Manuscript accepted on :27-11-2023
Published online on: 01-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Doaa Sayed Rashwan and Dr. Sharath B.S
Second Review by: Dr. Shoheb Shaikh
Final Approval by: Dr. Patorn Piromchai
Dilnoza Kh. Muratova1* , Nurali A. Ergashev2
, Nurali A. Ergashev2 and Muzaffar I. Asrarov2
and Muzaffar I. Asrarov2 
1Department of Biophysics, National University of Uzbekistan named after Mirzo Ulugbek., Tashkent, Uzbekistan.
2Institute of Biophysics and Biochemistry, National University of Uzbekistan named after Mirzo Ulugbek., Tashkent, Uzbekistan.
Corresponding Author E-mail: dilnozaxasanovnam@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2808
Abstract
Dysfunction of the mitochondria of various tissues causes the development of most pathological processes, including ischemia. In recent years, great attention has been paid to the use of plant biologically active substances in the prevention and treatment of pathological processes related to mitochondrial dysfunction. This is very relevant in relation to ischemic diseases and is of scientific and practical importance in the search for new pharmacological agents that correct the functions of damaged mitochondria for their treatment. The mitochondrial permeability transition pore (mPTP) actively participates in the regulation of mitochondrial functions, in the development of various pathological conditions and, at the same time, targets for various drugs and some biologically active substances. In vitro experiments evaluated the effects of alkaloids talatisamine and 14-O-benzoyltalatisamine on rat liver and heart Ca2+-dependent mPTP and lipid peroxidation (LPO) induced by Fe2+/ascorbate system. The investigated diterpenealkaloids inhibited the opening of the Ca2+-dependent mPTP in the membranes of rat liver and heart mitochondria. It was found that 14-O-benzoyltalatizamine inhibits the Ca2+-dependent conductance pore of rat liver and heart mitochondria more strongly than talatisamine. To compare the effects of 14-O-benzoyltalatisamine on rat liver and heart mPTP, concentrations from 1 μM to 200 μM were investigated. At these concentrations, liver mPTP was reliably inhibited by 10% to 81% and heart mPTP by 3.6% to 71.5% relative to control. The high sensitivity of diterpene alkaloids to the Ca2+-dependent permeability transition pore of liver mitochondria compared to heart mitochondria indicates their tissue specificity. The investigated alkaloids exhibited antioxidant properties by inhibiting Fe2+/ascorbate-induced mitochondrial suppression (LPO process) and MDA formation in membranes. LPO induced by Fe2+/ascorbate system in mitochondrial membranes was more actively inhibited by 14-O-benzoyltalatisamine. According to the results of the research, acylation of the hydroxyl group at the C-14 position of talatisamine by benzoyl chloride caused a rise in molecular activity of the derivative due to the introduction of the benzoyl group.
Keywords
lipid peroxidation; mitochondria; 14-О-benzoyltalatisamine; permeability transition pore; talatisamine .
Download this article as:| Copy the following to cite this article: Muratova D. K, Ergashev N. A, Asrarov M. I. Effect of Talatisamine and its Derivate 14-O-Benzoyltalatisamine on Functional State of Rat Liver and Heart Mitochondria. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Muratova D. K, Ergashev N. A, Asrarov M. I. Effect of Talatisamine and its Derivate 14-O-Benzoyltalatisamine on Functional State of Rat Liver and Heart Mitochondria. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/4azinEF |
Introduction
Leading scientific centers, universities and laboratories recognize that the dysfunction of mitochondria of various tissues causes the development of many pathological processes, including ischemia1-5. Much attention is being paid to the use of local plant biologically active substances, including alkaloids, in the prevention and treatment of pathological processes related to mitochondrial dysfunction. This is very relevant in relation to ischemic diseases and is of scientific and practical importance in the search for new pharmacological agents that correct the functions of damaged mitochondria for their treatment. This is very relevant in relation to ischemic diseases and is of scientific and practical importance in the search for new pharmacological agents that correct the functions of damaged mitochondria for their treatment. Despite the achieved results, there are insufficient studies aimed at determining the effects of diterpene alkaloids on cells and mitochondria, repairing membrane and molecular damage.
In our country, in order to create effective drugs for the prevention and treatment of liver, cardiovascular, neurodegenerative and other diseases based on plant compounds, scientific research is being conducted to determine their mechanisms of action6-11.
Mitochondria are important intracellular structures that not only produce energy necessary for the cells to function, but also play a role in various pathological processes. In the double-membrane system of mitochondria of living organisms, there are mechanisms that ensure the transport of metabolites, various ion channels, and transporters, critically involved in the development of pathological processes. Functional structures located in mitochondria and membranes are the main targets for various pathological effects1-4. Mitochondrial functions that can be corrected by drugs are implemented by structures that form PTP, the activity of which is regulated by Ca2+ ions, cyclophilin D, membrane potential, etc. Pharmacons-modulators of the PTP state of mitochondria are known: cyclosporin A, ubiquinone, boncrecat, buterol, sodium hydrosulfide, progesterone, doxorubicin, 1-O-benzoylnapelline, songorine etc5,9,11. The Ca2+-dependent transition of PTP to the open state increases the permeability of the inner membrane of mitochondria, resulting in a decrease in the transmembrane potential (Δψm), swelling of the organelles, followed by tear and burst of the outer membrane and the release of proapoptotic factors12. One of the mechanisms that leads to mitochondrial disorders is the critical increase in lipid peroxidation, which can disturb the integrity of cell membranes, increase non-selective membrane permeability, uncouple oxidative phosphorylation and ATP hydrolysis. Diterpene alkaloids is a new class of compounds with fascinating pharmacological properties. In particular, talatisamine and its derivatives have antiarrhythmic, hypotensive, N-cholinoblocking, curare-like, spasmolytic, anti-inflammatory, anesthetic, vasoprotective, anticancer and other effects7,10,13.
Since the mechanism of action of talatisamine and its derivative 14-O-benzoyltalatisamine on mitochondria has not been studied so far, in this study we have tested for the effect of these diterpene alkaloids on the LPO and mPTP state of rat liver and heart mitochondria.
Materials and Methods
Animals
White outbred male rats, weighing 180-220 g, were housed under standard laboratory conditions (20°C–24°C, natural light cycle and 65% humidity. Food and water were available ad libitum).
Isolation of intact mitochondria
Differential centrifugation was used to isolate intact rat liver mitochondria, as described previously14. Briefly, rat livers were homogenized in a following solution (in mM): sucrose – 250, Tris-HCl – 10, EDTA – 1, pH 7.4, and centrifuged at 1500´g for seven min (0-2°C). Sedimentation of intact mitochondria was achieved by supernatant centrifugation (6000´g) for 15 min (0-2°C). The supernatant was discarded, and the resulting pellet was re-suspended in a small volume (10 mitochondria:1 solution) of medium containing: sucrose – 250 mM, Tris-HCl – 10 mM and placed on ice.
Rat heart mitochondria were also isolated using differential centrifugation. Rat hearts were homogenized in a solution containing (in mM): sucrose – 300, Tris-HCl – 10, EDTA – 2, albumin 0.2%, pH 7.4. To isolate a sufficient amount of mitochondria, 5 or 6 rat hearts were isolated and placed in a chilled isolation medium. Laboratory equipment and petri dishes were stored in a freezer, and the procedure was carried out on ice. Rat hearts were cleaned of adipose tissue, blood and other large blood vessels in a chilled 0.9% KCl solution. The cleaned heart was washed again, dried using filter paper, the heart mass was determined and cut into small pieces using scissors. After that, the minced heart tissue was placed in a homogenizer and homogenized using a teflon pestle, poured with a 10:1 ratio of separation medium. Because the heart tissue is composed of transverse muscles, it was more homogenized than the liver tissue. The homogenate was centrifuged at 1500 g for 7 min (0-2°C). At this stage, heavy aggregates settle. In the next step, centrifugation was performed at 6000 g for 20 min (0-2°C). Isolated mitochondria were washed in medium without EDTA and albumin. Mitochondria isolation procedures were carried out under cold conditions. Mitochondria were kept on ice during the experiments. The Lowry method by Peterson15 was used to determine mitochondrial protein content.
A method for determining the state of the mitochondrial permeable pore
Mitochondrial folding kinetics were investigated by studying the state of the Ca2+-dependent permeable pore of the mitochondrial membrane. The optical density of swollen mitochondria was measured at 540 nm. The temperature of the incubation medium was 26°C, and the protein content was 0.3-0.4 mg/ml. To determine PTP permeability in mitochondria, the following incubation solution was used (mM): sucrose – 200, EGTA – 0.02, succinate – 5, Tris – 20, HEPES – 20, KH2PO4 – 1, rotenone – 0.002, oligomycin – 1 μg/ml, pH 7.4 12.
LPO measurement
The LPO process induced by Fe2+/ascorbate was recorded by photometric inhibition of liver mitochondrial swelling in the solution containing (mM): KCl – 125, Tris-HCl – 10, protein content 0.4 mg/ml, pH 7.4.
10 μM FeSO4 and 200 μM ascorbate were added to induce mitochondrial swelling. All experiments were carried out at the temperature of 24–26°C to preserve the integrity of mitochondria during incubation. The antioxidant properties of the tested drugs were measured by inhibition of Fe2+/ascorbate-induced mitochondrial swelling at 540 nm. This method was chosen because of the reported linear correlation between the LPO induced by the Fe2+/ascorbic acid and swelling of mitochondria 16.
The intensity of LPO was assessed by measuring the concentration of malondialdehyde (MDA). LPO was induced by adding 10 μM FeSO4 and 200 μM ascorbate to the incubation solution containing (in mM): 125 KCl, 10 Tris-HCl, with pH adjusted to 7.4. LPO products were separated with thiobarbituric acid (TBA), and the reaction was stopped by 0.220 ml of 70% (w/v) trichloroacetic acid (TCA). Next, the suspension containing mitochondria was centrifuged at 15000 g for 15 min. Then, 2 ml of supernatant was taken and poured in 1 ml of 75% TBА (w/v). 2 ml of H2O and 1 ml of TBА were added to the control solution. The mixture was incubated in a water bath at 300C for 30 min and optical density was detected again at 540 nm. The final concentration of MDA was calculated using the molar extinction coefficient (e=1.56×105 M-1 cm-1) according to the following formula17: nmol MDA/mg protein = D/ 1.56×30. 1 ml of incubation medium contained 0.3–0.4 mg of mitochondrial protein.
Drugs and Reagents
EDTA was from Sandoz, Switzerland, Tris-HCl was from Serva, Germany; KH2PO4, MgSO4, K2HPO4, succinate, sucrose, FeSO4, ascorbic acid, KCl, TBA and trichloroacetic acid were from Chemreaktivsnab, Russia; EGTA, HEPES, diazoxide, olygomycin, rotenone, ATP, CaCl2 were from Sigma, USA; and cyclosporine A was from Wako, China.
Diterpene alkaloid talatisamine (C24H39NO5) was isolated from Aconitum talassicum18. The structural formulas of talatisamine and its derivatives 14-O-benzoyltalatisamine (C31H43NO6) were drawn using the ChemOffice 2002 and Chem Draw Ultra 7.0 software (Figure. 1).
 |
Figure 1: Structural formulas of diterpene alkaloids |
Statistical Analysis
Statistical significance between groups was calculated based on the Student’s t-test using OriginPro 8.6 software (OriginLab, Northampton, Massachusetts, USA). P<0.05 was considered statistically significant.
Results and Discussion
In a number of pathologies, it has been found that the mPTP transitions to an open state, and the long duration of this process leads to a violation of cell function and structure. This, in turn, requires the correction of these pathological conditions with biologically active drugs. Therefore, it is necessary to search for local bioactive substances that inhibit mPTP and at the same time activate mechanisms that protect the cell from negative factors, namely ischemia, hypoxia, “overload” of Ca2+ ions, oxidative stress, etc. In order to study the membrane activity properties of the studied alkaloids, the effects of talatisamine and 14-O-benzoyltalatisamine diterpene alkaloids in different concentrations on the PTP state of the rat liver and heart mitochondria were studied in vitro. A concentration of 10 µM of Ca2+ ions was used as an inducer of mitochondrial swelling. Due to the addition of Ca2+ to the incubation medium, mitochondrial swelling and the transition of mPTP to a high-conductance state were observed. This condition was taken as 100% as a control (Figure 2A). In subsequent experiments, the effects of talatisamine and its derivative 14-O-benzoyltalatisamine alkaloids on the mPTP of rat liver and heart were studied. It was found that a 10 μM concentration of talatizamine alkaloid inhibited rat liver mPTP by 24.5±1.7% and rat heart mPTP by 18±2.1%, respectively, compared to control. When the concentration of talatisamine alkaloid was increased to 50, 100 and 200 μM incubation medium, the mPTP of rat liver and heart were 46±2,1%, 52.8±2,3%, 56.8±1,9%, and 31±3,0%, 39±1,7%, 44±1,5% were reliably inhibited. The half-maximum inhibitory concentration (IC50) of talatisamine was 78±3.6 μM in liver mitochondria (Figure 2A).
In order to predict which component of the PTP of the rat liver and heart mitochondria of the studied diterpene alkaloids affect, it was compared with the half-maximal inhibitory concentration of CsA (0.2 μM) in the experiments. During the studies, the effect of talatisamine on the Ca2+-dependent swelling of the rat liver and heart mitochondria in the presence of CsA in the incubation medium was investigated (Figure 2B). Similar to the above experiments, in this study, mitochondrial inhibition by 10 μM Ca2+ was taken as a control (100.0±3.4%). A concentration of 10 µM of this alkaloid significantly inhibited the mPTP of the rat liver and heart by 86.5±1.6% and 64.5±2.1%, respectively. Talatisamine concentrations of 50, 100, and 200 μM reliably inhibited rat liver mPTP by 88.4%, 90.6%, 94.6% and heart mPTP by 72%, 80%, 87.5%, respectively.
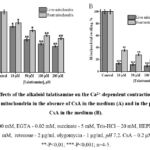 |
Figure 2: Effects of the alkaloid talatisamine on the Ca2+-dependent contraction of rat liver and heart mitochondria in the absence of CsA in the medium (A) and in the presence of CsA in the medium (B). |
To compare the effects of 14-O-benzoyltalatizamine, a derivative of talatisamine, on the mPTP of the rat liver and heart, concentrations from 1 μM to 200 μM were investigated (Figure 3A). Compared to the control, liver mPTP was reliably inhibited from 10% to 81% and heart mPTP from 3.6% to 71.5% at these concentrations. The half-maximum inhibitory concentration (IC50) of 14-O-benzoyltalatisamine was 38.6±3.2 and 85.6±3.5 µM in liver and heart mitochondria (Figure 3A).
It was found that the level of inhibition of this channel increased in the presence of the half-maximal inhibitory concentration of CsA in the medium. The effect of 14-O-benzoyltalatisamine on the Ca2+-dependent opening of the permeability pore of rat liver and heart mitochondria in the presence of CsA in the medium was studied. In the presence of 1, 10, 50, 100 and 200 µM concentrations of 14-O-benzoyltalatisamine in the medium, rat liver reliably inhibited mPTP by 32.2%, 90%, 92%, 95% and almost 100%, respectively, compared to the control. In the presence of the above concentrations of 14-O-benzoyltalatisamine in the medium, rat heart mPTP was reliably inhibited by 21%, 73.6%, 85%, 91%, and almost 96%, respectively, compared to the control (Figure 3B). Therefore, the increased inhibitory effect of 14-O-benzoyltalatisamine on mPTP in the presence of CsA in the incubation medium suggests that this alkaloid acts on this channel.
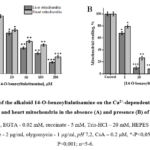 |
Figure 3: Effects of the alkaloid 14-O-benzoyltalatisamine on the Ca2+-dependent contraction of rat liver and heart mitochondria in the absence (A) and presence (B) of CsA. |
Talatisamine at a concentration of 200 μM inhibited liver mitochondrial mPTP by 56.8% compared to control, and heart mitochondrial mPTP by 44%. At this concentration, it was found that 14-O-benzoyltalatisamine inhibited liver mitochondrial mPTP by 81% and heart mitochondrial mPTP by 71.5% compared to the control. These alkaloids inhibited liver mitochondria more effectively than heart mitochondria. Talatisamine alkaloid was observed to be effective in the presence of CsA in the medium starting from a concentration of 10 μM.
This shows that the inhibitory properties of these alkaloids are more strongly manifested in the presence of the half-maximal inhibitory concentration of CsA in the medium. Hence, these alkaloids can activate cyclophilin D (CyP-D), a component of the PTP.
Thus, it was found that 14-O-benzoyltalatisamine has a more active effect on the Ca2+-dependent contraction of rat liver and heart mitochondria than talatisamine. Talatisamine had no effect at 1 μM concentration, but 14-O-benzoyltalatisamine had an effect starting at 1 μM concentration. The introduction of a benzoyl group into the talatisamine alkaloid may have increased its activity. By introducing a benzoyl group at the C-14 position, the relaxant activity of this alkaloid was found to increase significantly10.
Several toxic gases, heavy metal salts, chemical reagents, inducers in the environment have a toxic effect not only on living organisms at the cellular level, but also at the level of mitochondria, destroying the barrier function of the membrane19. In this condition, the lability of the mitochondrial membranes increases and the membrane potential decreases. As a result, membrane damage accelerates, and the formation of free radicals increases, which causes various pathologies20.
Oxidative stress, which disrupts normal cellular signaling mechanisms, plays an important role in cells and tissues damage21. Among the factors that damage the mitochondrial functional system, the accumulation of free radicals in the cell, as well as high concentrations of H2O2, accelerates the process of LPO in the mitochondrial membrane, causing the cellular antioxidant system to derail20.
There is an increasing demand for biologically active substances extracted from plants to overcome various pathological processes that cause LPO processes23. Detection of malondialdehyde (MDA), a product of LPO, which reacts with thiobarbituric acid in cells and tissues, is one of the ways to study LPO in biological systems24-25. During LPO, the amount of MDA directly represents the level of oxidative stress in cells and tissues. Therefore, in the next part of the study, the effect of diterpene alkaloids and their derivatives on the LPO process was investigated.
Addition of Fe2+/ascorbate to incubation medium during the experiment resulted in a dramatic increase in MDA formation in mitochondria (3.15 nmol MDA/mg protein), and this was taken as a control (100%) to the MDA formation process in rat liver mitochondria with the addition of LPO process inducers Fe2+/ascorbate to the incubation medium the effect of talatisamine alkaloid and its derivative 14-O-benzoyltalatisamine at different concentrations was studied (Figure 4). It was found that talatisamine alkaloid decreases MDA formation in rat liver mitochondria in a concentration-dependent manner, that is, MDA formation at a concentration of 50 μM was reduced by 8±2.9% compared to the control, and by 21.8±3.5% at a concentration of 100 μM, 150 μM and reduced by 33±3.7% and 40.5±3.2% at 200 μM concentrations, respectively. When 14-O-benzoyltalatisamine was also tested at these concentrations, it was found to reduce MDA formation by 25±3.4%, 41.5±3.7%, 70±2.9% and 81±3.4% compared to the control.
Thus, it was found that talatisamine alkaloid and its derivative at the studied concentrations, inducers of the LPO process, decrease the formation of MDA through the Fe2+/ascorbate system in a concentration-dependent manner. The half-maximal inhibitory concentration of LPO in the membranes of liver mitochondria for 14-O-benzoyltalatisamine was IC50=115.2±3.1 µM (Figure 4).
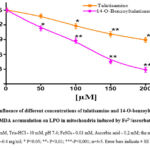 |
Figure 4: Influence of different concentrations of talatisamine and 14-O-benzoyltalatisamine, MDA accumulation on LPO in mitochondria induced by Fe2+/ascorbate. |
The products formed during the LPO process in cells and tissues in physiological and pathological conditions are activated as a protective signaling system in the body6. The LPO process leads to a disruption of the membrane structure, a change in permeability, a decrease in potential, separation of oxidative phosphorylation and ATP hydrolysis, and a decrease in the rate of electron transfer along the respiratory chain. Depending on the elimination of these processes by biologically active substances, it is possible to show the level of their activity. Similarly, biologically active substances extracted from plants are antioxidant compounds.
Therefore, according to the research results, it was found that 14-O-benzoylatisamine alkaloid is twice as active as talatisamine alkaloid in inhibiting the formation of MDA.
The products formed during the LPO process in cells and tissues in physiological and pathological conditions are activated as a protective signal system in the body25. It is known that the LPO process leads to a change in the permeability of biomembranes in mitochondria, a decrease in the membrane potential, the separation of oxidative phosphorylation processes, and the hydrolysis of ATP. Depending on the elimination of these processes by biologically active substances, it is possible to show the degree of their activity. Similarly, biologically active substances extracted from plants are antioxidant compounds.
Therefore, in subsequent studies, the antioxidant activity of diterpene alkaloids was also studied in the liver mitochondria model. In subsequent in vitro experiments, the antioxidant activity of the studied alkaloids was determined in the model of liver mitochondria contraction, where Fe2+/ascorbate-induced mitochondrial contraction amplitude (t-5min, ∆A540=0.330±0.012) was taken as 100%. In this study, the effects of talatisamine alkaloid on the liver mitochondrial LPO process were examined at concentrations of 10, 25, 50, 75 and 100 µM, 7.0±2.3%, 18.7±1.9%, 34.3±2.6% , was found to be inhibited by 49.9±2.1% and 62±1.8%. According to the results of the study, it was found that talatisamine had a relatively stronger inhibition at a concentration of 100 μM (Figure 5A). According to the results of the study, it was found that talatisamine had a relatively stronger inhibition at a concentration of 100 μM (Figure 5A). During the studies, the effect of 14-O-benzoyltalatisamine on the LPO process in the mitochondrial membrane was studied (Figure 5B). In contrast to talatisamine, 14-O-benzoyltalatisamine began to significantly affect the LPO process at a concentration of 10 μM. It was found to inhibit the LPO process at this concentration by 29±2.5% compared to the control. As the concentration of 14-O-benzoyltalatisamine in the environment increased, its inhibitory effect on LPO began to be strongly manifested. Incubation medium concentrations of 25, 50, 75, and 100 μM of 14-O-benzoyltalatizamine inhibited LPO by 38±2.1%, 59±2.9%, 73±1.7%, and 91±2.3%, respectively, compared to control was found to inhibit (Figure 5B).
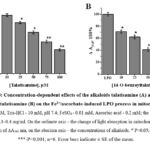 |
Figure 5: Concentration-dependent effects of the alkaloids talatisamine (A) and 14-O-benzoyltalatisamine (B) on the Fe2+/ascorbate-induced LPO process in mitochondria. |
A concentration of 100 µM of 14-O-benzoyltalatisamine alkaloid was found to maximally inhibit the LPO process in rat liver mitochondria membrane compared to the control. These mitochondrial suppression results were consistent with the results of experiments in which diterpenoid alkaloids reduced mitochondrial MDA formation. From the studies, it should be noted that the antioxidant activity of diterpenoid alkaloids – talatisamine and 14-O-benzoyltalatisamine showed relatively weaker antioxidant activity compared to 1-O-benzoylnapelline6.
Today, diterpene alkaloids are gaining importance as one of the classes of promising substances for the creation of new pharmacological drugs. Diterpene alkaloids have various physiological activities. For example: antiarrhythmic26, antispasmodic7, regenerative27, antimetastatic28, antidepressant29, antipyretic, anxiolytic, antioxidant, anti-inflammatory, wound healing30, relaxant31 effects were studied. A number of mechanisms of action of diterpene alkaloids have been studied, but mechanisms of action at the membrane level of a rat liver and heart mitochondria have not been studied.
In our research, it was found for the first time that talatisamine and its derivative 14-O-benzoyltalatisamine inhibit the Ca2+-dependent opening of the mitochondrial permeable pore in rat liver and heart, Fe2+/ascorbate-induced mitochondrial swelling (LPO process) and inhibit the formation of MDA in membranes. The antioxidant activity of talatisamine and 14-O-benzoyltalatisamine alkaloids and the inhibition of Ca2+-dependent opening of the mitochondrial pore open the prospects for the creation of new cytoprotective agents.
Mitochondria are the target organelles that control apoptosis, while the mPTP is particularly noteworthy as it performs an important regulatory function in cell life. Therefore, the mPTP is a target for various pharmaceutical agents and biologically active substances1. Since mPTP inhibitors have the property of stabilizing mitochondrial membranes, it is one of the urgent tasks to study the effect of biologically active compounds on the state of mPTP. A small increase in Ca2+ ions in mitochondria can cause a small amount of matrix degradation, which is observed with increased activity of oxidative phosphorylation and electron transport chain. An excess of Ca2+ ions in the mitochondria leads to the opening of the mPTP, which causes pathological strangulation32.
Currently, several pharmacological agents that modulate mPTP activity, such as CsA and adriamycin (doxorubicin), have undergone clinical trials. The classical inhibitor of mPTP, CsA, inhibits mitochondrial contraction caused by Ca2+ ions33 and switches the pore to the closed state. The inhibitory effect of CsA on mPTP prevents cardiomyocyte damage and inhibits palmitate-induced apoptosis in them. Perhaps, due to the inhibition of mPTP, CsA can prevent cell necrosis from the damaging effects of pH in ischemia-reperfusion, the toxicity of Ca2+-ionophores, and oxidative stress. The PTP-inhibiting property of CsA can be used for cell preservation in normothermic transplantation34. CsA has immunosuppressive properties and blocks transcription of cytokine genes by activated T-cells. CsA forms a complex with cyclophilin D by inhibiting the peptidylprolyl-cis-trans-isomerase activity of cyclophilin D35. Inhibition of cyclophilin D by major inhibitors of PTP prevents Ca2+-induced shock in liver mitochondria due to normalization of matrix Ca2+. CsA and its analogues have the effect of reducing the functional activity of mPTP, acting as an inhibitor by changing the value of peptidyl-prolyl cis-trans isomerase enzyme activity against cyclophilin D in mitochondria36.
The obtained results show that talatisamine and 14-O-benzoyltalatisamine alkaloid reliably inhibit mPTP. The inhibitory effect on rat liver and heart mPTP may be mediated by the activation of cyclophilin D. It can be explained that the introduction of the benzoyl group into the structure increases the inhibitory property of cyclophilin D and leads to the inhibition of mPTP. Thus, the above-mentioned alkaloids inhibit mPTP in vitro and have a stabilizing effect on the membranes of rat liver and heart mitochondria.
Thus, in our studies, it was found that the degree of mitochondrial swelling caused by the addition of a low concentration of Ca2+ was higher in liver mitochondria than in heart mitochondria. This indicates that liver tissues with high regenerative properties are less resistant to apoptosis caused by induced Ca2+. Studies in this area show that brain37-38 and heart39 mitochondria are more resistant to Ca2+-dependent permeability pore opening than liver cells. This process is explained by tissue specificity rather than the amount of Ca2+ exposed. When exposed to low concentrations of Ca2+, heart mitochondria are more resistant to the deleterious effects of Ca2+ than liver mitochondria, consistent with literature data39.
Thus, the obtained results confirm the existence of a tissue-specific feature of cell protection against the occurrence of Ca2+-dependent apoptosis and necrosis processes.
Pharmacological modulators that have an inhibitory effect on mPTP can be used in cases of hypoxia and ischemia, including in the treatment of cardiovascular diseases. On the contrary, those that modulate the open state of mPTP, that is, inducers that stimulate apoptosis, can be used in the treatment of oncological diseases.
Therefore, the researched diterpene alkaloids prevent the pathologies developed due to the dysfunction of mitochondria and have a corrective effect on them. In this process, diterpene alkaloids interact with receptors and signaling systems located in mitochondrial membranes. These alkaloids reduced the negative effects of Fe2+/ascorbate by protecting mitochondrial membranes to some extent from damage. These alkaloids lead to the recovery of the membrane potential, oxidative phosphorylation process and ATP synthesis as a result of the recovery of the membrane structure to a certain extent during the LPO process, and the acceleration of the speed of electron transmission along the respiratory chain.
The investigated alkaloids exhibited antioxidant properties by inhibiting Fe2+/ascorbate-induced mitochondrial suppression (LPO process) and MDA formation in membranes.
Conclusion
Studies have shown that 14-O-benzoyltalatisamine inhibits the Ca2+-dependent permeable pore of rat liver and heart mitochondria more strongly than the diterpenoid alkaloid talatisamine. The half-maximum inhibitory concentration (IC50) of talatisamine in liver mitochondria is 78±3.6 μM, the half-maximum inhibitory concentration (IC50) of 14-O-benzoyltalatisamine in liver mitochondria is 38.6±3.2 and in heart mitochondria was 85.6±3.5 μM. The sensitivity of the Ca2+-dependent conductive pore of liver mitochondria to diterpene alkaloids was found to be higher than that of heart mitochondria. These alkaloids exhibited antioxidant properties by inhibiting the LPO process and MDA formation in the mitochondrial membrane. Acylation of the hydroxyl group at the C-14 position of talatisamine in the presence of benzoyl chloride resulted in an increase in the overall activity of the alkaloid 14-O-benzoyltalatisamine. This diterpene alkaloid may be the basis for creating new effective cardio-, cyto- and hepatoprotective agents in the future.
Therefore, these diterpene alkaloids inhibit the Ca2+-dependent permeable pore, prevent apoptosis, and exert a stabilizing effect on mitochondrial membranes, and in the process of LPO, reduce the harmful effects of Fe2+/ascorbate and protect the membranes to some extent from damage.
Ethical considerations
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and within the guidelines of the local ethics committee‘s “Bioethical regulations for the use of laboratory animals in scientific research” (BEC/IBB-NUU/2019/02/22), the Institute of Biophysics and biochemistry at the National university of Uzbekistan named after Mirzo Ulugbek.
Acknowledgments
We would like to express our appreciation to the scientific team of the alkaloids laboratory of the Institute of Plant Substances Uzbek Academy of Sciences for kindly providing diterpene alkaloids for the research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Sources
There are no funding sources.
References
- Szewczyk A., Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev., 2002; 54: 101-127.
CrossRef - Di L.F., Canton M., Menabo R., Kaludercic N., Bernardi P. Mitochondria and cardioprotection // Heart Fail. Rev.,2007; 12: 249-260.
CrossRef - Szeto H.H. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusioninjury. Antioxid Redox Signal. 2008; 10: 60-619.
CrossRef - Smith R.A.J., Hartley R.C., Cochemé H.M., Murphy M.P. Mitochondrial pharmacology. Trends in Pharmacological Sciences. 2012; 33: 341-352.
CrossRef - Levchenkova O.S., Novikov V.E., Pogilova E.E. Mitochondrial pore as a pharmacological target. Smolensk state medical academy. 2014; 13: 24-33 (in Russian language).
- Muratova D.Kh., Ergashev N.A., Sobirov J.J., Kurbanov U.Kh., Asrarov M.I. Effects of diterpene alkaloids on lipid peroxidation in mitochondria. Nova Biotechnol Chim., 2021; 20: 1-8.
CrossRef - Dzhakhangirov F.N., Tursunkhodzhaeva F.M., Sultankhodzhaev M.N., Salimov B.T. Spasmolytic activity of diterpenoid alkaloids and their derivatives. Chem. Nat. Comp., 2013; 49: 702-706.
CrossRef - Ernazarov Z.M., Pozilov M.K., Asrarov M.I., Zhurakulov S.N. Effects of dihydroquercetin, 1-aryltetrahydroisoquinoline, and conjugate on the functional condition mitochondrial membrane of the rat liver// Nova Biotechnologica et Chimica. – 2023. – V.22.(1) – P.1-8.
CrossRef - Muratova D.Kh, Ergashev N.A, Shkinev A.V, Asrarov M.I, Kurbanov U.K. The effects of songorine on the activity of the ATP-dependent K+-channel and the state of the megapore of rat liver mitochondria. Exp. Clin. Phar., 2021; 84: 12-15 (in Russian language).
- Mirzayeva Y.T., Usmanov P.B. The role of the Na+/Ca2+ exchanger in the relaxation of the rat aorta caused by the alkaloid 14-O-benzoylthalatizamine. Acad. Scien. Rep. Uzb., 2016; 6: 73-77.
- Asrarov M.I., Shkinev A.V., Pozilov M.K., Ergashev N.A. The effect of the diterpenoid salvifolin on the state of the mitochondrial pore of the heart of rats with alloxan-induced diabetes. Problems of biological, medical and pharmaceutical chemistry. 2018; 21: 44-48 (in Russian language).
CrossRef - He L., Lemasters J.J. Heat shock suppresses the permeability transition in rat liver mitochondria. J.Biol.Chem., 2003; 278: 16755-16760.
CrossRef - Merve Demirbağ Karaali and Elanur Aydın Karataş. Investigation of the potential anticancer effects of napelline and talatisamine dirterpenes on experimental brain tumor models. Cytotechnology., 2020; 72: 569-578.
CrossRef - Schneider W.C., Hogeboom G.H. Cytochemical studies of mammalion tissues: the isolation of cell components by differential centrifugation. Cancer. Res., 1951; 11: 1-22.
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem., 1977; 83: 346-356.
CrossRef - Almeida A.M., Bertoncini C.R. Mitochondrial DNA damage associated with lipid peroxidation of the mitochondrial membrane induced by Fe2+-citrate. An. Acad. Bras. Cienc., 2006; 78: 505-514.
CrossRef - Devienne K.F., Cálgaro-Helena A., Dorta D.J., Prado I.M.R., Raddi M.S.G., Vilegas W., Uyemura S.A., Santos A.C., Curti C. Antioxidant activity of isocoumarins isolated from Paepalanthus bromelioides on mitochondria. Phytochemistry. 2007; 68: 1075-1080.
CrossRef - Eshmatov Zh.M., Sultankhodzhaev M.N., and Nigmatullaev A.M.. Accumulation dynamics of alkaloids in Aconitum talassicum. Chem. Nat. Comp., 2011; 47: 149-150.
CrossRef - Belyaeva E.A., Glazunov V.V., Korotkov S.M. Cyclosporin A – sensitive transition permeability pore is Cd2+-induced dysfunction of isolated rat liver mitochondria: doubts no more. Arch Biochem Biophys., 2002; 405: 252-264.
CrossRef - Shilov A.M., Melnik M.V., Chubarov M.V. Complex antioxidants in the prevention and treatment of diseases of the cardiovascular system. Russian medical news. 2004; 4: 45-49 (in Russian language).
- Lankin V.Z., Konovalova G.G., Tikhaze A.K., Nedosugova L.V. Influence of natural dicarbonyls on the activity of antioxidant enzymes in vitro and in vivo. Biomedical Chemistry. 2012; 58: 727-736.
CrossRef - Dubinina E.E. The role of reactive oxygen species as signal molecules in tissue metabolism under conditions of oxidative stress. Voprosy med. chemistry., 2001; 47: 561-581 (in Russian language).
- Murilo P., Tatiana B.A., Lilian C.P., Mariana F.F.B., Raíssa S.F., Daniel J.D Baicalein can be a great antioxidant, but it can impair mitochondrial bioenergetics and cause cytotoxicity at high concentrations. Applied Research in Toxicology. 2015; 1: 9-18.
- Ikatsu H., Nakajima T., Murayama N., Korenaga T. Flow-injection analysis for malondialdehyde in plasma with the thiobarbituric acid reaction. Clinical chemistry. 1992; 38: 2061-2065.
CrossRef - Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity.2014; 1-31.
CrossRef - Dzhakhangirov F.N., Sultankhodzhaev M.N., Tashkhodzhaev B., Salimov B.T. Diterpenoid alkaloids as a new class of antiarrhythmic agents. Structure-activity relationship // Chem. Nat. Compd. – 1997. – 33.P.190-202.
CrossRef - Nesterova Yu.V., Povetieva T.N., Suslov N.I., et al. Regeneratory Characteristics of Extract and Isolated Diterpene Alkaloids of Aconitum baicalense // Bull. Exp. Biol. Med. – 2012. – Vol.152, №4. P.439-443.
CrossRef - Kruczynski A., Poli M., Dossi R., Chazottes E., Berrichon G., Ricome C., Giavazzi R., Hill B.T., Taraboletti G. Anti-angiogenic, vascular-disrupting and anti-metastatic activities of vinflunine, the latest vinca alkaloid in clinical development // Eur. J. Cancer. – 2006. – 42. P.2821-2832.
CrossRef - Nesterova Y.V., Povetieva T.N., Suslov N.I., Semenov A.A., Pushkarskiy S.V. Antidepressant activity of diterpene alkaloids of Aconitum baicalense Turcz // Bull. Exp. Biol. Med. – 2011. – 151. P.425-428.
CrossRef - Khan H., Nabavi S.M., Sureda A., Mehterov N., Gulei D., Beridan-Neagoe I., Taniguchi H., Atanasov A.G. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum // Eur. J. Med. Chem. – 2018. – 153. P.29-33.
CrossRef - Yesimbetov A.T., Zaripov A.A., Begdullayeva G.S et al Effects of diterpene alkaloids zongorin and 1–O–benzoylnapellin on соntractile activity of rat aorta smooth muscle // Central Asia Journal of Medicine. – 2019. – 19. P.35-44.
- Szabo I., Zoratti M Mitochondrial channels: ion fluxes and more // Physiol Rev. –2014. – 94. P.519-608.
CrossRef - Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress // Biochemical Journal. – 1988. – 255. P.357–360.
- Bernardi P., Lisa F. D. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection // J Mol Cell Cardiol. – 2015. – 78. P.100–106.
CrossRef - Nicolli A., Basso E., Petronilli V., Wenger R.M & Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel // J. Biol. Chem. – 1996. –271.P.2185–2192.
CrossRef - Lemasters J.J., Theruvath T.P, Zhong Z. et al. Mitochondrial calcium and the permeability transition in cell death // Biochim Biophys Acta. – 2009. – Vol. 1787(11). P.1395-1401.
CrossRef - Kobayashi T., Kuroda S., Tada M., Houkin K., Iwasaki Y., Abe H. Calcium-induced mitochondrial swelling and cytochrome c release in the brain: its biochemical characteristics and implication in ischemic neuronal injury. Brain Res., 2003; 960: 62–70.
CrossRef - Panov A, Dikalov S, Shalbuyeva N, et al. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol., 2007; 292: 708–718.
CrossRef - Endlicher R, Krivakova P, Lotkova H, Milerova M, Drahota Z, Červinkova Z. Tissue specific sensitivity of mitochondrial permeability transition pore to Ca2+ions. Acta medica (Hradec Králové). 2009; 52: 69–72.
CrossRef







