catch-up of birth weight in the first week of life. J Altern Complement Med. 2011; 17(2):139–42.
CrossRef - Damanik R, Wahlqvist ML, Wattanapenpaiboon N. Lactagogue effects of Torbangun, a Bataknese traditional cuisine. Asia Pac J Clin Nutr. 2006; 15(2):267–74.
CrossRef - El Sakka A, Salama M, Salama K. The Effect of Fenugreek Herbal Tea and Palm Dates on Breast Milk Production and Infant Weight. J Pediatr Sci. 2014; 6:e202.
- Dehkhoda N, Valizadeh S, Jodeiry B, Hosseini MB. The effects of an educational and supportive relactation program on weight gain of preterm infants. J Caring Sci. 2013; 2(2):97–103.
- Abdoua RM, Fatheyb M. Evaluation of early postpartum fenugreek supplementation on expressed breast milk volume and prolactin levels variation. Egyptian Pediatric Association Gazette. 2018; 66(3):57-60.
CrossRef - Fugh-Berman A.”Bust enhancing” herbal products. Obstet Gynecol. 2003; 101(6):1345-49.
CrossRef - Tabares FP, Jaramillo JV, Ruiz-Cortes ZT. Pharmacological overview of galactogogues. Vet Med Int. 2014; 602894.
CrossRef - Saleh AA, Ahmed EAM, Ebeid TA. The impact of phytoestrogen source supplementation on reproductive performance, plasma profile, yolk fatty acids and antioxidative status in aged laying hens. Reprod Domest Anim. 2019; 54(6):846-54.
- Azouz HM. Effects of dietary turmeric and fenugreek powder supplementation on productive performance of local laying hens. Egyptian Poultry Science Journal. 2020; 20(1):243-58.
- Awadein NB, Eid YZ, Abd El-Ghany FA. Effect of dietary supplementation with phytoestrogens sources before sexual maturity on productive performance of mandarah hens. Poult Sci. 2010; 30(3):829-46.
- Ghani JM, Sharba IR, Shamran SJ. Antioxidant role of origanum Vulgare aqueous extract against reproductive toxicity assessment of fenugreek in female mice. Eur asia J Biosci. 2020; 14:1335-40.
- Modaresi M, Mahdian B, Jalalizand A. The Effect of Hydro-Alcoholic Extract of Fenugreek Seeds on Female Reproductive Hormones in Mice. Int Conf Appl Life Sci. 2012; 437-43.
- Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, et al. Efficacy of anovel fenugreek seed extract (Trigonella foenum-graecum, furocyst (T.M.)) in polycysticovary syndrome (PCOS). Int J Med Sci. 2015; 12(10):825-31.
CrossRef - Ben Hameid AS, Al-Sindi TA, Allow AK, Nafie EM, Alahmad BE, Faisal GG. Substantial effect of fenugreek seeds aqueous extract on serum estradiol level in ovarian hyper stimulation syndrome- rat model. Oman Med J. 2019; 34:238-43.
CrossRef - Wilborn C, Taylor L, Poole C, Foster C, Willoughby D, Kreider R. Effects of a purported aromatase and 5α- reductase inhibitor on hormone profiles in college-age men. Int J Sport Nutr Exerc Metab. 2010; 20(6):457-65.
CrossRef - Hilles A, Allow A, Mahmood S. Evaluation and comparison of the antifertility potential activity and adverse effects of Trigonella foenum-graecum seeds and combined oral contraceptive pills in female rats. Int J Reprod Contraception, Obstet Gyneco. 2016; l5:680-88.
CrossRef - Mazalzadeh F, Hekmat K, Namjouyan F, Saki A. Effect of Trigonella foenum (fenugreek) vaginal cream on vaginal atrophy in postmenopausal women. J Family Med Prim Care. 2020; 9:2714-19.
CrossRef - Safary M, Hakimi S, Mobaraki-Asl N, Amiri P, Tvassoli H, Delazar A. Comparison of the Effects of Fenugreek Vaginal Cream and Ultra Low-Dose Estrogen on Atrophic Vaginitis. Current Drug Delivery. 2020; 17(9):815-22.
CrossRef - Geberemeskel GA, Debebe YG, Nguse NA. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J Diabetes Res. 2019; 8507453.
CrossRef - Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric. 2015; 18(2):260–69.
CrossRef - Kalailingam P, Kannaian B, Tamilmani E, Kaliaperumal R. Efficacy of natural diosgenin on cardiovascular risk, insulin secretion, and beta cells in streptozotocin (STZ)-induced diabetic rats. Phytomedicine. 2014; 21:1154-61.
CrossRef - Harijono TE, Ariestiningsih AD, Wardani NAK. The effect of crude diosgenin extract from purple and yellow greater yams (Dioscorea alata) on the lipid profile of dyslipidemia rats. Emirates Journal of Food and Agriculture. 2016; 28:506-12.
CrossRef - Bahadur S, Roy A, Chanda R, Baghel P, Saha S, Choudhury A. Assessment of Some Phytochemcial and Physicochemical Properties of Fenugreek Seed Mucilage. Research J. Pharm. and Tech. 2016; 9(9):1321-24.
CrossRef - Boban PT, Nambisan B, Sudhakaran PR. Hypolipidaemic effect of chemically different mucilages in rats: a comparative study. Brit J Nutr. 2006; 96(06):1021-9.
- Mandegary A, Pournamdari M, Sharififar F, Pournourmohammadi S, Fardiar R, Shooli S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti- inflammatory effects. Food Chem Toxicol. 2012; 50(7):2503-7.
CrossRef - Akbari M, Rasouli H, Bahdor T. Physiological and pharmaceutical effect of fenugreek: a review. IOSR Journal of Pharmacy. 2012; 2(4):49-53.
CrossRef - Hao S, Xu R, Li D, Zhu Z, Wang T, Liu K. Attenuation of Streptozotocin-Induced Lipid Profile Anomalies in the Heart, Brain, and mRNA Expression of HMG-CoA Reductase by Diosgenin in Rats. Cell Biochem Biophys. 2015; 72:741-49.
- Zhou HT, Yu XF, Zhou GM. Diosgenin inhibits angiotensin II-induced extracellular matrix remodeling in cardiac fibroblasts through regulating the TGF-β1/ Smad3 signaling pathway. Mol Med Rep. 2017; 15:2823-28.
CrossRef - Scuderi G, Contestabile MT, Caterina Gagliano C, Iacovello D, Scuderi L, Avitabile T. Effects of phytoestrogen supplementation in postmenopausal women with dry eye syndrome: a randomized clinical trial. Canadian Journal of Ophthalmology. 2012; 47(6):489-92.
CrossRef - Gagliano C, Amato R, Scollo D, Ortisi E, Caruso S, Reibaldi M. Effect of Fenugreek on Severe Evaporative Dysfunctional Tear Syndrome. ARVO Annual Meeting Abstract. 2012; 53(14):5321.
CrossRef - Qadir MI, Cheema BN. Phytoestrogens and Related Food Components in the Prevention of Cancer. Critical Reviews™ in Eukaryotic Gene Expression. 2017; 27(2):99-112.
CrossRef - Sebastian K.S., Thampan R.V. Differential effects of soybean and fenugreek extract on the growth of MCF-7 cells. Chem Biol Interact. 2007;170(2):135-43.
CrossRef - Raju J, Patlolla JMR, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004; 13(8):1392-98.
CrossRef - Devasena T, Menon VP. Fenugreek affects the activity of β-glucuronidase and mucinase in the colon. Phytotherapy Research. 2003; 17(9):1088–91.
CrossRef - Najdi RA, Hagras MM, Kamel FO, Magadmi RM. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr Health Sci. 2019; 19(1):1594–601.
CrossRef - Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Canadian Journal of Diabetes. 2014; 38(6):409-14.
CrossRef - Al-Habori M, Raman A. Antidiabetic and hypocholesterolemic effects of fenugreek. Phytotherapy Research. 1998; 12(4):233–42.
- Xue W, Lei J, Li X, Zhang R. Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutrition Research. 2011; 31(7):555-62.
CrossRef - Sayed AAR, Khalifa M, Abd El-Latif FF. Fenugreek attenuation of diabetic nephropathy in alloxan – diabetic rats. J Physiol Biochem. 2012; 68:263-9.
CrossRef - Belaid-Nouira Y, Bakhta H, Haouas Z, Flehi-Slim I, Neffati F, Najjar MF. Fenugreek seeds, a hepatoprotector forage crop against chronic AlCl3toxicity. BMC Vet Res. 2013; 9:22.
CrossRef - Badr MI. Effect of fenugreek seeds on kidney structure and some physiological parameters in rats. Middle East J Appl Sci. 2017; 7(4):967-73.








