Jaelyne Iona Tauro1 , Anshula Anilkumar1
, Anshula Anilkumar1 , Layla Jameel Shamlooh1
, Layla Jameel Shamlooh1 , Zavia Evangeline Kitherian1
, Zavia Evangeline Kitherian1 , Abid Shaheer Karanghadan2
, Abid Shaheer Karanghadan2 and Nelofar Sami Khan2*
and Nelofar Sami Khan2*
1Bachelor of Biomedical Science, Gulf Medical University, Ajman, UAE
2Department of Biochemistry, Department of Biomedical Sciences, Gulf Medical University, Ajman, UAE.
Corresponding Author E-mail: neloferkhan@gmu.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2824
Abstract
Background: Fluctuating levels of biomarkers of inflammation and oxidative damage are observed during different phases of the menstrual cycle. Recent studies suggest an involvement of oxidative stress (OS) and inflammation in the development of Polycystic ovary syndrome (PCOS). As obesity increases the risk of PCOS, the present study aims to compare these biomarkers among young females across different Body Mass Index (BMI) groups. Objectives: To determine variations in the concentrations of Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC) as biomarkers of oxidative stress, and high sensitivity C-reactive protein (hs-CRP) as a biomarker of inflammation, and compare among normal and obese young females during the phases of menstrual cycle. Methods:The study included 37 females (20 normal and 17 obese) aged 18 – 22 years. Serum analysis for hs-CRP, MDA, and TAC were performed. Paired and Independent sample T-tests were appropriately used comparing the parameters between early follicular (EFP) and mid-luteal phase (MLP) among the normal and obese subjects. Results: Significant differences were seen in the concentrations of hs-CRP, MDA, and TAC during EFP and MLP of the menstrual cycle among the normal and obese females. An elevated concentration of hs-CRP and MDA, and reduced TAC were observed in the obese compared to normal throughout the menstrual cycle. More than 82.5% of obese subjects having the hs-CRP above normal is alarming, increasing their risk of future CVD and PCOS. Conclusion: Our findings warrant clinical evaluation with prevention strategies for our obese young females. Also, the findings recommend future elaborate research including various biological parameters connected to inflammation and oxidative stress, resolving the etiology of hormonal disorders causing reproductive issues like PCOS in women.
Keywords
High sensitivity C-reactive protein; Inflammation; Menstrual cycle; Oxidative stress; Polycystic ovary syndrome
Download this article as:| Copy the following to cite this article: Tauro J. I, Anilkumar A, Shamlooh L. J, Kitherian Z. E, Karanghadan A. S, Khan N. S. Influence of Body Mass Index on the Markers of Inflammation and Oxidative Stress among Young Females during Menstrual Cycle. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Tauro J. I, Anilkumar A, Shamlooh L. J, Kitherian Z. E, Karanghadan A. S, Khan N. S. Influence of Body Mass Index on the Markers of Inflammation and Oxidative Stress among Young Females during Menstrual Cycle. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/487DIDy |
Introduction
C-reactive protein (CRP) belongs to acute-phase reactants as its level increases during inflammation and infection. It is primarily produced by liver cells, but also by endothelial and smooth muscle cells of coronary arteries, macrophages, adipose tissues, and many other inflamed tissues throughout the body. Comparatively higher levels of CRP are observed among females than males exhibiting gender variation, however, increases with age in both genders. Increased CRP among healthy women is considered a predictor of cardiovascular events and myocardial infarction.1 In women after menopause, the increased risk of cardiovascular diseases is attributed to the low levels of endogenous estrogen. In women of the menstruating age group, although cardiovascular events are rare, they correspond to the minimal estrogen level phase i.e. early follicular phase. Estrogen is reported to be a regulator of inflammation since estrogen released in the blood exerts anti-inflammatory effects.1 Several studies have shown that estrogen decreases the CRP while progesterone elevates it i.e., CRP level is negatively associated with estrogen and positively with progesterone.1,2,3
Increased production of free radicals and reactive oxygen species (ROS), and insufficient antioxidant capacity result in conditions of oxidative stress. Excessive ROS produced by severe oxidative stress can damage DNA, and proteins, and cause other cell injuries.4 However, ROS produced by the preovulatory follicle is an important inducer of ovulation.5 Obese people are reported to have higher levels of oxidative stress than nonobese people, which could be due to a variety of factors such as chronic inflammation, hyperglycemia, or antioxidant defense system impairment.6 Malondialdehyde (MDA) is a lipid peroxidation indicator, a final by-product of oxidative damage to cell membrane unsaturated fatty acids. It has been used as an effective biomarker of lipid oxidation.7
To combat the oxidative stress induced damages, the body has evolved several defense systems, including preventive and repair processes, and an antioxidant system. Antioxidants are molecules that can convert reactive molecules into relatively stable and inert substances. Total antioxidant capacity (TAC) is the ability of serum to reduce generation and scavenge free radicals and reactive oxygen species. Studies suggest a reciprocal relationship between body fat content and antioxidant capacity.8 Also, antioxidant status varies in different phases of menstrual cycle.9
Iron is involved in important processes in the body, including growth and development, metabolism, and transport of oxygen. Iron deficiency is the most common form of anemia, resulting in symptoms as shortness of breath, palpitations, reduced physical capacity, and decreased intestinal blood flow leading to malabsorption and motility disorders. One of the common causes of iron-deficiency anemia is menstruation. Levels of iron in the blood can vary over the different phases of the menstrual cycle.10 Heavy menstrual blood loss can deplete the body’s iron stores.11
Globally 5-10% of young females are affected by hormonal-related reproductive problems like Polycystic ovary syndrome (PCOS), more commonly seen among obese. Free radical-induced damages are now recognized as key events in the pathophysiology of many disorders including PCOS.12 According to recent research, PCOS patients had considerably lower levels of serum TAC than normal women, which could indicate higher oxidative stress.13 Also, studies suggest that women with PCOS have higher levels of inflammatory markers.14 Understanding the mechanisms of increased generation of free radicals, ROS, and inflammatory biomarkers leading to the development of PCOS is important to plan strategies for prevention, early detection, and therapy of PCOS.
Rationale
Obesity increases the incident risk for PCOS and some recent studies point out the connection of inflammation and oxidative stress in causing PCOS. A literature search revealed scanty information in this regard among young females, therefore we intend to conduct this pilot study to explore more information and compare normal and obese young participants.
Objectives
To compare the levels of markers of inflammation, oxidative stress, total antioxidant capacity, and hemoglobin status among young females during different phases of the menstrual cycle.
To observe if there is any difference in the measured parameters between normal and obese young females.
Methods
Design of study
This pilot study was planned as a prospective, short period of follow-up with no intervention. The cohort was followed during the menstrual cycle from day 2 representing the early follicular phase (EFP) to day 21, representing the mid-luteal phase (MLP).
Study Population
This study was conducted on female students at Gulf Medical University. The inclusion criteria were as follows: a) Females between the ages of 18 to 22; b) Belongs to either normal BMI category (range 18.5 – 24.9 Kg/m2) or obese (≥ 30 Kg/m2). The exclusion criteria were as follows: using oral contraceptives; pregnancy; diagnosed with any chronic disease; sick in the previous two weeks; use of vitamins/mineral supplements; and any form of smoking/vaping
Sample Size
It was a pilot study conducted on a total of 37 participants, 20 were with normal BMI, and 17 were obese.
Study Settings
Gulf Medical University, Thumbay Medi-city, Ajman.
Study Duration
It was a six-month-long study, conducted from January to June 2023, including sample collection, lab investigations, data entry, analysis, and report preparation.
Ethical Issues
This research study got approval from the Institutional Review Board (IRB) of Gulf Medical University, ethical approval Ref. no. IRB/COM/STD/44/JULY-2022. Each participant provided the consent by signing the consent form. Throughout the study, access to the collected data was limited to the supervisor, ensuring utmost confidentiality.
Study Instrument
Relevant information was collected from the study participants using a validated instrument in the form of a questionnaire. It included questions to collect basic information like age, marital status, length and regularity of cycles, the existence of any medical issues, medication or supplement intake, smoking status, and to know if they had been sick recently. Subjects that fit the inclusion criteria were selected based on this instrument.
Methodology
Information related to the purpose and procedure of research along with the questionnaire link was emailed to around 300 female students at Gulf Medical University. The responses allowed us to recruit subjects based on inclusion and exclusion criteria. After obtaining the signed consent, height and weight were measured and BMI was calculated for each participant. Subjects not fitting any of the two BMI groups were excluded and so achieved a total sample of 37 participants. The first day of their last menstrual period was recorded and each participant was followed individually to collect blood samples on day 2 and day 21 of their menstrual cycle. On the respective days, temperature and blood pressure were measured, and blood samples were collected in two vials, one for hemoglobin, and the second to obtain serum for high-sensitivity CRP (hs-CRP), MDA, and TAC estimation. Serum samples were prepared immediately to prevent hemolysis. hs-CRP estimation was outsourced and hemoglobin was estimated in Thumbay Labs as and when the sample was collected, with a maximum turnaround time of 4 hours. Serum samples for the quantitative estimation of MDA and TAC were stored in four aliquots at -80°C. Once all samples were collected, MDA and TAC were measured as per the set protocol.
Quantitative estimation of hs-CRP
hs-CRP test was used to accurately quantify the lower concentration range (0.5-10 mg/L) and capture even the slightest fluctuation in the levels of CRP, utilizing the immunoturbidimetry method on a Beckman Coulter AU700.
Quantitative estimation of MDA
A modified colorimetric method using Thiobarbituric Acid Reactive Substances (TBARS) was used to estimate MDA levels.15,16 Addition of 10 μL of 5% BHT per mL serum prevented further oxidation. Thiobarbituric acid (TBA) reacts with the MDA forming a colored MDA-TBA adduct, quantified at the wavelength of 532 nm. The MDA standard curve was prepared and used to calculate MDA concentrations in the serum samples of our participants.
Estimation of TAC
A green chromophore (ABTS+) generated by the oxidation of 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) in the presence of ferryl-myoglobin radical, absorbs at a wavelength of 640 nm. The presence of antioxidants in the serum sample inhibits the generation of ABTS+ radicals in an inverse relationship.17 Serum total antioxidant capacity in the samples was reported as Trolox equivalent (TE), using the Trolox Standard Curve.
Estimation of Hemoglobin
Hemoglobin was measured by sodium lauryl sulfate-methemoglobin method using the Beckman Coulter DxH 800/900 automated analyzer.
Statistical Analysis of Data
Using a paired sample T-test, parameters between day 2 and day 21 of the menstrual cycle were compared via Statistical Software SPSS (28.0.1.1). Mean values of parameters were compared between the normal and obese participants using an independent sample T-test. A P-value of ˂0.05 was considered statistically significant.
Results
Subject Information
The questionnaire used to select the study population received responses from 60 students. A medical condition, such as asthma, diabetes, or allergy, was present in 15% of respondents, 6.7% were smokers, and 6.6% were using nutritional supplements, hence were excluded. Subjects in the overweight BMI category (25 – 29.9 Kg/m2) were also excluded. Six subjects were afraid of needles and declined to give blood. Therefore, the final sample included 37 participants, with 20 falling into the normal BMI and 17 into the obese category.
Table 1 shows the data on BMI, age, and menstrual cycle length. Comparison of body temperature during EFP (Day 2) and MLP (Day 21) among each BMI group and between the normal and obese BMI categories shows no significant difference.
Table 1: General characteristics of the sample population
|
|
Group A (Normal BMI) |
Group B (Obese BMI) |
|
Number of Participants |
20 |
17 |
|
BMI (Mean ± SD) |
21.88 ± 2.6 |
33.14 ± 2.4 |
|
Age (Mean ± SD) |
20.3 ± 1.59 |
20.0 ± 1.97 |
|
Menstrual cycle (day) |
28.75 ± 3.76 |
29.23 ± 3.53 |
hs-CRP level as a biomarker of inflammation
Participants were distributed among the CVD risk groups based on hs-CRP levels, shown in Table 2.
Table 2: Based on hs-CRP levels, participants distributed among the CVD risk groups
|
hs-CRP (mg/L) |
Normal Group |
Obese Group |
||
|
Day 2 |
Day 21 |
Day 2 |
Day 21 |
|
|
<1.0 mg/L (Low Risk) |
50% |
60% |
23.5% |
17.6% |
|
1–3 mg/L (Average Risk) |
30% |
20% |
47.1% |
47.1% |
|
>3.0 mg/L (High risk) |
20% |
20% |
29.4% |
35.3% |
Table 3 show the comparison of mean hs-CRP levels between the EFP (day 2) and MLP (day 21) of the menstrual cycle among the normal and obese participants. A significantly higher level was seen among obese subjects compared to normal BMI on each of the observed days. Also, significantly higher levels of hs-CRP on day 2 among normal BMI, and on day 21 among obese BMI was observed.
Table 3: Comparison of serum hs-CRP levels on Day 2 and Day 21 of menstrual cycle across BMI Groups
|
|
Serum hs-CRP (mg/L) |
P-Value |
|
|
Day 2 |
Day 21 |
||
|
Group A (Normal BMI) |
1.63 ± 1.57 |
1.37 ± 1.54 |
˂0.001 |
|
Group B (Obese BMI) |
2.96 ± 2.77 |
3.21 ± 2.72 |
˂0.001 |
|
P-Value |
0.011 |
0.012 |
|
The correlation coefficient of BMI to serum hs-CRP levels on day 2 and day 21 was 0.417 and 0.482 respectively, indicateing a moderate positive association.
Malondialdehyde level as a biomarker for oxidative stress
Table 4 show a significant difference in serum MDA values, with the level being significantly higher on day 21 compared to day 2 and higher among obese compared to the normal BMI group.
Table 4: Comparison of serum MDA levels on Day 2 and Day 21 of menstrual cycle across BMI Groups.
|
|
Serum MDA (µM/L) |
P-Value |
|
|
Day 2 |
Day 21 |
||
|
Group A (Normal BMI) |
3.581 ± 1.66 |
4.9 ± 2.15 |
0.02 |
|
Group B (Obese BMI) |
4.79 ± 1.33 |
6.49 ± 2.37 |
<0.001 |
|
P-Value |
0.01 |
0.04 |
|
Based on the correlation coefficient between serum MDA and BMI, a moderate positive correlation was seen on day 2 (0.340) and a weak positive on day 21 (0.252).
TAC as a marker for the antioxidant activity
Table 5 show a significant difference in serum TAC values on Day 2 between the normal and obese subjects, with normal BMI having higher TAC. Also, among the normal BMI group, TAC was significantly higher on day 2 compared to day 21.
Table 5: Comparison of serum Total Antioxidant Capacity (TAC) on Day 2 and Day 21 of menstrual cycle across BMI Groups.
|
|
Serum TAC (TE/L) |
P-Value |
|
|
Day 2 |
Day 21 |
||
|
Group A (Normal BMI) |
323.67 ± 47.88 |
314.5 ± 70.51 |
0.058 |
|
Group B (Obese BMI) |
310.98 ± 100.12 |
281.96 ± 107.64 |
0.962 |
|
P-Value |
0.032 |
0.392 |
|
Based on the correlation coefficient of serum TAC on day 2 (-0.174) and day 21 (-0.147), there is a weak negative association between BMI and TAC suggesting an inverse relationship.
Hemoglobin as a marker of anemia
Table 6 show a significant difference in the hemoglobin levels between day 2 and day 21 of the menstrual cycle among each group based on BMI. However, no significant difference can be seen between normal and obese groups on either of the days. The levels of hemoglobin increase from day 2 to day 21 in the normal category, while there is a dip in the level among the obese group.
Table 6: Comparison of Blood Hemoglobin on Day 2 and Day 21 of menstrual cycle across BMI Groups
|
|
Blood Hemoglobin (g/dL) |
P-Value |
|
|
Day 2 |
Day 21 |
||
|
Group A (Normal BMI) |
12.12 ± 1.33 |
12.28 ± 1.54 |
˂0.001 |
|
Group B (Obese BMI) |
12.06 ± 1.73 |
11.97 ± 1.72 |
˂0.001 |
|
P-Value |
0.247 |
0.397 |
|
Based on the correlation coefficient of BMI to the levels of hemoglobin on days 2 (-0.103) and 21 (-0.164), a weak negative correlation can be seen.
Table 7 summarizes the findings of hs-CRP, MDA, TAC, and hemoglobin, comparing between day 2 and day 21 of each group and also comparing between the two groups i.e. normal and obese.
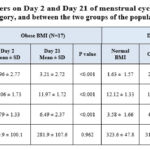 |
Table 7: Comparison of laboratory parameters on Day 2 and Day 21 of menstrual cycle among Normal BMI category, obese BMI category, and between the two groups of the population |
Discussion
In this study, levels of hs-CRP, oxidative damage, total antioxidant capacity, and hemoglobin were assessed and compared between the phases of the menstrual cycle among young females with normal and obese BMI groups.
Inflammation
Results generated for the inflammation marker showed a significant difference in serum hs-CRP values on day 2 and day 21 of the menstrual cycle among the normal and obese groups (Table 3). Among the obese category, hs-CRP levels were comparatively elevated than the normal BMI. Excess adipose tissue in obese induces chronic low-grade inflammation by secreting proinflammatory adipocytokines like TNF-α, IL-1, and IL-6 and reducing the level of adiponectin, an anti-inflammatory adipokine.18,19 Increased levels of hs-CRP synthesized in response to increased expression of adipocytokines have an important role in the atherogenic process in obese people who are more susceptible to cardiovascular diseases.18,19 Moreover, several studies reported a rise of inflammatory markers including hs-CRP among women with PCOS, more often seen in obese females compared to normal BMI.20,21 hs-CRP level of more than 1.0 mg/L in approximately 82.5% of our obese participants (Table 2), indicates high chances of developing cardiovascular disorders and PCOS.
Comparing the inflammation marker between the phases of the menstrual cycle, our results showed higher levels of hs-CRP during the EFP compared to MLP in the normal BMI category (Table 3), which is supported by several studies. Vashishta et al studied regularly menstruating females with a mean BMI of 23 ± 3.81 and showed follicular phase had significantly raised CRP level than the luteal phase.22 Another study conducted by Gursoy et al on a sample with a mean BMI of 23.2 ± 3.6 also reported CRP levels in EFP being significantly higher than the luteal phase.2 Another study conducted by Wander et al on non-obese females also showed high CRP during menses, the estrogen level being negatively associated with CRP, while progesterone demonstrated a positive association.3 However, contrasting these findings, a study by Saxena et al on twenty healthy females demonstrated CRP levels do not vary significantly between the early follicular and early luteal phases.23 Blum et al showed normal-weight females having higher CRP in the luteal phase compared to the overweight group.24 Among the obese, our results show higher levels of hs-CRP during the MLP compared to EFP. An extensive literature search did not reveal any study comparing the hs-CRP among obese young females during the EFP and MLP. Therefore, any support for this result is currently not available. The variation in the results in different studies can be attributed to the day of sample collection as the hormonal variation during the cycle influences the level of CRP, during menses both estrogen and progesterone are low, estrogen peaks during late follicular, and progesterone increases during luteal phase along with a second surge of estrogen.1
Oxidative stress
Our study indicates obese females experience higher levels of OS compared to normal BMI (Table 4). Elevated levels of serum MDA among the obese population,25 and specifically among obese females compared to the normal BMI groups, are reported by other studies also.26 Visceral fat accumulation promotes a pro-oxidant and proinflammatory environment, significantly contributing to obesity-associated diseases.6 Obesity-induced chronic low-grade inflammation, mediated by TNF-α, IL-1, and IL-6, increases the production of ROS through the activation of several biochemical pathways including mitochondria and NADPH oxidase systems.6
Our research findings also suggest that levels of MDA in the blood are higher during the MLP compared to the EFP (Table 4). Similar results were demonstrated in other studies that investigated the reactive oxygen metabolites throughout the menstrual cycle and discovered they start increasing from day 6, peak at day 15, and then start declining to reach a minimum level on day 2.27 Another study reported significantly lower levels of hydrogen peroxide and thiobarbituric acid reactive substances as markers of OS in urine during the follicular stage when compared to the luteal segment of the cycle.28 The peak OS during the central phase of the cycle i.e. period of ovular maturation and possible implantation, corresponds to the estrogen and lieutining hormone peaks, while the progesterone peak seems to correspond with an OS recovery phase.27 The increased cellular activity for the increased energy production during the ovulatory phase subsequently increases the production of free radicals and reactive oxygen metabolites.27 It has been observed that oxidative stress affects women for approximately two-thirds of the menstrual cycle.27 The role of ROS produced by the preovulatory follicle in inducing ovulation is also reported, and inhibition of ROS has been found to disrupt the ovulation process.5 This indicates a significant role of OS in the physiological events of the menstrual cycle. However, it is important to note that the absence of significant differences in oxidative stress markers during different phases of the menstrual cycle is also reported.29
Total Antioxidant Capacity
Discussing the biological variation of antioxidant status with the menstrual cycle, our results show a weak negative correlation between TAC and BMI. These findings correspond to Chaves et al., who reported decreased TAC among obese compared to normal subjects.30 Singh et al. further explained obesity can result in a reduction in antioxidants due to an increase in ROS production.31 To understand the effects of obesity in otherwise healthy females, Chrysohoou et al. conducted the ATTICA study in Greece and discovered that obese or overweight females have 10% less serum TAC than normal-weight females.8
Several studies report an increase in serum TAC from the late follicular phase to the early luteal phase.9,32 According to Michos et al., total antioxidant status is maximum during the ovulation time which corresponds to estrogen peak and then starts declining.33 In our study we did not collect the sample during the ovulation time, hence did not get the peak value, and the differences in TAC during the EFP and MLP turned out to be non-significant (Table 5).
The young obese females in our study have significantly higher hs-CRP, lower TAC, and higher OS compared to normal healthy females. Similar findings are reported among young obese PCOS patients, thus warranting clinical evaluation with prevention strategies for our study participants.34
Hemoglobin
Regarding hemoglobin, the findings in this study show lower levels in the EFP compared to the MLP in the normal BMI category (Table 6). This is in agreement with the patterns seen by Chandra et al,35 and Shilpa et al.36 Blood loss during menstruation causes a deficit in iron stores and may contribute to iron deficiency. The increased hemoglobin concentrations in the luteal phase may be due to enhanced erythropoiesis to make up for the blood loss during menstruation.36
In the obese category, hemoglobin was higher in the EFP than in the MLP (Table 6). Similar results were reported in another study, explaining increased adipocytes to be a source of circulating estrogens and the variations in the levels of estrogen and progesterone influence the plasma volume. Fluid retention due to the presence of estrogen during the luteal phase causes hemodilution, reducing hemoglobin levels.37,38 However, loss of blood during regular menstruation does not significantly impact the hemoglobin level thus maintaining oxygen-carrying capacity.38
Our study also reports higher mean levels of hemoglobin in the normal BMI compared to the obese category during both follicular and luteal phases, though not proven to be statistically significant (Table 6). The negative correlation between BMI and hemoglobin among young female university students is also reported by Ahad et al.,39 and Acharya et al.40 Study by Cepeda-Lopez et al. indicatedinflammation related to adiposity decreases iron absorption irrespective of the body iron store status.41 Hepcidin hormone found in the liver is reported to be a negative regulator of iron level.42 Among the obese, elevated levels of proinflammatory cytokines may increase the generation of hepcidin by the hepatocytes and adipocytes. Elevated hepcidin inhibits the gene for ferroportin, a protein involved in transporting iron from inside the cells to outside, reaching the blood. High hepcidin also decreases iron absorption from the intestine and increases the traping of iron in macrophages and the reticuloendothelial system.42 In addition, inflammation upregulates the formation of an iron-binding protein, lipocalin-2, causing iron sequestration in the adipocytes.43 Thus, adiposity-induced low-grade inflammatory state can be an explanation for slightly lower mean hemoglobin observed in obese females compared to normal BMI in our study.
Limitations
It was a pilot study, hence the sample size was small. We chose only one marker to assess the inflammation, oxidative stress, antioxidant, and iron status. Also, the cyclical variation of female reproductive hormones influences all these parameters. Hence, a larger study monitoring more parameters related to inflammation, oxidative stress, antioxidant status, and iron status, including reproductive hormone levels, assessing at several points during the menstrual cycle will help clearly understand the physiological variations and the influence of BMI.
Conclusion
According to the results of our study, significant differences were observed in the concentrations of MDA, TAC, hs-CRP, and hemoglobin during EFP and MLP of the menstrual cycle among normal and obese young females. An increase in levels of hs-CRP, a biomarker of inflammation, and MDA as a biomarker of oxidative damage, and a reduction in total antioxidant capacity were noticed among the obese category compared to the normal BMI during both MLP and EFP of the menstrual cycle. As increased inflammation and oxidative stress are also observed in PCOS, it warrants clinical evaluation with prevention strategies for our study participants. Also, the results of this study could serve as a starting point for further elaborate research including biological parameters connected to oxidative stress and inflammation unraveling the etiology of hormonal disorders like PCOS in women.
Acknowledgments
We appreciate the guidance of Dr. Anusha Sreejith, Assistant Professor in Demography, Department of Community Medicine, Gulf Medical University, in conducting statistical analysis.
Conflict of Interest
The authors declared no conflicts of interest. This research was entirely supported by Thumbay Labs and Gulf Medical University, Ajman, UAE.
References
- Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: The BioCycle Study. Am J Epidemiol. 2012;175(5):423–31.
CrossRef - Gursoy AY, Caglar GS, Kiseli M, Pabuccu E, Candar T, Demirtas S. CRP at early follicular phase of menstrual cycle can cause misinterpretation for cardiovascular risk assessment. Interv Med Appl Sci. 2015;7(4):143–6.
CrossRef - Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138–46.
CrossRef - Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017.
CrossRef - Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):1–18.
CrossRef - Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab Syndr Relat Disord. 2015;13(10):423–44.
CrossRef - Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014.
CrossRef - Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas I, Papademetriou L, Economou M, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: The ATTICA study. Nutr Metab Cardiovasc Dis. 2007;17(8):590–7.
CrossRef - Nasorllahi S, Tavilani H, Mehrabi N, Neghab N. Evaluating Activity of Antioxidant Enzymes during Human Menstrual Cycle. Canon J Med. 2019;1(1):44–9.
- Ofojekwu MJN, Nnanna OU, Okolie CE, Odewumi LA, Isiguzoro IOU, Lugos MSD. Hemoglobin and serum iron concentrations in menstruating nulliparous women in Jos, Nigeria. Lab Med. 2013;44(2):121–4.
CrossRef - Kocaoz S, Cirpan R, Degirmencioglu AZ. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pakistan J Med Sci. 2019;35(2):365–70.
CrossRef - Mohammadi M. Oxidative stress and polycystic ovary syndrome: A brief review. Vol. 10, International Journal of Preventive Medicine. 2019. 1–7.
CrossRef - Kanafchian M, Esmaeilzadeh S, Mahjoub S, Rahsepar M, Ghasemi M. Status of Serum Copper, Magnesium, and Total Antioxidant Capacity in Patients with Polycystic Ovary Syndrome. Biol Trace Elem Res. 2020;193(1):111–7.
CrossRef - Rudnicka E, Duszewska AM, Kucharski M, Tyczyński P, Smolarczyk R. Oxidative Stress And Reproductive Function – Oxidative stress in polycystic ovary syndrome. Reproduction. 2022;164(6):F145–54.
CrossRef - Yagi K. Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol. 1998;108:107–10.
CrossRef - Al-Najar AAH, Tabassum T, Thaliffdeen FL, Kadhum SJ, Karanghadan AS, Khan NS. Electronic Cigarettes Versus Tobacco Poly Use: Reduced Oxidative Stress but Similar Inflammatory Effect. New Emirates Med J. 2023;4(1).
CrossRef - Kambayashi Y, Binh NT, Asakura HW, Hibino Y, Hitomi Y, Nakamura H, et al. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplte. J Clin Biochem Nutr. 2009;44(1):46–51.
CrossRef - Mulyamin W, Kurniawan LB, Adnan E, Widaningsih Y, Idris I, Santoso A, et al. Body mass index as the most influential factor of high-sensitivity C-reactive protein in non-diabetic adults. Universa Med. 2021;40(1):22–8.
CrossRef - Csige I, Ujvárosy D, Szabó Z, István L, Paragh G, Harangi M, et al. Review Article The Impact of Obesity on the Cardiovascular System. 2018;2018.
CrossRef - Rudnicka E, Kunicki M, Suchta K, Machura P, Grymowicz M, Smolarczyk R. Inflammatory Markers in Women with Polycystic Ovary Syndrome. Biomed Res Int. 2020;2020.
CrossRef - Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HED, Amer S. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int J Mol Sci. 2021;22(5):1–31.
CrossRef - Vashishta S, Gahlot S, Singh A, Goyal R. Impact of menstrual cycle phases on C-reactive protein concentrations. Int J Res Med Sci. 2017;5(3):1090.
CrossRef - Saxena AR, Seely EW and Goldfine AB. Cardiovascular Risk Factors and Menstrual Cycle Phase in Premenopausal Women. J Endocrinol Invest. 2013;35(8):715–9.
- Blum CA, Mu B, Huber P, Kraenzlin M, Schindler C, Geyter C De, et al. Low-Grade Inflammation and Estimates of Insulin Resistance during the Menstrual Cycle in Lean and Overweight Women. 2015;90(November):3230–5.
CrossRef - Adnan MT, Amin MN, Uddin MG, Hussain MS, Sarwar MS, Hossain MK, et al. Increased concentration of serum MDA, decreased antioxidants and altered trace elements and macro-minerals are linked to obesity among Bangladeshi population. Diabetes Metab Syndr Clin Res Rev. 2019;13(2):933–8.
CrossRef - Amin MN, Siddiqui SA, Uddin MG, Ibrahim M, Uddin SMN, Adnan MT, et al. Increased Oxidative Stress, Altered Trace Elements, and Macro-Minerals Are Associated with Female Obesity. Biol Trace Elem Res. 2020;197(2):384–93.
CrossRef - Cornelli U, Belcaro G, Cesarone MR, Finco A. Analysis of oxidative stress during the menstrual cycle. Reprod Biol Endocrinol. 2013;11(1):2–7.
CrossRef - Karowicz-Bilinska A, Plodzidym M, Krol J, Lewinska A, Bartosz G. Changes of markers of oxidative stress during menstrual cycle. Redox Rep. 2008;13(5):237–40.
CrossRef - Ishikawa A, Matsushita H, Shimizu S, Morita N, Hanai R, Sugiyama S, et al. Impact of Menopause and the Menstrual Cycle on Oxidative Stress in Japanese Women. J Clin Med. 2023;12(3).
CrossRef - Chaves TR, Lima RPA, Ribeiro MR, Boico VF, De Lima Ferreira FEL, Da Conceição Rodrigues Gonçalves M, et al. Association between values of anthropometric indicators, Total Antioxidant Capacity and Malondialdehyde in adults: A population-based study. Nutr Clin y Diet Hosp. 2021;41(3):47–57.
CrossRef - Singh K, Singh S. Comparative study on malondialdehyde and certain antioxidants in north west obese Indians. J Cardiovasc Dis Res. 2015;6(3):138–44.
CrossRef - Greene R, Pisano MM. Analytical and biological variation of biomarkers of oxidative stress during the menstrual cycle. Biomarkers. 2008;13(2):160–83.
CrossRef - Michos C, Kiortsis D, Evangelou A, Karkabounas S. Antioxidant protection during the menstrual cycle: The effects of estradiol on ascorbic-dehydroascorbic acid plasma levels and total antioxidant plasma status in eumenorrhoic women during the menstrual cycle. Acta Obstet Gynecol Scand. 2006;85(8):960–5.
CrossRef - Khashchenko E, Vysokikh M, Uvarova E, Krechetova L, Vtorushina V, Ivanets T, et al. Activation of systemic inflammation and oxidative stress in adolescent girls with polycystic ovary syndrome in combination with metabolic disorders and excessive body weight. J Clin Med. 2020;9(5).
CrossRef - Chandra S, Kaushik N, Gupta N. Study of Iron Status Indicators in Different Phases of Menstrual Cycle in Females of Lower Socio-Economic Group. Ann Int Med Dent Res. 2016;3(1):46–9.
CrossRef - Shilpa N, Itagi V, Rani R. A Study of Hemoglobin Concentration in Different Phases of Menstrual Cycle. Int J Physiol. 2018;6(2):90.
CrossRef - Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction – implications for pulmonary arterial hypertension. Pulm Circ. 2020;10(3).
CrossRef - Melissa Faith Keller SL. The Effect of the Menstrual Cycle on Hemoglobin Mass. FASEB J. 2019;33(S1):840.
CrossRef - Ahad F, Jaan I, Gowhar M. Hemoglobin concentration in relation to body mass index among undergraduate medical students – A cross-sectional institutional study. Natl J Physiol Pharm Pharmacol. 2020;10(8):1.
CrossRef - Acharya S, Patnaik M, Mishra S, Panigrahi A. Correlation of hemoglobin versus body mass index and body fat in young adult female medical students. Natl J Physiol Pharm Pharmacol. 2018;8(9):1371.
CrossRef - Cepeda-Lopez AC, Osendarp SJM, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93(5):975–83.
CrossRef - Means RT. Hepcidin and cytokines in anaemia. Hematology. 2004;9(5–6):357–62.
CrossRef - Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang J, Tso AWK, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53(1):34–41.
CrossRef








