Asep Sukohar1* , Dwi Aulia Ramdini1
, Dwi Aulia Ramdini1 , Citra Yuliyanda Pardilawati1
, Citra Yuliyanda Pardilawati1 and Suharyani2
and Suharyani2
1Department of Pharmacy, Faculty of Medicine, University of Lampung, P.O 35145, Indonesia
2Faculty of Medicine, University of Lampung, P.O 35145, Indonesia
Corresponding Author E-mail: asepsukohar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2794
Abstract
Background: One of the major causes of death in the world is cancer. The cancer frequently affects in women especially breast cancer and cervical cancer. Many anticancer drugs have been developing throughout time due to the side effect of cancer treatments. Current study, plants have been extensively explore for their bioactive compound that is effective as anticancer drug candidates. Gnetum gnemon L. plant contains a bioactive compound that is beneficial for health and can be developed as an anticancer agent. Objective: The aim of this study was to investigate the potential of Gnetum gnemon L. seed extract as an antioxidant and anticancer in two cells line, MCF-7 and HeLa cells. Methods: The antioxidant evaluated through the DPPH (2,2-Diphenyl-1-picrylhydrazyl) and MTT (3-(4,5-dimethylthiazol-2-ly)-2,5-diphenyltetrazolium bromide) assays conducted for cytotoxicity. Phytochemical qualitative screening identified the flavonoids, tannins, and terpenoids. Results: The result of the DPPH assay was 543.19 ± 11.43 μg/mL and the MTT assay with IC50 value 316 ± 45.76 μg/mL, 489.57 ± 4.03 μg/mL on MCF-7 and HeLa cells respectively. Based on these findings, antioxidant activity of the Gnetum gnemon L. seed extract against MCF-7 and HeLa cancer cells line defined as moderate cytotoxicity. Conclusion: The percentage (%) cell viability of MCF-7 and HeLa cells decreased as the concentration of the extracts increased. Further investigation is needed to make a quantitative analysis of these compounds and their mechanism of action as anticancer activities.
Keywords
Anti-cancer; Cytotoxicity; DPPH assay; Gnetum gnemon L
Download this article as:| Copy the following to cite this article: Sukohar A, Ramdini D. A, Pardilawati C. Y, Suharyani S. Investigation of Antioxidant and Anticancer Activity againts MCF-7 and HeLa Cancer Cells of Melinjo (Gnetum gnemon L.). Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Sukohar A, Ramdini D. A, Pardilawati C. Y, Suharyani S. Investigation of Antioxidant and Anticancer Activity againts MCF-7 and HeLa Cancer Cells of Melinjo (Gnetum gnemon L.). Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3Srn7p1 |
Introduction
Cancer remains one of the leading causes of morbidity and mortality globally. There are many diseases caused by oxidative stress such as cancer 1 . It occurs from a multiple-step process which includes initiation, promotion, and progression stages, called carcinogenesis 2. The incidence rate of cancer rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. From 36 different types of cancer, cancer mainly affects women in the form of breast and cervical cancer 3. In present, various treatments i.e chemotherapy, radiotherapy, surgery, and chemically derived drugs are used as cancer treatment 4. However, those modality treatments have some side effects 5. Therefore, discovery and development studies were still required to find new and effective anticancer drugs 6.
Generally, plants have been used widely to prevent and treat cancer for a long period 7. Plants medicine has been recognized as a source of potential therapeutic due to their biologically active compounds. Thus, many anticancer drugs and antioxidants are derived from natural sources 8. Over 60% of chemotherapeutic drugs are identified and isolated from plants or their synthetic derivatives 6. Plant-derived agents have an important role in the treatment of cancer due to producing a wide range of chemical constituents called a secondary metabolite (9). For instance, among the many plants, Gnetum gnemon L. (G. gnemon) local name: Melinjo has been identified with the presence of secondary metabolite and polyphenols 10,11. Melinjo (G. gnemon) is a gymnospermae plant native to Southeast Asia whose seeds and fruits are commonly used in Indonesian cuisine 11. The seed flour of melinjo was evaluated for nutritional composition, antioxidant activity and functional properties. It consisted primary utilitarian groups such as: amines, amides, amino acids, polysaccharides, carboxylic acids, esters and lipids 12.
Data from preclinical studies show the potential of the melinjo seeds as antioxidants, decreasing uric acid, antimicrobial, anti-obesity, and anti-cancer 13,14,11,15. Therefore, extensive investigation of the potential of the seeds as anticancer agents is necessary. Thus, thoroughly evaluating cytotoxic activity and screening raw extracts of the seeds would confirm these effects.
Material and Methods
Chemical and Reagents
The antioxidant was performed by colorimetric through DPPH assay and MTT assay were conducted for cytotoxicity. The DPPH and MTT kit was purchased from Sigma-Aldrich. All chemicals and solvents were analytical grades and obtained from commercial sources.
Materials Collection and Identification
The edible parts of G.gnemon were selected because they are widely consumed. G.gnemon seeds were harvested in March 2021 from the Pesawaran Regency of Lampung Province, Indonesia. The botanical identification of G.gnemon was performed at the Botanical Laboratory, Faculty of Mathematics and Natural Sciences, University of Lampung, Lampung, Indonesia.
Preparation and Extraction
Based on our previous study 16, seeds were air-dried in air shade at room temperature, then ground to a uniform powder. The powder of dry seeds G. gnemon (300 g) was macerated in 1.2 L of ethanol for three days and then filtered. The thick extracts were obtained by concentrating with a rotary evaporator at 40°C and stored at 4°C until being used. For qualitative phytochemical analysis, the residue was reconstituted in a solvent before testing.
Qualitative Phytochemical Analysis
The ethanol seed extract was subjected to the qualitative phytochemicals screening of some chemical compounds i.e. alkaloids, flavonoids, saponins, tannins, and triterpenoids. Mayer test for identifying alkaloids, Shinoda test, Foam test, Braemer’s test, and Salkowski test for flavonoids, saponin, tannin and terpenoid respectively 17,18.
DPPH Antioxidant Activity Determination
The DPPH was used to determine the free radical scavenging activity 19, 20. A total 2 mL of DPPH (50 ppm in ethanol) was mixed with 2 mL extract. This was incubated for 30 min at room temperature (23-25°C) and protected from light. The absorbance was measured with a spectrophotometer at 517 nm 16 then the IC50 value (µg/mL) determination was followed. Experiments were conducted in triplicates.
Cell Culture
MCF-7 and HeLa cell lines were used during the experiment. Cells were cultured in a complete growth medium: Roswell Park Memorial Institute (RPMI 1640) media supplemented with 10% fetal bovine serum (FBS), antibiotics (100 I.U/mL penicillin and 100 µg/mL streptomycin) at 37°C, 5% CO2 incubator humidity. Cells were cultured in healthy conditions and exponentially growing cells (-80% confluency) were used for experiments.
Cytotoxicity Assay
The cytotoxicity of the ethanol extract on MCF-7 and HeLa cells was measured using an MTT assay. The cells were seeded in 96-microwell plates a density of the cell 2×104 cells/well then incubated for 24 hours (h). A total 100 µL amount of extract was added to test wells and the microplates were incubated for 24 h. In addition, removing the supernatant and add 20 μL of MTT solution to each well and incubated for 3 h. The supernatant was added with 100 μl DMSO and dissolved formazan crystals. The amount of formazan crystal was measured at wavelength 570 nm 21. The percentage of the cell viability of the cells treated with ethanol extract was calculated according to the formula:
% Cell viability = (Abssample– Abscontrol)/Abscontrolx100%
The inhibitory concentration (IC) 50 value was calculated from a graph plotting % cell viability against the concentration of the sample.
Observation of Morphological Changes
MCF-7 and HeLa cells were seeded in 96-microwell plates at the density of the cell 2×104 cells/well then incubated for 24 h. They were then treated with the extract and cell morphology was observed and analyzed after 24 h incubation.
Statisctic Analysis
All data analysis were performed using Microsoft Excel software 2016 and the result was presented as mean ± SD with triplicate (n=3).
Results
Sample extraction
The G.gnemon seeds extract was obtained by evaporation of ethanol as a solvent and the percentage by weight yield was estimated at 0.55% based on the solvent used. The qualitative phytochemical investigation result found flavonoid, tannin, and terpenoid (Table 1).
Tabel 1: Qualitative Phytochemical screening of G.gnemon L. seed extracts.
|
Constituents |
Test |
Result |
|
Alkaloid |
Mayer test |
– |
|
Flavonoid |
Shinoda test |
+ |
|
Saponin |
Frothing test / Foam test |
– |
|
Tanin |
Braemer’s test |
+ |
|
Terpenoid |
Salkowski test |
+ |
Abbreviations: (+), present;(−), absence; N, Not indicated.
DPPH Antioxidant Activity Determination
The most common method to determine the antioxidant activity of various compounds is the DPPH assay 22. This assay was measured by changing the purple ethanol solution of DPPH. The antioxidant agents can convert DPPH into 1-1 diphenyl-2-picryl hydrazine in the yellow molecule, by transferring electrons or hydrogen 8. The result of antioxidant activity as mean ± SD was 543.19±11.43 µg/mL in 3 replications (Figure 1). The ascorbic acid as a standard with a coefficient correlation (R2) of 0.9993 has IC50 5.42 ± 0.009 µg/mL.
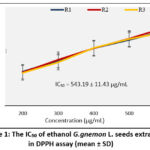 |
Figure 1: The IC50 of ethanol G. gnemon L. seeds extract in DPPH assay (mean ± SD). |
Cytotoxicity assay
The cell viability on MCF-7 and HeLa cells was assessed in vitro through MTT assay. The extract is revealed as moderate cytotoxicity (Figure. 2). According to ISSO: 10993-5 standard, percentages of cell viability over 80% are considered as non-cytotoxicity; within 80%–60% weak; 60%–40% moderate and underneath 40% strong cytotoxicity respectively following above 80% as non-cytotoxicity, within 80-60% as a weak, 60-40% moderate and below 40% strong cytotoxicity 23. Base on Figure. 2 indicated that after being treated with the extract for 24 h, significantly decreased the % cell viability (p<0.05) at the final concentration, up to 47.55% and 62% for MCF-7 and HeLa cells respectively.
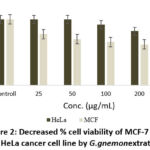 |
Figure 2: Decreased % cell viability of MCF-7 and HeLa cancer cell line by G. gnemon extrats. |
The cytotoxicity of the extract (IC50) was determined in comparison to the anticancer drug doxorubicin (Table.2). The IC50 values obtained refer to 50% of cells inhibited by extracts. Based on Table 2. Shown that G.gnemon extract has IC50 300-400 µg/mL for both cancer cells. Moreover, the extract indicates moderate cytotoxic (100-1000 µg/mL) 24.
Table 2: Result of cytotoxicity against MCF-7 and HeLa cell lines
|
|
Cytotoxic activity (IC50, µg/mL) |
|
|
MCF-7 (mean ± SD) |
HeLa (mean ± SD) |
|
|
G.gnemon extract |
316.19 ± 45.76 |
489.57 ± 4.03 |
|
Doxorubicin |
0.14 ± 0.12 |
2.38 ± 3.5 |
The IC50 in MTT assay (mean ± SD) of three independent experiments, n=3
Observation of Morphological Changes
We evaluated the morphology changes of both breast cancer and HeLa cells with microscopically examined after 24 hours given extract treatment. A major change in cell morphology was observed in cell shrinkage, cell wall blebbing (Figure.3)
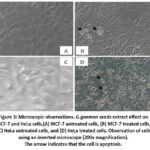 |
Figure 3: Microscopic observations. G.gnemon seeds extract effect on MCF-7 and HeLa cells. |
(A) MCF-7 untreated cells, (B) MCF-7 treated cells, (C) HeLa untreated cells, and (D) HeLa treated cells. Observation of cells using an inverted microscope (200x magnification). The arrow indicates that the cell is apoptosis.
Morphological changes were revealed after MCF-7 and HeLa were treated with G.gnemon seeds extract compared to untreated cells. Figure (A) shows that untreated MCF-7 cells appear healthy, normal, and confluent cells while treated cell (B) it appeared that cell vacuolization was formed, and the cells appeared to be smaller and rounded. Crude extract of G.gnemon also induced cell death in HeLa cells. HeLa cells without treatment showed a flat polygonal shape with good permeability, complete nucleoli, clear and adherence (C) while in treated cells (D), the cell was observed shrinkage, the shape became fusiform cells or long circles, and some cells had started to undergo apoptosis.
Discussion
There are many anticancer drugs that have been developed over time. However due to side effects of cancer treatments, a new anti-cancer substance that is more effective and less side effects is needed. A large number of plants are well known as alternative medicine that considered less side effects. Our previous study has been conducted several plant medicines including robusta coffee 25, Polygonum pulchrum 26, Annona muricata Linn 27, Jatropha gossypifolia 28. In the recent study, a part of species G.gnemon L was used to evaluate for its antioxidant and anticancer activity. These plants had been intensively studied for their therapeutic properties. We observed the presence of various phytochemicals (Table 1) and these phytochemicals showed antioxidant and anticancer activities. Phytochemical compounds of natural products as antioxidants have a main role as radical scavenging that produce certain diseases. The reactive oxygen species (ROS) is a chemically reactive molecule in cells and associated with a variety of biological processes, such as cell proliferation, differentiation and programmed cell death ROS has a relationship with the oncogene function and suppression function 29. Concisely, ROS will results in G1 phase inhibition leading to a significant influence on cell proliferation 16. Antioxidant presence deactivates free radicals by donating hydrogen atoms to free radicals that are dominant to scavenge radicals 30. Those are explanations of how antioxidant prosperity toward on underlying cancer mechanism. An uncontrolled increase in cell proliferation and decreased cellular apoptosis are characteristics of cancer. Approaches for treating cancer should be apoptosis induction and growth inhibition of tumor cells 31.
Some parts of G. gnemon plant are known to have anticancer activity, especially in leaves and seeds. Recent studies there were several reports the extract of G. gnemon leaves was indicated have high IC50 values. The ethanol extract of G. gnemon leaves on previous studied showing the IC50 values was at high potential toxicity 32. Moreover, Bioactivity assay of gnetumal and p-coumaric acid as a bioactive compound of this plant indicated possessed more potent tyrosinase inhibitory activity, with IC50 values of 31.6 and 2.3 mM, respectively, than that of a positive control kojic acid 33. In our study presented G.gnemon seed extract have anticancer activity as moderate IC50 values. A different result was shown by Narayan et al. which presented IC50 value as potential toxicity 34. We presumably it could cause the different type and specification of sample testing, in which the amount of compound was known. The preparation sample testing would expressed a different result. The specific isolation compound of plants can understand clearly the mechanism and further investigation on anticancer candidates. Such as like our studied that performed the bioactive from Robusta Coffee such as caffeine and chlorogenic acid against on cell line Hep-G2 35,36.
The antioxidant activity were carried out to assess the capacity of plant extracts to scavenge free radicals. Compare with the previous study regarding the antioxidant activity of melinjo seed have been reported by Supriyadi et al. tested with the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay, this study shown that the melinjo seed has very weak category for antioxidant activity 37. This may be due to differences in the method of the assay. Extracts showing low antioxidant that measured use one method should not be discarded as poor sources of antioxidant without having been compared with other methods 38. Phenol compounds in the melinjo seed is the main component to have the potential for antioxidant activity 39. High levels of phenol could be considered as a good source of antioxidants that will act in the prevention of many diseases e.g carcinomas 40.
According to the literature, mechanism of action flavonoids are anti-cell proliferation, induced apoptosis and cell cycle arrest, inhibition angiogenesis and suppression of metastasis 41, 42. Terpenoid through induced apoptosis intrinsic pathway 43. Tannins induced G1 arrest, phosphorylation of the tumor suppressor protein p5344. As known G. gnemon has several anticancer activities with a certain mechanism such as inhibits endothelial senescence 45. In our report studied that fraction of G. gnemon seed showed antioxidant and cytotoxic activity against HeLa cell lines 16. In vivo studies exhibited Gnetin C as another bioactive compound that has lead activity over the stilbenoid. Gnetin C-treated tumors showed reduced mitotic activity and angiogenesis and a significant increase in apoptosis compared to all the other groups. The data suggest that Gnetin C is more vigorous in declining tumor progression in prostate cancer xenografts than Res or Pter 46. Beside phytochemical compounds, these plants are rich in resveratrol and gnetin C content 10. Resveratrol is a natural polyphenolic phytoalexin that has been shown to have antioxidant activity and anticancer properties. Resveratrol suppresses cell proliferation and induced apoptosis through mitochondrial and p53 signaling pathways in human cervical carcinoma 47. In a previous study, gnetin C induced apoptosis through inhibits the mTOR and MAPK pathway in acute myeloid leukemia (AML) 48. Morphological changes of the cancer cell are beginning event during the induction of apoptosis i.e. cell blebbing, shrinkage, nuclear fragmentation, chromatin condensation, and so on 21, 49, in this study presented in Figure 3.
Recently, some phytochemical compounds were identified by qualitative methods and the anticancer activity of the extract was classified as moderate cytotoxicity. These studies exhibit that G. gnemon is a natural source with potent activity as anticancer candidates. Many bioactivities of this plant need to be investigated and developed with in vitro and in vivo assay. Further investigation needs to be performed quantitative analysis and mechanism of action as anti-cancer of these plant compounds.
Conclusion
In the light of these findings, it is apparent that G. gnemon or melinjo seeds could be considered as a vital source of antioxidants. It is observed that extract of G. gnemon had significant anticancer activity in different cancer cell lines using in vitro models. We investigated the phytochemical, antioxidant and cytotoxicity of the G. gnemon seed extract against the MCF-7 and HeLa cells line. Our findings revealed that the extract was of moderate cytotoxicity. The % cell viability of MCF-7 and HeLa cells decreased as the concentration of the extracts increased.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding Sources.
References
- Islam S, Nasrin S, Khan MA, Hossain ASMS, Islam F, Khandokhar P, et al. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement Altern Med. 2013;13(1):1.
CrossRef - Meiyanto E, Larasati YA. The Chemopreventive Activity of Indonesia Medicinal Plants Targeting on Hallmarks of Cancer. Adv Pharm Bull. 2019;9(2):219–30.
CrossRef - Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S, et al. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2020;10(1):1–30.
CrossRef - Greenwell M, Rahman PKSM. Medicinal Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res. 2015;6(10):4103–12.
- Scaria B, Sood S, Raad C, Khanafer J, Jayachandiran R, Pupulin A, et al. Natural health products (NHP’s) and natural compounds as therapeutic agents for the treatment of cancer; mechanisms of anti-cancer activity of natural compounds and overall trends. Int J Mol Sci. 2020;21(22):1–32.
CrossRef - Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. Scientific World Journal. 2014;1–12.
CrossRef - Wang H. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med Chem. 2012;12(10):1281–305.
CrossRef - Soltanian S, Sheikhbahaei M, Mirtadzadini M, Kalantari Khandani B. Evaluation of anticancer, antioxidant and antibacterial properties of methanol extract of three Acantholimon Boiss. species. Avicenna J Phytomed. 2020;10(6):641–52.
- Kooti W, Servatyari K, Behzadifar M, Asadi-Samani M, Sadeghi F, Nouri B, et al. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J Evid Based Complementary Altern Med. 2017;22(4):982–95.
CrossRef - Kato E, Tokunaga Y, Sakan F. Stilbenoids isolated from the seeds of melinjo (Gnetum gnemon L.) and their biological activity. J Agric Food Chem. 2009;57(6):2544–9.
CrossRef - Oniki K, Kawakami T, Nakashima A, Miyata K, Watanabe T, Fujikawa H, et al. Melinjo seed extract increases adiponectin multimerization in physiological and pathological conditions. Sci Rep. 2020;10(1):1–13.
CrossRef - Bhat R, Binti Yahya N. Evaluating belinjau (Gnetum gnemon L.) seed flour quality as a base for development of novel food products and food formulations. Food Chem [Internet]. 2014 Aug 1 [cited 2023 May 24];156:42–9. Available from: https://pubmed.ncbi.nlm.nih.gov/24629936/
CrossRef - Kunarto B, Sutardi S, Supriyanto, Anwar C com. Antioxidant activity of melinjo ketan (Gnetum gnemon L., ’Ketan’) seed extract at various ripening stages and ethanol solvent concentration. Int J Adv Sci Eng Inf Technol. 2019;9(4):1344–51.
CrossRef - Konno H, Kanai Y, Katagiri M, Watanabe T, Mori A, Ikuta T, et al. Melinjo (Gnetum gnemon L.) seed extract decreases serum uric acid levels in nonobese Japanese males: A randomized controlled study. Evidence-based Complementary and Alternative Medicine. 2013;1–9.
CrossRef - Narayanan NK, Kunimasa K, Yamori Y, Mori M, Mori H, Nakamura K, et al. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in acolon-26 tumor-bearing mouse model in vivo. Cancer Med. 2015;4(11):1767–80.
CrossRef - Sukohar A, Suharyani, Sutyarso, Busman H, Nurcahyani N, Kurniawaty E. Antioxidant and Cytotoxic Activities of Melinjo (Gnetum gnemon L.) Seed Fractions on HeLa Cell Line an In Vitro. Pharmacognosy Journal. 2022 May 1;14(3):559–64.
CrossRef - Kausar F, Kim KH, Farooqi HMU, Farooqi MA, Kaleem M, Waqar R, et al. Evaluation of antimicrobial and anticancer activities of selected medicinal plants of Himalayas, Pakistan. Plants. 2022;11(1):1–15.
CrossRef - Ali S, Khan MR, Sajid M, Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med. 2018;18(1):1–15.
CrossRef - Sukohar A, Busman H, Nurcahyani N, Kurniawaty E. Antioxidant and Cytotoxic Activities of Melinjo ( Gnetum gnemon L .) Seed Fractions on HeLa Cell Line an In Vitro. Pharmacogn J. 2022;14(3):559–64.
CrossRef - Nurhayati B, Rahayu IG, Rinaldi SF, Zaini WS, Afifah E, Arumwardana S, et al. The antioxidant and cytotoxic effects of Cosmos caudatus Ethanolic Extract on Cervical Cancer. Indonesian Biomedical Journal. 2018;10(3):243–9.
CrossRef - Hosseini K, Jasori S, Delazar A, Asgharian P, Tarhriz V. Phytochemical analysis and anticancer activity of Falcaria vulgaris Bernh growing in Moghan plain, northwest of Iran. BMC Complement Med Ther. 2021;21(1):1–10.
CrossRef - Kumar P, Sucheta S, Sudarshana Deepa V, Selvamani P, Latha S. Antioxidant activity in some selected Indian medicinal plants. Afr J Biotechnol. 2008;7(12):1826–8.
CrossRef - A, Ciorîta, C, Zăgrean-Tuza, A CM. The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules. 2021;26(3040):1–21.
CrossRef - Prayong P, Barusrux S, Weerapreeyakul N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia. 2008;79(7–8):598–601.
CrossRef - Sukohar A, Herawati H, Witarto AB, FWirakusumah F, Sastramihardja HS. ROLE OF CHLOROGENIC ACID FROM LAMPUNG ROBUSTA COFFEE AGAINST GENE EXPRESSION OF MIRNA (MICRO RNA) 146 A ON HEPATOCELLULAR CARCINOMA CELLS. International Journal of Research in Pharmaceutical and Nano Sciences [Internet]. 2013 [cited 2023 Jun 14];2(6):776–84. Available from: www.uptodateresearchpublication.com
CrossRef - SAHIDIN, Nohong, Sani A, Anggrenimanggau M, Sukohar A, Widodo H, et al. RADICAL SCAVENGING ACTIVITY OF TRITERPENE STEROIDS FROM STEM OFPOLYGONUM PULCHRUM Bl. Int J Pharm Pharm Sci [Internet]. 2014 Aug 31 [cited 2023 Jun 14];350–4. Available from: https://journals.innovareacademics.in/index.php/ijpps/article/view/1408/10249
CrossRef - Muhartono, Sukohar A, Sutyarso, Kanedi M. Anti-Proliferative and Apoptotic Effects of Mucoxin (Acetogenin) in T47D Breast Cancer Cells. Biomedical and Pharmacology Journal [Internet]. 2016 Aug 21 [cited 2023 Jun 14];9(2):491–8. Available from: https://biomedpharmajournal.org/vol9no2/anti-proliferative-and-apoptotic-effects-of-mucoxin-acetogenin-in-t47d-breast-cancer-cells/
CrossRef - Asep S, Hening H, Gema SP, Gigih S, Widya MC, Sahidin. Anticancer activity of jatrophone an isolated compound from Jatropha gossypifolia plant against hepatocellular cancer cell Hep G2 1886. Biomedical and Pharmacology Journal. 2017;10(2):667–73.
CrossRef - Checa J, Aran JM. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J Inflamm Res [Internet]. 2020 Dec 2 [cited 2023 Jun 13] ;13:1057–73. Available from: https://www.dovepress.com/ reactive-oxygen-species-drivers-of-physiological-and-pathological-proc-peer-reviewed-fulltext-article-JIR
CrossRef - Xiao Z, He L, Hou X, Wei J, Ma X, Gao Z, et al. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods [Internet]. 2021 Apr 1 [cited 2023 Jun 13];10(4). Available from: /pmc/articles/PMC8070355/
CrossRef - Hegazy MGA, Imam AM, Abdelghany BE. Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumor Biology [Internet]. 2020 May 1 [cited 2023 Jun 13];42(5). Available from: https://journals.sagepub.com/doi/full/ 10.1177/1010428320918685
CrossRef - Dutta PP, Bordoloi M, Roy S, Narzary B, Gogoi K, Bhattacharyya DR, et al. Antiplasmodial Activity of Gnetum gnemon Leaves and Compounds Isolated from them. [cited 2023 Jun 10]; Available from: https://florafaunaweb.nparks.gov.sg/
- Le TH, Van Do TN, Nguyen HX, Dang PH, Nguyen NT, Nguyen MTT. A new phenylheptanoid from the leaves of Gnetum gnemon L. https://doi.org/101080/1478641920201753055 [Internet]. 2020 [cited 2023 Jun 10];35(21):3999–4004. Available from: https://www.tandfonline.com/doi/abs/ 10.1080/14786419.2020.1753055
CrossRef - Narayanan NK, Kunimasa K, Yamori Y, Mori M, Mori H, Nakamura K, et al. Antitumor activity of melinjo (Gnetum gnemon L.) seed extract in human and murine tumor models in vitro and in a colon-26 tumor-bearing mouse model in vivo. Cancer Med [Internet]. 2015 Nov 1 [cited 2023 Jun 14];4(11):1767–80. Available from: https://pubmed.ncbi.nlm.nih.gov/26408414/
CrossRef - Sukohar A, Muhartono. Comparative effects of chlorogenic acid and doxorubic in against expression of caspase3 in cell lines Hep-G2. J Chem Pharm Res [Internet]. 2015 Jan 31 [cited 2023 Jun 13];7(1):187–92. Available from: https://www.jocpr.com/abstract/comparative-effects-of-chlorogenic-acid-and-doxorubic-in-against-expression-of-caspase3-in-cell-lines-hepg2-6239.html
- Sukohar A, Herawati H, Sibero HT, Gigih S, Graharti R, Riyan W, et al. Effects of caffeine againts expression on Mir-423-3p in Cell Lines Hep-G2. Biomedical and Pharmacology Journal. 2018;11(1):429–35.
CrossRef - Supriyadi A, Arum LS, Nugraha AS, Ratnadewi AAI, Siswoyo TA. Revealing antioxidant and antidiabetic potency of melinjo (Gnetum gnemon) seed protein hydrolysate at different stages of seed maturation. Current Research in Nutrition and Food Science. 2019 Aug 1;7(2):479–87.
CrossRef - Chaves N, Santiago A, Alías JC. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants (Basel) [Internet]. 2020 Jan 1 [cited 2023 Jun 14];9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31952329/
CrossRef - Sari M, Rahmawati SI, Izzati FN, Bustanussalam, Putra MY. Antioxidant Activity of Ethanolic Extract of Peel and Seed Melinjo (Gnetum gnemon) Based on Color Variations. Proceedings of the 1st International Conference for Health Research – BRIN (ICHR 2022) [Internet]. 2023 Mar 1 [cited 2023 Jun 14];255–65. Available from: https://www.atlantis-press.com/proceedings/ichr-22/125984792
CrossRef - Khalid M, Amayreh M, Sanduka S, Salah Z, Al-Rimawi F, Al-Mazaideh GM, et al. Assessment of antioxidant, antimicrobial, and anticancer activities of Sisymbrium officinale plant extract. Heliyon. 2022 Sep 1;8(9):e10477.
CrossRef - Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8(16):122–46.
CrossRef - Amawi H, Ashby CR, Tiwari AK. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin J Cancer. 2017;36(1):1–13.
CrossRef - Hasanudin H, Rifayani KS, Supriadi G, Kurnia D, Adhita D. Potential of Terpenoid Bioactive Compound Isolated from Papua Ant Nest as an Alternative Ovarian Cancer Treatment. Open J Obstet Gynecol. 2015;05(07):406–11.
CrossRef - Youness RA, Kamel R, Elkasabgy NA, Shao P, Farag MA. Recent advances in tannic acid (gallotannin) anticancer activities and drug delivery systems for efficacy improvement; a comprehensive review. Molecules. 2021;25(6).
CrossRef - Ota H, Akishita M, Tani H, Tatefuji T, Ogawa S, Iijima K, et al. Trans-resveratrol in Gnetum gnemon protects against oxidative-stress- induced endothelial senescence. J Nat Prod [Internet]. 2013 Jul 26 [cited 2023 Jun 10];76(7):1242–7. Available from: https://pubs.acs.org/doi/pdf/10.1021/np300841v
CrossRef - Gadkari K, Kolhatkar U, Hemani R, Campanelli G, Cai Q, Kumar A, et al. Therapeutic Potential of Gnetin C in Prostate Cancer: A Pre-Clinical Study. Nutrients 2020, Vol 12, Page 3631 [Internet]. 2020 Nov 26 [cited 2023 Jun 10];12(12):3631. Available from: https://www.mdpi.com/2072-6643/12/12/3631/htm
CrossRef - Li L, Qiu RL, Lin Y, Cai Y, Bian Y, Fan Y, et al. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol Lett. 2018;15(6):9845–51.
CrossRef - Espinoza JL, Inaoka PT. Gnetin-C and other resveratrol oligomers with cancer chemopreventive potential. Ann N Y Acad Sci. 2017;1403(1):5–14.
CrossRef - Tuama AA, Mohammed AA. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi J Biol Sci. 2019;26(3):600–4.
CrossRef








