Dinesh Yasothkumar1* , Selvaraj Jayaraman2, Karthikeyan Ramalingam1
, Selvaraj Jayaraman2, Karthikeyan Ramalingam1 and Pratibha Ramani1
and Pratibha Ramani1
1Department of Oral and Maxillofacial Pathology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India.
2Department of Biochemistry, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, Tamil Nadu, India.
Corresponding Author E-mail: drdineshdentist@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2795
Abstract
Introduction: Pongamia pinnata Merr., a member of the Fabaceae family, is extensively spread throughout tropical Asia. Historically, several parts of P. pinnata have been utilised in the indigenous medicinal systems of several cultures. Anti-inflammatory and antioxidant properties are basic requisites in treating any oral mucosal lesions. This study aims to investigate the antioxidant and anti-inflammatory properties of these leaf extracts. Materials and methods: pinnata seeds were collected in Chennai, Tamil Nadu, India. For the extraction, shade-dried P. pinnata plant seeds were utilized. P. pinnata seed ethanolic extract was analysed qualitatively for the content of phenols, tannins, saponins, proteins, and acids, among other phytochemicals. Using the DPPH test and suppression of albumin denaturation, the anti-oxidant and anti-inflammatory activity of P. pinnata seed extract was determined. Results: The phytochemical analysis of P. pinnata seed extract revealed the presence of phenols, tannins, saponins, proteins, and acids. The anti-oxidant and anti-inflammatory activity of P. pinnata seed extract was considerably higher at 500 g, as shown by the DPPH test and albumin denaturation inhibition. Conclusion: The results of this study show that P. pinnata seed extracts have both anti-inflammatory and antioxidant properties. Considering the results, P.pinnata shows high potential for management of oral mucosa lesions. Further clinical research needs to be done to analyse the effect of these properties.
Keywords
Anti inflammatory; Antioxidant; Ethanolic Extract; P. pinnata; Seed
Download this article as:| Copy the following to cite this article: Yasothkumar. D, Jayaraman S, Ramalingam K, Ramani P. In vitro Anti-Inflammatory and Antioxidant Activity of Seed Ethanolic Extract of Pongamia pinnata. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Yasothkumar. D, Jayaraman S, Ramalingam K, Ramani P. In vitro Anti-Inflammatory and Antioxidant Activity of Seed Ethanolic Extract of Pongamia pinnata. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/40x6NVY |
Introduction
The functional food and nutraceutical potential of phytochemical therapeutics is generating a great deal of interest today 1. Bioactive compounds in these natural compounds contain anticarcinogenic action and provide several health-promoting benefits 2. Numerous medicinal plants are known to generate bioactives with antioxidant and antibacterial properties. The phenolic acids, which hinder the growth of infections and cause minimal damage to host cells, are also intriguing prospects for the development of novel antimicrobial medications. As a result, there is a rising interest in the development of several plant-derived medicines with different biological activities for the treatment of diverse infectious illnesses 3.
Pongamia pinnata (L.) Pierre, a member of the Fabaceae family, is extensively dispersed Historically, the parts of P. pinnata were utilised in the indigenous medicinal systems of several cultures. It has been observed that several primary phenolic compounds are found in diverse plant sections 4,5. Karanjin, a furanoflavone extracted from this plant’s seeds, has superior therapeutic properties. Extracts of the seed oil of P. pinnata were active against both Gram-positive and Gram-negative bacteria 6,7.
Oral mucosal lesions, also known as OML, are any abnormal changes that occur on the surface of the oral mucosa. These changes can manifest as pigmented, ulcerative, red and white characteristics, or any swelling or developmental fault variations. Oral mucosal ulceration is an inflammatory lesion caused due to disintegration of the oral epithelium. 8,9,6 It has been established that medicinal plants that are abundant in a number of different chemical ingredients are quite beneficial in the treatment of OML. Several oral mucosal lesions are treated by anti-inflammatory and antioxidants like retinol, lycopene etc. 10. Upon inflammation, toxins are released that cause cell damage and the body produces substances that activate the immune system. About half of analgesics are anti-inflammatory medicines, which treat pain by reducing inflammation as opposed to opioids 11. Diclofenac is the typical anti-inflammatory medication, and its negative effects have been recorded 12.
In turn, the antioxidant capacity is dependent on the total amount of polyphenolic compounds, essential oils, and other components 13. Herbs are regarded as an excellent source of natural antioxidants, although very little research has been conducted on their potential use as antioxidants 14,15. The majority of studies have been conducted on these leaves, with the exception of their anti-inflammatory properties. Therefore, the purpose of this study is to extract the essence of P. pinnata’s therapeutic properties using ethanol as a solvent. This study’s objective is to investigate the antioxidant and anti-inflammatory properties of the seed extracts of P.pinnata in invitro methods.
Materials and Methods
Collecting and extracting plants
P. pinnata seeds were gathered in Chennai, Tamil Nadu, India. For the extraction, shade-dried P. pinnata plant seeds were used. The ethanol extract was made by soaking the seed powder in water and then evaporating it. The collection of dry powder for subsequent examination.
Evaluation of the phytochemical properties of P.pinnata seed extract
In this study, the content of phenols, tannins, saponins, proteins, and acids in the seed ethanolic extract of P.pinnata was examined qualitatively using phytochemical techniques (Harbone & Baxter, 1993). 16
Test for phenols
In a test tube, a tiny amount of ethanolic extract is combined with 1 mL of water, 1 to 2 drops of iron III chloride (FeCl3) are added, and a black colour change is noticed.
Test for tannins
3 drops of lead sub-acetate solution were mixed with 1 ml of filtrate. creamy-coloured gelatin consistency implies tannins.
Test for saponins
Foam test: After forcefully shaking a 1 ml sample of the extract with water, persistent foam was detected.
Test for proteins
One millilitre of ninhydrin was dissolved in one millilitre of acetone, followed by the addition of a little amount of extract. The presence of proteins led to the development of a purple hue.
Test for acids
This is measured by boiling 3.0 g of the powder in 50 cc of 10% sulfuric acid for 5 minutes; the resulting ammonia is then freed, distilled into 0.1 N acid, and titrated.
DPPH radical scavenging efficacy of ethanolic P.pinnata seed extracts
Using the approach of Hatano et al., the DPPH radical scavenging was evaluated (1989). Briefly, 1.0 ml of DPPH solution was added to 1.0 ml of extract at doses ranging from 0.1 to 0.5 mg/ml. After 50 minutes at room temperature, the mixture’s activity was measured at 517 nm. As a standard, identical amounts of ascorbic acid were utilised. Using the following formula, the capacity to scavenge the DPPH radical was computed and represented as a percentage (%).

In an Albumin denaturation inhibition assay, ethanolic seed extracts of P.pinnata demonstrate an anti-inflammatory effect in vitro.
The anti-inflammatory efficacy was investigated using inhibition of albumin denaturation analysis, as determined by Leela Prakash and Mohan Dass’s approach (2010). The pH of the reaction mixture, which contained the test extracts as well as a 1% aqueous solution of bovine albumin fraction, was adjusted by adding a small amount of 1N hydrochloric acid. After heating the sample extracts for 20 minutes at 51 degrees Celsius after having been incubated at 37 degrees Celsius for 20 minutes, the turbidity was measured at 660 nanometers. The experiment was carried out three times. The proportion of protein denaturation inhibition was computed as follows:

Statistical analysis using a one-way ANOVA in SPSS software v25, with a significance threshold of (p<0.05) considered.
Results
In the present study, results of the DPPH radical scavenging activity of P.pinnata seed ethanolic extract showed a significant (p<0.05) dose-dependent potential activity by inhibiting DPPH radical formation (figure 3). Inhibitory activity showed 10.74, 20.83, 36.71, 53.4 and 74.9% at 100, 200, 300, 400 and 500µg respectively. Protein denaturation inhibition activity of P.pinnata seed ethanolic extract showed a significant (p<0.05) dose-dependent potential anti-inflammatory activity (figure 4) such as 33,38,42,53 and 68% respectively whose activity was near to that of the standard drug diclofenac activity (14.8, 26.4, 37.1, 64.4 and 92%) and this study clearly indicates that P.pinnata seed extract exhibits a potential anti-inflammatory activity.
 |
Figure 1: P. pinnata seed extraction preparation. |
 |
Figure 2: Phytochemical screening of P.pinnata seed extract. |
 |
Figure 3: In vitro antioxidant potential of P.pinnata seed ethanolic extract by DPPH radical scavenging activity |
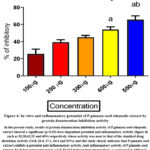 |
Figure 4: In vitro anti inflammatory potential of P.pinnata seed ethanolic extract by protein denaturation inhibition activity |
Discussion
In developing nations, pharmaceutical programmes continue to play a vital role as basic care therapeutic solutions. While phytochemical agents are predominantly used as a treatment for minor diseases at home, several medical practitioners like Siddha, Ayurveda and Unani have gained attention and shown promising cures for chronic diseases 17,18. Herbalists often do not treat acute psychological or physical illnesses; rather, the objective of herbal therapy is to promote long-term health improvements 19.
The results were consistent with the previous study, which characterised P.pinnata leaf methanol extracts (400 mg/kg) as effective anti-inflammatory agents, comparable to the present study 20,21. The DPPH test is a standard technique for measuring the ability of antioxidant molecules to scavenge free radicals by neutralising the stable, coloured DPPH radical. The radicals generated by DPPH are neutralised by plant extracts containing antioxidants, offering an ambitious foundation for future in vivo investigations. According to studies, phytochemicals such as phenolics and flavonoids can donate hydrogen and neutralise DPPH radicals 22,2324.
Compared to the various concentrations, anti-inflammatory activities were clear in our investigation. Using the albumin denaturation assay, the strongest antiinflammatory effect was discovered at 50µl. In an in vitro investigation, Srinivasan et al assessed the antiinflammatory activity of a 70% ethanolic extract of P.pinnata leaves in rats and found significant antiinflammatory activity without any ulcerogenic potential. 25. Singh et al aimed to assess anti-inflammatory potential of ethanolic seed extract of P.pinnata in rats and found the best anti-inflammatory activity against Bradykinin and prostaglandin E1 induced inflammation and minimal effects against histamine and serotonin induced inflammation 26. Rekha et al also studied the characterization and anti-inflammatory properties of P.pinnata seed extract and found a significant increase in antioxidant and lipoxygenase inhibitory activity of ketone and oxide. The derivatives of P.pinnata showed higher anti-inflammatory activity in rat models 7. Dinesh et al showed that a herbal combination of 3 herbal plants showed superior antioxidant and antiinflammatory activities 27,28.
Several oral lesions are treated by topical application of gels and topical agents with antiinflammatory and antioxidant properties. Phytochemical agents like curcumin, aloe vera, etc show superior antioxidant and antiinflammatory properties29. Curcumin is predominantly studied its therapeutic use of oral lesions and wound healing 30,31. Plants like P.pinnata which are commonly and easily found and are being less explored in the field of medical research. Hence, an attempt was made to study its antioxidant and antiinflammatory properties. The presence of metabolites like tannins, saponins, acids, phenols and proteins in P.pinnata is the reason for high anti-inflammatory and antioxidant properties. Further research is needed to implement the ethanolic seed extract of P.pinnata as a therapeutic agent for oral mucosal lesions.
Conclusion
It was obvious from the investigation that the ethanolic seed extract of P.pinnata contained both antioxidant and anti-inflammatory properties. The anti-oxidant and anti-inflammatory properties of P.pinnata seed extract were considerably higher at 500µg, as shown by the DPPH test and suppression of albumin denaturation, respectively. Additional research is required to confirm the therapeutic effects of these plant extracts. Further extensive research on the utilisation of P.pinnata seeds in the treatment of oral mucosal lesions using this plant’s bioactives in in-vivo models is required in order to develop particular applications and synthesise novel and powerful medications of natural origin.
Acknowledgement
We thank BPG Lab, Saveetha Dental College and Hospitals, SIMATS for the use of their research laboratory facilities.
Conflict of Interest
No conflicts of interest with regard to the current work.
Funding Sources
There is no funding Sources.
References
- Akbar S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications. Springer; 2020.
CrossRef - Msomi NZ, Simelane MBC. Herbal Medicine. Herbal Medicine. Published online 2019. doi:10.5772/intechopen.72816
CrossRef - Chaudhry B. A Handbook of Common Medicinal Plants Used in Ayurveda. Kojo Press; 2019.
- Thakur S, Kaurav H, Chaudhary G. KARANJ (PONGAMIA PINNATA) – AN AYURVEDIC AND MODERN OVERVIEW. Asian Journal of Pharmaceutical and Clinical Research. Published online 2021:14-21. doi:10.22159/ajpcr.2021.v14i6.41367
CrossRef - Supriyanto NFN, Silvikultur D, Fakultas Kehutanan, Institut Pertanian Bogor. Morphological Diversity of Fruits, Seeds and Seedlings of Pongamia (Pongamia pinnata (L.) Pierre) in Java Island. Jurnal Perbenihan Tanaman Hutan. 2017;5(2):103-114. doi:10.20886/bptpth.2017.5.2.103-114
CrossRef - Antony JVM, Ramani P, Ramasubramanian A, Sukumaran G. Particle size penetration rate and effects of smoke and smokeless tobacco products – An invitro analysis. Heliyon. 2021;7(3):e06455.
CrossRef - Rekha MJ, Bettadaiah BK, Muthukumar SP, Govindaraju K. Synthesis, characterization and anti-inflammatory properties of karanjin (Pongamia pinnata seed) and its derivatives. Bioorg Chem. 2021;106:104471.
CrossRef - Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2019;48(4):299-306.
CrossRef - R H, Hannah R, Ramani P, Ramanathan A, Jancy MR, Gheena S, et al. CYP2 C9 polymorphism among patients with oral squamous cell carcinoma and its role in altering the metabolism of benzo[a]pyrene. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2020;130(3):306-312. doi:10.1016/j.oooo.2020.06.021
CrossRef - Sundaram R, Nandhakumar E, Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol Mech Methods. 2019;29(9):644-653.
CrossRef - Ponnulakshmi R, Shyamaladevi B, Vijayalakshmi P, Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol Mech Methods. 2019;29(4):276-290.
CrossRef - Daniyal M, Wang W. Molecular pharmacology of inflammation: Medicinal plants as antiinflammatory agents. Inflammation and Natural Products. Published online 2021:21-63. doi:10.1016/b978-0-12-819218-4.00005-5
CrossRef - Raj Preeth D, Saravanan S, Shairam M, Selvakumar N, Selestin Raja I, Dhanasekaran A, et al. Bioactive Zinc(II) complex incorporated PCL/gelatin electrospun nanofiber enhanced bone tissue regeneration. Eur J Pharm Sci. 2021;160:105768.
CrossRef - Ambreen M. Antioxidant Potential of Medicinal Plants: Natural Antioxidants.; 2012.
- Pereira DAM. Medicinal Plants: Antioxidant Properties, Traditional Uses and Conservation Strategies. Nova Science Pub Incorporated; 2014.
- Waterman PG. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Biochemical Systematics and Ecology. 1993;21(8):849. doi:10.1016/0305-1978(93)90098-c
CrossRef - Parimelazhagan T. Medicinal Plants: Promising Future for Health and New Drugs. CRC Press; 2018.
- Prithiviraj N, Yang GE, Thangavelu L, Yan J. Anticancer Compounds From Starfish Regenerating Tissues and Their Antioxidant Properties on Human Oral Epidermoid Carcinoma KB Cells. In: PANCREAS. Vol 49. LIPPINCOTT WILLIAMS & WILKINS TWO COMMERCE SQ, 2001 MARKET ST, PHILADELPHIA …; 2020:155-156.
- Joshi MC. Hand Book of Indian Medicinal Plants. Scientific Publishers; 2019.
- Khatri TT, Patel MG, Ram VR. Phytochemicals, Silicates, Borates Determination from P. Pinnata. LAP Lambert Academic Publishing; 2015.
- Ajibade MA, Akhigbemen AM, Okolie NP, Ozolua RI. Methanol leaf extract of Paullinia pinnata exerts sleep-enhancing and anticonvulsant effects via a mechanism involving the GABAergic pathway. Epilepsy Res. 2022;183:106943.
CrossRef - Tayade S, More K, Gawande P. Antioxidative properties of Mucuna nivea (Roxb.) DC by DPPH Assay. SSR Institute of International Journal of Life Sciences. 2020;6(6):2687-2693. doi:10.21276/ssr-iijls.2020.6.6.3
CrossRef - Salihović M, Pazalja M, Ajanović A. Antioxidant Activity of Watermelon Seeds Determined by DPPH Assay. Kemija u industriji. 2022;(5-6). doi:10.15255/kui.2021.064
CrossRef - Divya M, Vijayakumar S. Traditional South Indian Herbal Plants for a Strong Immune System. Traditional Herbal Therapy for the Human Immune System. Published online 2021:245-254. doi:10.1201/9781003137955-9
CrossRef - Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78(2-3):151-157.
CrossRef - Singh RK, Pandey BL. Anti-inflammatory activity of seed extracts of Pongamia pinnata in rat. Indian J Physiol Pharmacol. 1996;40(4):355-358.
- Dinesh Y, Abilasha R, Ramani P, Rajeshkumar S. Assessment of Cytotoxic, Antioxidant, Thrombolytic, Anti Inflammatory and Antimicrobial Activity of Curcuma longa Linn, Cissus quadrangularis and Boerhaavia diffusa Herbal Mixture – An In vitro Study. J Pharm Res Int. Published online December 23, 2021:1766-1777.
CrossRef - Dinesh Y, Abilasha R, Ramani P, Kumar SR. In-vitro Bioactivity of Herbal Mixture Curcuma longa Linn, Cissus quadrangularis and Boerhaavia diffusa – An In vitro Study. In: Challenges and Advances in Pharmaceutical Research Vol. 3. Book Publisher International (a part of SCIENCEDOMAIN International); 2022:140-154.
CrossRef - Princeton B, Santhakumar P, Prathap L. Awareness on Preventive Measures taken by Health Care Professionals Attending COVID-19 Patients among Dental Students. Eur J Dent. 2020;14(S 01):S105-S109.
CrossRef - Subramanyam D, Gurunathan D, Gaayathri R, Vishnu Priya V. Comparative evaluation of salivary malondialdehyde levels as a marker of lipid peroxidation in early childhood caries. Eur J Dent. 2018;12(1):67-70.
CrossRef - Alsawalha M, Rao CV, Al-Subaie AM, Haque SKM, Veeraraghavan VP, Surapaneni KM. Novel mathematical modelling of Saudi Arabian natural diatomite clay. Mater Res Express. 2019;6(10):105531.
CrossRef








