Alia Khwaldeh1* , Nour Al-Sawalha2
, Nour Al-Sawalha2 , Shefa' Aljabali 1
, Shefa' Aljabali 1 , Ziad Shraideh3
, Ziad Shraideh3 , Sokiyna Ababneh1
, Sokiyna Ababneh1 and Roba Bdeir4
and Roba Bdeir4
1Faculty of Allied Medical Sciences, Department of Medical Laboratory Sciences, Jadara University, Irbid, Jordan.
2Faculty of Pharmacy, Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
3Faculty of Science, Department of Biological Sciences, the University of Jordan, Amman, Jordan.
4Faculty of Nursing, Department of Allied Health Sciences, Al-Balqa Applied University, Al-Salt, Jordan
Corresponding Author E-mail:a.khawaldeh@jadara.edu.jo
DOI : https://dx.doi.org//10.13005/bpj/2787
Abstract
The current study investigated the potential positive impact of EMPA, an antidiabetic medication, on hepatocytes and liver outcomes in STZ-induced diabetic rats. Male Wistar rats were randomly assigned into four groups: control, DM (received 40mg/kg streptozotocin IP injection), DM+EMPA (received 40mg/kg streptozotocin and 10 mg/kg EMPA), and EMPA (received 10 mg/kg EMPA). Here, liver functional tests were assessed spectrophotometrically, while histological analysis of liver tissues was evaluated using light microscopy. Treated diabetic rats significantly reduced AST levels compared to treated control rats (p < 0.05). DM rats, with or without EMPA treatment, showed significantly elevated ALT levels compared to control rats (p < 0.005). Also, LDH levels were found to be lower in both treated and untreated diabetic rats compared to control rats (p < 0.0001; p < 0.05, respectively), while ALP levels were higher in both groups of diabetic rats relative to control rats (p < 0.0001; p < 0.005). Interestingly, the data showed clear trends indicating that empagliflozin-treated diabetic rats had improved liver parameters compared to untreated diabetic rats, although statistically significant differences were not observed. Remarkably, histological examination showed significant sinusoidal dilation and infiltration of inflammatory cells in hepatocytes in diabetic rats, whereas treated diabetic rats exhibited a normal hepatocyte arrangement with minor sinusoidal dilation. Altogether, the observed results suggest that EMPA may possess a protective effect on hepatocytes, thereby highlighting its potential as a therapeutic intervention for diabetes-related liver complications.
Keywords
Diabetes; EMPA; Hepatocytes, Histopathology; Liver Outcomes; STZ
Download this article as:| Copy the following to cite this article: Khwaldeh A, Al-Sawalha N, Aljabali S, Shraideh Z, Ababneh S, Bdeir R. Empagliflozin: Potential Protective Effects on Hepatocytes and Liver Outcomes in Streptozotocin -Diabetic Rats. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Khwaldeh A, Al-Sawalha N, Aljabali S, Shraideh Z, Ababneh S, Bdeir R. Empagliflozin: Potential Protective Effects on Hepatocytes and Liver Outcomes in Streptozotocin -Diabetic Rats. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3Rp8OjK |
Introduction
Diabetes is a chronic disease that is characterized by hyperglycemia (blood glucose: > 126 mg/dL) due to insufficient insulin production, insulin resistance, or increased glucagon production 1,2 Diabetes mellitus (DM) is a significant worldwide problem; in 2019, 9.3% of the world’s population was affected. The percentage is presumed to rise to 10.2% by 2045 3. Uncontrolled DM leads to nephropathy, retinopathy, neuropathy, and cardiovascular complications 4-7 pathogenesis of these complications involves several proposed mechanisms, with oxidative stress being one of the most widely accepted factors. When there is an imbalance between the production of reactive oxygen species (ROS) and the body’s defense mechanisms against antioxidants8, it is known as oxidative stress. Research has indicated that oxidative stress plays a crucial role in the onset and progression of complications associated with diabetes 9, 10.
EMPA is an inhibitor of (SGLT2) sodium-glucose cotransporter 2 11-14 and is a novel therapeutic approach agent for managing type 2 DM 10-13. It reduces glucose reabsorption in the kidney tubules, lowering blood glucose levels 15-18. Clinical studies have demonstrated that EMPA improves clinical outcomes and reduces mortality in diabetic patients with cardiovascular and chronic kidney diseases 19, 20. Studies have demonstrated that EMPA can reduce oxidative stress in the heart and kidneys of diabetic animal models. It has also been observed to decrease lipid peroxidation in patients with diabetes.21, 22. The effects of EMPA interconnected mechanisms and outcomes associated with different factors, such as Histone Deacetylases (HDACs) in deacetylases, mediate the effects of EMPA and SGLT2 inhibition in the context of DPN.23,24 Assess HDACs modulate gene expression and epigenetic changes related to neuroprotection and nerve regeneration in diabetic conditions and glycerophospholipids in modulating neuronal membrane structure and function, their role in the DPN context, and their potential interaction with EMPA and SGLT2 inhibition. The combined influence of EMPA, SGLT2 inhibition, histone deacetylases, and glycerophospholipids contributes to the amelioration of DPN in rats. The impact on nerve conduction, pain perception, and neuroinflammatory processes, considering central and peripheral nerve function. Role of miR-21, TRAF3IP2, and RECK in mediating the effects of EMPA and SGLT2 inhibition on DPN. These factors influence neuroinflammation, extracellular matrix remodeling, and neuronal survival in diabetic neuropathy.23, 24
Various research studies have shown the beneficial effects of EMPA on liver health. For instance, EMPA treatment significantly improved liver outcomes in hereditary hypertriglyceridemic rats by reducing cell senescence markers and attenuating oxidative stress 25. Clinical trials in patients with non-alcoholic fatty liver disease (NAFLD) without diabetes demonstrated that EMPA treatment significantly improved liver steatosis and fibrosis compared to placebo 26, 27 Another study indicated that the treatment with EMPA showed improvement in markers of fibrosis and liver steatosis. Suggesting its potential benefit in managing liver-related conditions 28. Additionally, EMPA exhibited hepatoprotective effects in rats with bile duct ligation-induced liver injury, highlighting its efficacy in protecting the liver against injury 29 A recent study showed that ursodeoxycholic acid leads to more reduction in insulin resistance and liver fibrosis scores in NAFLD patients with type 2 diabetes compared with EMPA treatment. However, both treatments managed the liver steatosis and achieved a significant regression in non-alcoholic fatty liver score30.
In addition, EMPA has an advantageous effect on the hematological system by restoring neutrophil function and count and allows the termination of G-CSF treatment, thereby improving patients’ quality of life by removing painful injections31. Moreover, the AMPK/SIRT-1 pathway regulates metabolism, apoptosis, inflammation, and mitochondrial function during oxidative stress. In thioacetamide-induced Liver fibrosis in Rats, EMPA showed an ant-fibrotic effect by inhibiting Hypoxia-inducible factor 1-alpha (HIF-1α) and stimulation AMP-activated protein kinase (AMPK)/Sirtuin-1 (SIRT-1) activity. EMPA is an SGLT-2 inhibitor that activates the AMP-activated protein kinase (AMPK)/mammalian target of the rapamycin (mTOR) signaling pathway, thus controlling the autophagy and oxidative stress in NAFLD32.
The effects of empagliflozin on the liver in conjunction with metallothionein and quercetin, and considering various related pathways and outcomes in the context of streptozotocin-induced diabetes mellitus in rats including Modulation of NF-κB/Nrf-2/PPAR-γ Interplay within the liver, the potential regulatory effects on inflammation, oxidative stress, and metabolic regulation, Normalized Pin1 Expression Level and AMPK Activation Assess how these changes affect cellular proliferation, apoptosis, and energy metabolism in the diabetic liver. SGLT2 Inhibitors and Lipotoxicity its influence on lipid metabolism, hepatic steatosis, and lipid-induced cellular stress responses, particularly in the diabetic condition.33, 34, 35
Despite existing studies supporting the positive impact of EMPA on liver outcomes, the present study investigates the potential protective effects of EMPA on hepatocytes and liver function in streptozotocin-induced diabetes in rats. This study’s results could enhance our comprehension of how EMPA acts as a hepatoprotective agent against diabetes-related complications.
Material and methods
Animals
58 male Wister rats aged 9-10 weeks were selected for this study. The Animal Care and Use Committee at Jordan University of Science and Technology approved the animal protocol. The rats were housed in a controlled environment with a 12-hour light/dark cycle at room temperature and provided ad libitum access to food and water. The rats were randomly assigned to one of four groups: Control (n=15), DM (n=13), DM+EMPA (Diabetic rats treated with EMPA, n=15), and EMPA (Control rats treated with EMPA, n=15).
Diabetes induction and empagliflozin treatment
Diabetic rats were included in the study. To reduce early death, the drinking water after STZ injection was supplemented with sucrose (15g/l) for 48 hours (205). However, two rats in the DM group died. The fasting blood glucose was monitored weekly using a glucose analyzer (Accu-check Guide Blood Glucose Meter, Germany). Rats in groups 2 and 3 were treated with (10 mg/kg) EMPA dissolved in 5% hydroxyethylcellulose by oral gavage using a 2.25 mm metallic gauge for 8 weeks after DM induction. Diabetes was induced by administering a single intraperitoneal injection of streptozotocin (STZ) (40 mg/kg) dissolved in sodium citrate buffer (pH 4.5). The fasting plasma glucose level was measured one day after STZ injection, and rats with fasting blood glucose levels >150 mg/dl (204). To prevent early mortality, the drinking water of diabetic rats was supplemented with sucrose (15 g/L) for 48 hours (205). EMPA treatment was initiated after 7 days of STZ injection at a dose of 10 mg/kg, administered orally using a 5% hydroxyethyl cellulose solution, and continued for 8 weeks.
Blood collection and biochemical analysis
Following the euthanization of the rats, blood samples were obtained. These blood samples were subsequently subjected to centrifugation, separating serum from other blood components for further biochemical analysis. The levels of lactate dehydrogenase (LDH) (AGAPPE, India), alanine aminotransferase (ALT), aspartate aminotransferase (AST) (Teco Diagnostics, Annaheim, CA, USA), total and direct bilirubin (BioLabo, France), and total protein (TP) (Abcam, USA), were evaluated using commercially available kits. The activity or concentration of each parameter was measured spectrophotometrically by UV/VIS single beam spectrophotometer (EMC-11D-V; EMCLAB instruments, Duisburg, Germany).
Histological examination
Liver tissues were harvested after sacrificing the rats. Liver tissues were washed with phosphate buffered saline to remove excess blood and then fixed in 10% formaldehyde for 48 hours. The tissues were dehydrated, cleared, infiltrated with paraffin wax, and embedded in paraffin blocks. Subsequently, thin sections (3-5 µm) were cut using a microtome, deparaffinized with xylene, stained with H and E and mounted with DPX for light microscopic (Leica inverted light mi-croscopy, Leica Microsystems, Wetzlar, Germany) examination.
Data analysis
To perform the statistical tests, we utilized GraphPad Prism 5.01 Computer Software from GraphPad Software Inc. Specifically, we employed the D’Agostino & Pearson omnibus and Shapiro-Wilk normality tests. To compare the different groups, either One Way ANOVA or Kruskal-Wallis tests were utilized. The data were presented as means ± standard error of the mean (SEM). At a ‘p‘ value of 0.05, the results were accepted as statistically significant.
Results and Discussion
Figure (1) demonstrates the levels of aspartate aminotransferase (a), alanine aminotransferase (b), Lactate dehydrogenase (c), and alkaline phosphatase (d) in the four experimental groups. All liver enzymes were measured in U/L. As shown in Figure (1a), AST concentration was significantly increased (p < 0.05) in rats treated with EMPA compared to control rats. However, when we compared the levels of the same enzyme between DM and rats treated with EMPA, the former group showed a statistically significant decline (p = 0.05). Figure (1b) shows that DM rats with and without treatment ha EMPA d a significant increase in ALT concentration (p < 00.5) when compared to control rats. Figure (1c) shows that LDH levels were significantly lower in DM rats treated with (p < 0.0001, p < 0.005) or without (p < 0.05, p < 0.005) EMPA compared to control groups treated with or without EMPA. As can be seen in Figure (1d), the levels of ALP were significantly higher in both DM rats (p < 0.0001) and DM+ EMPA (p < 0.005) versus control rats. In addition, the DM group showed higher levels of ALP (p < 0005) versus normal rats treated with EMPA.
Figure (2) illustrates the levels of total protein (g/dL) in the four tested groups; DM rats without treatment, DM+ EMPA, rats treated with EMPA, and control rats. As reflected in the figure, there were no significant differences (p = 0.1037) in the total protein concentrations between all groups.
Levels of bilirubin (mg/dL) in DM rats, DM+ EMPA rats, EMPA rats, and control rats are illustrated in Figure (3). As can be seen in the figure, even though there was an increase in the bilirubin levels in the tested groups versus the control group, this elevation was not statistically significant (p = 0.1165).
Figure (4) represents the histological examination of liver tissues harvested from the study groups using microscopy. In figure (4a), the liver section from the control group exhibited a normal liver structure, including a central vein, portal vein, sinusoids, and hepatocytes forming hepatic lobules. In figure (4b), the liver section from the DM rat group demonstrated dilated sinusoids and infiltration of inflammatory cells, along with congestion of blood within the sinusoids. However, in figure (4c), the liver section from the DM+ EMPA showed a normal arrangement of hepatocytes with slight dilation of sinusoids.
Figure (1)
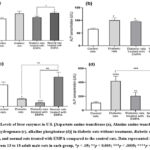 |
Figure 1: Levels of liver enzymes in U/L [Aspartate amino transferase (a), Alanine amino transferase (b), Lactate dehydrogenase (c), alkaline phosphatase (d)] in diabetic rats without treatment, diabetic rats treated with EMPA, and normal rats treated with EMPA compared to the control rats. |
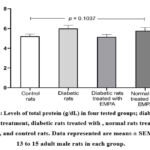 |
Figure 2: Levels of total protein (g/dL) in four tested groups; diabetic rats without treatment, diabetic rats treated with , normal rats treated with EMPA, and control rats. |
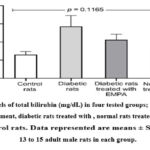 |
Figure 3: Levels of total bilirubin (mg/dL) in four tested groups; diabetic rats without treatment, diabetic rats treated with , normal rats treated with EMPA, and control rats. |
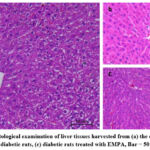 |
Figure 4: Histological examination of liver tissues harvested from (a) the control rats,(b) untreated diabetic rats, (c) diabetic rats treated with EMPA, Bar = 50 µm, H & E. |
The impact of diabetes on various organ systems is a pressing concern in light of the escalating global prevalence of this metabolic disorder36. While much attention has been given to the renal and cardiovascular complications of diabetes, the effect on liver is highly important. The liver’s functions are essential for metabolic regulation, detoxification, and nutrient storage. Unfortunately, diabetes can have a harmful impact on this vital organ. Liver complications, including hepatic steatosis, inflammation, fibrosis, and impaired liver function, can significantly contribute to the overall burden of diabetes-related complications.
In terms of liver enzyme levels, analyzed data showed that empagliflozin treated diabetic rats had lower AST and LDH levels when compared to the diabetic group. These enzymes are commonly used as biochemical markers for liver damage 37, 38. This decrease in AST and LDH levels suggests that empagliflozin has beneficial for liver health. The observed reduction in AST and LDH levels may be attributable to empagliflozin’s ability to improve hepatic cellular function and energetic status 39, enhance glucose utilization 40, and reduce inflammation 41.
The observed increase in ALT levels in diabetic rats treated with empagliflozin may be influenced by the drug’s effects on hepatic metabolism. Empagliflozin is known to modulate gluconeogenesis and hepatic glucose output 25, which can impact alanine levels and subsequently affect ALT concentrations 42. Additionally, empagliflozin’s ability to increase fatty acid oxidation 43 and decrease hepatic steatosis may also contribute to changes in ALT levels 44, reflecting alterations in hepatic lipid turnover.
Diabetes disrupts the balance of calcium and phosphate 45, 46, which can influence ALP levels 47. Furthermore, the presence of hepatic inflammation and hepatocyte damage associated with diabetes can contribute to the release of ALP into the bloodstream. Although empagliflozin’s effects on glucose metabolism and hepatic function may help mitigate these disturbances, they may not fully normalize ALP levels in the diabetic condition. Another point, it is important to note that ALP is not a specific indicator of liver damage and can be elevated in various clinical conditions, including active bone formation 48, disorders affecting blood calcium levels 49, and vitamin D deficiency 38. Given that, factors other than liver damage may contribute to the observed elevation in ALP levels in both diabetic rats and diabetic rats treated with empagliflozin. Empagliflozin causes a reduction in liver fat in type 2 diabetes patients compared with control group50.Also, it lowers the blood glucose level. Of note, EMPA treated-rats showed a significant smaller Atherosclerotic plaque area in the aortic valve51. The beneficial effect of EMPA through inhibiting the p38 MAPKα and ERK1/2 activities causes an enhancement of the anti-fibrotic action of metformin52.
In regard of the effect of EMPA in the digestive system, it has been found to reduce the uric acid level in type 2 diabetic patients and thus eliminates the need for antigout therapy53.Additionally, empagliflozin reduces gluconeogenesis and increasing glycogenesis by stimulation renal mRNA expression of phosphoenolpyruvate carboxykinase, gluconeogenic enzyme54.
Despite the lack of significance in some statistical tests, clear trends indicate improved liver parameters in diabetic mice treated with empagliflozin compared to untreated diabetic mice. Further studies are needed for a longer duration of empagliflozin treatment to draw better conclusions.
Drawing attention to an intriguing comparison, this study compared liver functional tests in control rats (non-diabetic) treated with the drug versus control rat. Interestingly, the control rats receiving the drug showed higher liver parameter values in comparison to the normal rats, indicating potential complications associated with empagliflozin administration. However, in diabetic rats, the drug appeared to alleviate the negative effects of diabetes on liver outcomes. This contrast highlights the importance of considering the diabetes context when assessing the drug’s impact on liver function.
In a previous study conducted in bile duct ligation-induced liver injury in rat,EMPA treatment reduced TNF-α, IL-6 and liver enzymes and increased Superoxide dismutase and glutathione peroxidase enzymes, therefore this shows the anti-inflammtory ,hepatoprotective, and antioxidant role of EMPA sequentially. Also, bile duct proliferation and fibrosis of liver are both reduced55. Another study showed that EMPA minimizes the lipid accumulation and oxidative stress in the liver and accordingly ameliorates the NAFLD condition. The mechanism of action of EMPA on hepatic lipid metabolism could be summarized in four points as following: (1) a reduction in accumulation of lipotoxic diacylglycerol and ectopic triacylglycerol56 (2) a decrease in the mRNA expression of lipogenic enzymes including fatty acid synthetase (fas) and stearoyl-CoA desaturase 1 (scd1). (3) Lowering the expression of sterol regulatory element-binding protein 1 (srebp1) and peroxisome proliferator-activated receptor-α (PPAR-α), and thus suppressing hepatic lipogenesis57.(4) The EMPA treatment in Type 2 diabetes mellitus patients causes a significant reduction in liver fat content 58.Another research showed that EMPA lowers the concentration of intrahepatic lactosylceramide and increases the unsaturated triglycerides in normal mice59
Several limitations are evident in this study that should be acknowledged. One limitation of this study is the use of an animal model, which may not fully represent the complexities of human diabetes and liver complications. The focus on biochemical parameters and histological examination provides valuable insights but may not capture the complete spectrum of liver function. Additionally, this study solely examined empagliflozin without comparing it to other treatments, limiting the assessment of its relative efficacy. Further investigations using diverse techniques, longer-term studies, and comparative analyses are needed to enhance the understanding of empagliflozin’s effects on diabetes-related liver complications.
A research study was conducted to observe the impact of Wattakaka volubilis leaf extract, a traditional Indian herb, on male Wistar rats. Diabetic rats exhibited necrosis of hepatocytes, congestion of blood in sinusoids, and infiltration of inflammatory cells with dilation of sinusoids 60. These pathological changes are indicative of liver damage and inflammation associated with diabetes. In light of this, exploring novel therapeutic interventions that can effectively manage diabetes-related liver complications is of utmost importance.
The present study investigated the potential influence of empagliflozin is an inhibitor of sodium-glucose cotransporter 2 (SGLT2). On liver outcomes in diabetic rats. The results revealed intriguing trends; indicating improvements in liver functional tests among diabetic rats treated with empagliflozin. In addition, histological examination revealed a restoration of the normal arrangement of hepatocytes and reduced sinusoidal dilation in the empagliflozin-treated diabetic group. Altogether, these findings offer valuable insights into the potential hepatoprotective effects of empagliflozin in the context of diabetes. These findings contribute to the existing body of knowledge and stimulate further exploration of the mechanisms underlying empagliflozin’s impact on liver outcomes.
According to the literature, few research studies have demonstrated the impact of empagliflozin on liver outcomes. For instance, a study by Trnovska and colleagues showed that empagliflozin treatment significantly improved liver outcomes in hereditary hypertriglyceridemic rats 25. These improvements are associated with reduced biomarkers of cell senescence, which refers to the deterioration and loss of cellular function over time. Additionally, empagliflozin attenuated oxidative stress biomarkers in the liver tissues 25. A research team conducted a study using randomized, double-blind, and placebo-controlled methods to examine how empagliflozin affects liver steatosis and fibrosis in patients with NAFLD but no diabetes. According to the study, empagliflozin treatment resulted in notable enhancements in liver steatosis and fibrosis when compared to the placebo group 26, 27 .The researchers also explored the possible protective effects of empagliflozin on liver injury induced by bile duct ligation in rats. 29. Through a combination of molecular docking analysis and in vivo experiments, the study revealed that empagliflozin demonstrated promising efficacy in protecting the liver against injury 29. These studies are in line with the findings of our current study.
Several explanations have been addressed and discussed here to elucidate the observed findings in the current study. For example, one possible explanation for restoration of the normal arrangement of hepatocytes and the reduction in sinusoidal dilation observed in diabetic mice following empagliflozin administration is its impact on hepatic inflammation, fibrosis, and oxidative stress. Empagliflozin has demonstrated anti-inflammatory effects 22, 40, attenuated hepatic fibrosis 24, and exhibited antioxidant properties in various models 20, 23. Hence, empagliflozin may contribute to the restoration of hepatocyte structure and improved sinusoidal dilation, and Hallmarks of Aging in the Liver which includes hepatocyte growth factor (HGF), Kupffer cells, endothelial cell dysfunction, hepatic stellate cells, and liver sinusoidal function61. Empagliflozin’s potential to inhibit inflammatory and apoptotic signaling pathways within the liver may contribute to the attenuation of liver damage and aging. Investigating the combined effects of empagliflozin with other agents, such as MET and JTXK, in enhancing antioxidant capacity and restoring liver cell activities can provide a comprehensive understanding of its synergistic actions in promoting liver health62, 63. Additionally, its effects on glucose and lipid metabolism 64, 65, along with improved insulin sensitivity, may further aid in the improvement of liver histology.
Conclusion
This study sheds light on the impact of EMPA on liver function and histological features in STZ -induced diabetic rats. Through liver functional tests, we observed significant improvements in parameters such as AST, ALT, LDH, and ALP levels among diabetic rats treated with EMPA compared to untreated diabetic rats. Moreover, histological examination revealed a restoration of the normal arrangement of hepatocytes and reduced sinusoidal dilation, infiltration of inflammatory cells, along with less congestion of blood within the sinusoids in the EMPA-treated diabetic group. Altogether, the data offer valuable insights into the potential in the context of diabetes.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
Financial support by Jadara University. Grant number is (R1- 122- 13-467)
References
- Blair M. Diabetes Mellitus Review. Urol Nurs., 2016;36(1):27-36.
CrossRef - Panari H, Vegunarani M. Study on complications of diabetes mellitus among the diabetic patients. Asia j. nurs. educ. res., 2016;6(2):171-82.
CrossRef - Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract., 2019;157:107843.
CrossRef - Wilson S. A study to assess the effect of awareness programme on compliance to insulin therapy among patients with diabetes mellitus (DM) in a selected community health centre, Thrissur district. Asia j. nurs. educ. res., 2016;6(4):464-70.
CrossRef - Parsa P, Ahmadinia-Tabesh R, Mohammadi Y. Assessment of the risk of coronary heart disease in diabetes patients type-II. Asia j. nurs. educ. res., 2019;9(2):267-70.
CrossRef - Khan Y, Gupta P, Bihari B, Verma VK. A Review on Diabetes and Its Management. Asian j. pharm. res. dev., 2013;3(1):28-33.
- Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med. Sci. Monit., 2006 ;12(7):130-47.
- Aljerf LO, Williams MI, Ajong AB, Onydinma UP, Dehmchi FA, Pham VT, Bhatnagar SU, Belboukhari NA. Comparative study of the biochemical response behavior of some highly toxic minerals on selenosis in rats. Rev Chim., 2021;72(2):9-18..
CrossRef - Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int. J. Mol. Sci.,2013;14(11):21525-50.
CrossRef - Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm J., 2016;24(5):547-53.
CrossRef - Mathew C, Varma S. Green Analytical Methods based on Chemometrics and UV spectroscopy for the simultaneous estimation of Empagliflozin and Linagliptin. Asian J. Pharm. Anal., 2022;12(1):43-8.
CrossRef - Eswarudu MM, Ouchitya G, Reddy NS, Deekshitha M, Babu PS. Empagliflozin, Linagliptin and Metformin Hydrochloride. Asian J. Pharm. Anal., 2023;13(1),
- Chandramore K. Review on Dipeptidyl Peptidase IV Inhibitors as a Newer Target for Diabetes Mellitus Treatment. Asian j. pharm. res. dev., 2017;7(4):230-8.
CrossRef - Kumar RS. SGLT-2 inhibitors-hope or hype?-an updated review. Asian j. pharm. res. dev., 2021;11(1):29-38.
CrossRef - Patil SD, Chaure SK, Kshirsagar S. Development and validation of UV spectrophotometric method for Simultaneous estimation of Empagliflozin and Metformin hydrochloride in bulk drugs. Asian J. Pharm. Anal., 2017;7(2):117-23.
CrossRef - Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol. Rev., 2013 93(1):137-88.
CrossRef - Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs. 2018; 78:1037-48.
CrossRef - Chinta R, Rohini P. Formulation development of Empagliflozin and Metformin hydrochloride extended release Tablets: Optimization of Formulation using statistical experimental design. Res J Pharm Technol., 2021;14(3):1201-8.
CrossRef - Liakos A, Karagiannis T, Athanasiadou E, Sarigianni M, Mainou M, Papatheodorou K, Bekiari E, Tsapas A. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab., 2014;16(10):984-93.
CrossRef - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med., 2015;373(22):2117-28.
CrossRef - Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med., 2016;375(4):323-34.
CrossRef - Malínská H, Hüttl M, Marková I, Miklánková D, Hojná S, Papoušek F, et al. Beneficial effects of empagliflozin are mediated by reduced renal inflammation and oxidative stress in spontaneously hypertensive rats expressing human c-reactive protein. Biomedicines., 2022;10(9):2066.
CrossRef - Andreadou I, Efentakis P, Balafas E, Togliatto G, Davos CH, Varela A, et al. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front. Physiol., 2017;8:1077.
- Huang, Haojun. Prediction and mechanisms of doxorubicin-induced cardiotoxicity: role of RARG and cardioprotective effects of SGLT2 inhibitor. Diss. University of British Columbia, 2022.
CrossRef - Abdelkader NF, Elbaset MA, Moustafa PE, Ibrahim SM. Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: a glucose-independent effect through AMPK signaling. Arch. Pharm. Res., 2022 ;45(7):475-93.
CrossRef - Trnovska J, Svoboda P, Pelantova H, Kuzma M, Kratochvilova H, Kasperova BJ, et al. Complex positive effects of SGLT-2 inhibitor empagliflozin in the liver, kidney and adipose tissue of hereditary hypertriglyceridemic rats: Possible contribution of attenuation of cell Senescence and Oxidative Stress. Int. J. Mol. Sci.,2021;22(19):10606.
CrossRef - Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, et al. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv. Ther., 2020;37:4697-708.
CrossRef - Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care., 2020;43(2):298-305.
CrossRef - Kahl S, Ofstad AP, Zinman B, Wanner C, Schüler E, Sattar N, et al. Effects of empagliflozin on markers of liver steatosis and fibrosis and their relationship to cardiorenal outcomes. Diabetes Obes. Metab., 2022;24(6):1061-71.
CrossRef - Shakerinasab N, Azizi M, Mansourian M, Sadeghi H, Salaminia S, Abbasi R, et al. Empagliflozin Exhibits Hepatoprotective Effects Against Bile Duct Ligation-induced Liver Injury in Rats: A Combined Molecular Docking Approach to In Vivo Studies. Curr. Pharm. Des., 2022;28(40):3313-23.
CrossRef - Elhini SH, Wahsh EA, Elberry AA, El Ameen NF, Abdelfadil Saedii A, Refaie SM, Elsayed AA, Rabea HM. The Impact of an SGLT2 Inhibitor versus Ursodeoxycholic Acid on Liver Steatosis in Diabetic Patients. Pharm J., 2022 ;15(12):1516.
CrossRef - Lédeczi Z, Pittner R, Kriván G, Kardon T, Legeza B. Empagliflozin restores neutropenia and neutrophil dysfunction in a young patient with severe congenital neutropenia type 4. J. Allergy Clin. Immunol. Pract., 2023;11(1):344-6.
CrossRef - ElBaset MA, Salem RS, Ayman F, Ayman N, Shaban N, Afifi SM, Esatbeyoglu T, Abdelaziz M, Elalfy ZS. Effect of empagliflozin on thioacetamide-induced liver injury in rats: role of AMPK/SIRT-1/HIF-1α pathway in halting liver fibrosis. Antioxidants., 2022; 11(11):2152.
CrossRef - Abdelhamid AM, Elsheakh AR, Abdelaziz RR, Suddek GM.
Empagliflozin ameliorates ethanol-induced liver injury by modulating NF-κB/Nrf-2/PPAR-γ interplay in mice. Life Sci, 2020;256:117908.
CrossRef - Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care., 2020 ;43(3):508-11.
CrossRef - Ghosh N, Chacko L, Bhattacharya H, Vallamkondu J, Nag S, Dey A, Karmakar T, Reddy PH, Kandimalla R, Dewanjee S. Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines., 2023; 7;11(4):1126
CrossRef - Aljerf L, Alhaffar I. Salivary distinctiveness and modifications in males with diabetes and Behçet’s disease. Biochem. Res. Int., 2017 21;2017
CrossRef - Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJ, Rahmani AH. The biochemical and clinical perspectives of lactate dehydrogenase: an enzyme of active metabolism. Endocr. Metab. Immune. Disord., 2020;20(6):855-68.
CrossRef - Zhang H, Uthman L, Bakker D, Sari S, Chen S, Hollmann MW, Coronel R, Weber NC, Houten SM, van Weeghel M, Zuurbier CJ. Empagliflozin decreases lactate generation in an NHE-1 dependent fashion and increases α-ketoglutarate synthesis from palmitate in type II diabetic mouse hearts. Front. cardiovasc. med., 2020;7:592233.
- Guerra S, Gastaldelli A. The role of the liver in the modulation of glucose and insulin in non alcoholic fatty liver disease and type 2 diabetes. Curr Opin Pharmacol., 2020;55:165-74.
CrossRef - Maayah ZH, Ferdaoussi M, Takahara S, Soni S, Dyck JR. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology., 2021;29:269-79.
CrossRef - Qian K, Zhong S, Xie K, Yu D, Yang R, Gong DW. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab. Res. Rev., 2015;31(6):562-71.
CrossRef - Nambu H, Takada S, Fukushima A, Matsumoto J, Kakutani N, Maekawa S, Shirakawa R, Nakano I, Furihata T, Katayama T, Yamanashi K. Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. Eur. J. Pharmacol., 2020;866:172810.
CrossRef - Park HS, Jang JE, Ko MS, Woo SH, Kim BJ, Kim HS, Park HS, Park IS, Koh EH, Lee KU. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J., 2016;40(5):376-85.
CrossRef - Kern M, Klöting N, Mark M, Mayoux E, Klein T, Blüher M. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism. 2016;65(2):114-23.
CrossRef - Tripathi N, Balai MK. A Study to assess the Quality of life among Persons living with Hypertension, Diabetes Mellitus and Arthritis in selected Village of Ludhiana, Punjab. Int. J. Nurs. Educ., 2018;6(4):379-82.
CrossRef - Wongdee K, Krishnamra N, Charoenphandhu N. Derangement of calcium metabolism in diabetes mellitus: negative outcome from the synergy between impaired bone turnover and intestinal calcium absorption. J Physiol Sci., 2017;67(1):71-81.
CrossRef - Vimalraj S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. 2020;754:144855.
CrossRef - Crincoli V, Cazzolla AP, Di Comite M, Lo Muzio L, Ciavarella D, Dioguardi M, et al. Evaluation of vitamin d (25OHD), bone alkaline phosphatase (BALP), serum calcium, serum phosphorus, ionized calcium in patients with mandibular third molar impaction. An Observational Study.Nutrients., 2021;13(6):1938.
CrossRef - Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia., 2018;61:2155-63.
CrossRef - Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia., 2017 ;60:364-76.
CrossRef - Abdelhamid AM, Youssef ME, Abd El-Fattah EE, Gobba NA, Gaafar AG, Girgis S, Shata A, Hafez AM, El-Ahwany E, Amin NA, Shahien MA. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci., 2021;286:120070.
CrossRef - Ferreira JP, Inzucchi SE, Mattheus M, Meinicke T, Steubl D, Wanner C, Zinman B. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA‐REG OUTCOME trial. Diabetes Obes Metab., 2022;24(1):135-41.
CrossRef - Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, Sharma K. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Renal Physiol., 2020 ;319(4):F712-28.
CrossRef - Shakerinasab N, Azizi M, Mansourian M, Sadeghi H, Salaminia S, Abbasi R, Shahaboddin ME, Doustimotlagh AH. Empagliflozin exhibits hepatoprotective effects against bile duct ligation-induced liver injury in rats: a combined molecular docking approach to in vivo studies. Curr. Pharm. Des., 2022;28(40):3313-23.
CrossRef - Wang Y, Ding Y, Sun P, Zhang W, Xin Q, Wang N, Niu Y, Chen Y, Luo J, Lu J, Zhou J. Empagliflozin-enhanced antioxidant defense attenuates lipotoxicity and protects hepatocytes by promoting FoxO3a-and Nrf2-mediated nuclear translocation via the CAMKK2/AMPK pathway. Antioxidants., 2022;11(5):799.
CrossRef - Hüttl M, Markova I, Miklankova D, Zapletalova I, Poruba M, Haluzik M, Vaněčkova I, Malinska H. In a prediabetic model, empagliflozin improves hepatic lipid metabolism independently of obesity and before onset of hyperglycemia. Int. J. Mol. Sci., 2021;22(21):11513.
CrossRef - Papaetis GS. Empagliflozin therapy and insulin resistance-associated disorders: effects and promises beyond a diabetic state. Arch. Med. Sci. Atheroscler. Dis., 2021;6(1):57-78.
CrossRef - Hossain MF, Khan NA, Rahman A, Chowdhury MF, Bari S, Khan MA, Masud UW, Zakia UB, Paul SP, Tasnim N. Empagliflozin Ameliorates Progression From Prediabetes to Diabetes and Improves Hepatic Lipid Metabolism: A Systematic Review. Cureus., 2022;14(8).
CrossRef - Gopal V, Mandal V, Tangjang S, Mandal SCJJoP. Serum biochemical, histopathology and SEM analyses of the effects of the Indian traditional herb Wattakaka volubilis leaf extract on wistar male rats. J. Pharmacopunct., 2014;17(1):13.
CrossRef - Hunt NJ, Kang SW, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of aging in the liver. Comput. Struct. Biotechnol. J., 2019;17:1151-61.
CrossRef - Perakakis N, Chrysafi P, Feigh M, Veidal SS, Mantzoros CS. Empagliflozin improves metabolic and hepatic outcomes in a non-diabetic obese biopsy-proven mouse model of advanced NASH. Int. J. Mol. Sci., 2021;22(12):6332.
CrossRef - Janić M, Cankar M, Šmid J, France Štiglic A, Jerin A, Šabovič M, Janež A, Lunder M. Empagliflozin-Metformin Combination Has Antioxidative and Anti-Inflammatory Properties that Correlate with Vascular Protection in Adults with Type 1 Diabetes. J. Diabetes Res., 2022
CrossRef - Hüttl M, Markova I, Miklankova D, Zapletalova I, Poruba M, Haluzik M, Vaněčkova I, Malinska H. In a prediabetic model, empagliflozin improves hepatic lipid metabolism independently of obesity and before onset of hyperglycemia. Int. J. Mol. Sci., 2021;22(21):11513.
CrossRef - Umamaheswari A. To compare the anti-inflammatory effect of oral hypoglycemic drugs in type 2 diabetes mellitus. Diss. PSG Institute of Medical Sciences and Research, Coimbatore, 2016.







