Amberkar Mohanbabu Vittalrao1 , Linthoinganbi laishram2, Meena Kumari K2*
, Linthoinganbi laishram2, Meena Kumari K2* , Mohandas Rao KG3
, Mohandas Rao KG3
1Department of Pharmacology, The Oxford Medical College and Research Hospital,Bengaluru , Karnataka, India.
2Department of Pharmacology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3Department of Anatomy, Department of Basic Medical Sciences, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Corresponcing Author E-mail:meena.kumari@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2778
Abstract
Sleep plays a vital role to help in normal biological functions that are required for normal cognitive functioning. This study was done to determine the cognition-modulating effects of coenzyme Q10 (CoQ10), ramipril, and vinpocetine on REM sleep-deprived acute insomniac rat models. A total of Forty-eight albino rats were divided into eight groups (Gr) (n=6).). Gr. 1 was REM control and Gr. 2 was REM sleep-deprived rats treated with water. Gr. 3 to Gr.8 - REM sleep-deprived rats were administered corn oil, donepezil, vinpocetine, coenzyme Q10+corn oil, ramipril, and (coenzyme Q10 + corn oil + ramipril) respectively. Except for the control Gr 1, REM sleep deprivation was induced in Gr. 2 and 8 daily for 7 days. All the rats were subjected to a Morris water maze (MWM) to test the navigation memory dysfunction after 7 days of acute insomnia. The rats were deprived of REM sleep by using a modified multiple platform method. The body weight of the animals was measured on day 1 and day 7. On day 1 and Day 2 acquisition trials, all groups of rats showed comparable latency time required to reach the hidden platform. However, on day 3 and Day 4, rats treated with coenzyme Q10, ramipril, and the combination (CoQ10+ Ramipril) showed a significant decrease in latency time (p<0.01). In the probe trial, sleep-deprived rats showed a significant decrease (p<0.001) in the percentage of time spent in the target quadrant as compared to the REM control. However, there was a significant increase in the percentage time spent in CoQ10, ramipril, the combination (CoQ10+ ramipril), donepezil, and vinpocetine as compared to sleep-deprived rats (p<0.05). At the end of day 7 of insomnia, when the body weight of rats was compared with day 1, there was a significant decrease in weight gain was seen with the sleep-deprived rats treated with corn oil, ramipril, vinpocetine, CoQ10, and (CoQ10+ramipril) (p<0.05). The present study shows that coenzyme Q10, ramipril, and their combination improve sleep deprivation induced cognition impairment.
Keywords
Acquisition Trial; Morris Water Maze; Probe Trial
Download this article as:| Copy the following to cite this article: Vittalrao A. M, laishram L, Kumari K. M, Rao K. G. M. Cognitive Enhancing Activities of Coenzyme Q10, Ramipril, and Vinpocetine Through Modulating Neuroinflammatory Response and Oxidative Damage Inflicted by Acute REM Sleep Deprivation in Rats. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Vittalrao A. M, laishram L, Kumari K. M, Rao K. G. M. Cognitive Enhancing Activities of Coenzyme Q10, Ramipril, and Vinpocetine Through Modulating Neuroinflammatory Response and Oxidative Damage Inflicted by Acute REM Sleep Deprivation in Rats. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3Rie1tW |
Introduction
Sleep is one of the fundamental conditions for quality of life¹. Sleep is very crucial in the formation and consolidation of memory.
Many sleep disorders lead to memory problems. Impairment of cognition is seen even after a single total night of sleep deprivation and can cause industrial, and transportation accidents and medical errors. REM and NREM sleep are the two phases of the sleep cycle. REM sleep has been reported mostly to be linked with memory and spatial learning by enhancing hippocampal consolidation2,3 and can also cause hyperphagia and weight loss4
Various neuropsychological studies and numerous behavioral studies on animals, such as the Morris water maze have been shown to cause cognition and memory deficits due to REM sleep deprivation2. With the increase in financial and social needs of the ‘modern global 24/7 society, more people work outside their regular working time and ignore sleep.
As per Reimund’s free radical flux theory, sleep promotes endogenous anti-oxidant mechanisms activities and decreases the production of free radicals in the brain3. Thus, sleep essentially plays the role of a facilitator of antioxidants. Sleep deprivation has been proven to have a pro-inflammatory effect and its effects extend to the brain where cytokines like IL-1ß, IL-6, and TNF-à are present5,6.
Ramipril is a potent ACE (ACE Ⅰ). Angiotensin Ⅱ is reduced by ACE Ⅰ in the brain which inhibits the release of Acetylcholine and has been proven to improve cognition7.
Coenzyme Q10, a quinone compound is an electron acceptor in the ETC in the mitochondria membranes and has a central role in the chemi- osmotic synthesis. Coenzyme Q10 plays an important role in the process of cellular energy supply via oxidative phosphorylation within mitochondria. Treatment with coenzyme Q10 has been proven to ameliorate cognitive deficits by modulating mitochondrial function 8.
Vinpocetine, a nootropic alkaloid synthesized from periwinkle acts as a neurotropic agent and reduces damage to the brain from ischemia, stroke, and trauma. It improves memory and increases blood flow and metabolism.
This study has been conducted to study the effect of Coenzyme Q10, Ramipril and vinpocetine on cognition enhancement in REM sleep-deprived insomniac rats.
Materials and Method
Animals
Forty-eight male Albino Wistar rats were selected for the study, which is locally bred in the Central Animal House Research Facility
To avoid bias due to sex differences. In the Morris water maze, Male Wistar rats were preferred. Three animals each were housed in a 41cm×28cm×14cm propylene cage. The animals are 8-10 weeks old and 200-250g weight, housed under controlled temperature conditions of 23±2ºC, the humidity of 50±5%, and light and dark cycle of 10-14 hours respectively. Each animal was housed separately in polypropylene cages containing sterile paddy husks (procured locally) as bedding throughout the study. Free access to sterile food (VRK nutritional solutions, Pune, India) and water were given ad libitum. The study has been carried out after it has been approved by Institutional Animal Ethics Committee (IAEC/KMC/40/2021 dated 06.03.2021).
The marking of animals for identification was done using picric acid. The animals were marked on the head, body, and tail using a cotton swab dipped in dilute picric acid. The markings were done accordingly as described in the experimental manual.
Drugs and Dosage
Ramipril (Lupin Ltd.), donepezil ( Intas Pharmaceuticals Ltd.), Coenzyme Q10 (Bulk Supplements), and Vinpocetine (Vivid Biotek Pvt Ltd.) were purchased from a local community pharmacy and were of analytical grade.
Table 1: showing various groups, drugs dose, and routes used
|
Group |
Drugs, Dose And Route |
Duration |
|
1 |
REM sleep control animals |
1 week |
|
2 |
REM sleep deprived |
1 week |
|
3 |
REM sleep deprived + 2ml corn oil p.o |
1 week |
|
4 |
REM sleep deprived with donepezil 10mg/kg/day p.o |
1 week |
|
5 |
REM sleep deprived with vinpocetine 2mg/kg/day |
1 week |
|
6 |
REM sleep deprived with coenzymeQ10 10mg/kg/day dissolved in corn oil p.o |
1 week |
|
7 |
REM sleep deprived with ramipril 10mg/kg/day p.o |
1 week |
|
8 |
REM sleep deprived with coenzyme Q10 10mg/kg/day dissolved in corn oil + ramipril 10 mg/kg /day p.o |
1 week |
Coenzyme Q10, an electron acceptor in the ETC, and ramipril, an ACE inhibitor are used in this study. Doses of Coenzyme Q10(10mg/kg), and ramipril (10 mg/kg) were taken from previous studies. Coenzyme Q10 50mg was dissolved in 30 ml corn oil and ramipril 50mg was dissolved in 30 ml distilled water.
Experimental groups
Forty-eight animals were divided into 8 groups equally
Experimental design
Prior to experimentation, the animals were allowed to acclimatize to the laboratory condition. Then, they were housed under a standard condition of 12h light/dark cycle and were given standard rat feed and water ad libitum.
REM sleep control
REM sleep control animals were evaluated using a modified multiple platform method. In this method, the control animals(n=6) to be studied were placed on a platform of diameter 6.5cm which is larger compared to the body size of the animal. This allows the relaxed position for REM sleep. This is done to exclude stress due to isolation.
REM sleep deprivation procedure
A modified multiple platform is used for REM sleep deprivation which is a modified version of the Inverted flower pot model. Here, the animals (REM sleep-deprived groups and treatment groups) are placed on a small platform of diameter 5.5cm surrounded by water. This platform is small compared to the size of the body of the animal. REM sleep was disturbed for 24hrs every day for 1 week. The animals received the drugs in above table 1 for 1 week. This model is based on the loss of muscle tone which accompanies REM sleep which is followed by the falling of the animals into the water. After sleep deprivation, the animal is subjected to learning and memory by the Morris water maze apparatus.
Spatial learning and memory Assessment
Morris Water Maze
Morris water maze is used for the evaluation of learning and memory. The apparatus consists of a round tank (165cm × 135 cm). The tank is filled with water and maintained at 25ºC. Milk powder was added to make it non-transparent. The tank was divided into four equal zones (Z1, Z2, Z3, Z4) and the escape platform was placed in one of the zones submersed in water (water level 1cm below the platform). As a cue, Black and White boards were placed. Throughout the learning session, the extra maze cue and platform were placed at a fixed position 9.
It consists of 2 phases-
Spatial task acquisition phase
Each animal was put through 4 consecutive trials with 2 min intervals, during which the study animal could leave from the platform and could remain there for 20secs for 4 consecutive days. Animals will then undergo a series of trials using any of the start positions (Z1, Z2, Z3, Z4) randomly. If the animals are unable to locate the platform even after 90secs then they are guided to the platform. The time taken by each animal to reach the platform was counted.
A preliminary test was conducted to familiarize the animals with the apparatus.
b)Probe trial
On the 5th day, the platform/island is removed and the animal is allowed to traverse the pool for 90 sec to assess reference memory. Time spent in the target zone was measured.
Biochemical Analysis- for GSH,MDA and acetylcholinesterase activity was done
Statistical Analysis
One-way ANOVA (Analysis of variance) followed by post hoc analysis using Tukey’s test was used for data analysis. Student’s paired t-test is used to compare the result before and after. The level of significance for any measure was set at p<0.05 at a confidence level of 95%. Mean ± standard error of mean was used for data expression.
Results
Morris water maze results
Acquisition trials
In day 1 and day 2 acquisition trials, all groups were comparable to the time required to reach the hidden platform. On day 3 and Day 4, there was a significant decrease (p<0.001) in latency in coenzyme Q10 and ramipril-treated rats. There was also a significant decrease in latency (p<0.001) in the combination group SD +CoQ10+ Ramipril. [Table 2]
Probe trial
The percentage of time spent in the target zone was calculated:
In the probe trial, as compared to the control, sleep-deprived rats showed a significant decrease (p<0.001) in the percentage spent in the target quadrant. Whereas there was a significant increase in percentage time spent in CoQ10, ramipril, and the combination of these two, donepezil and Vinpocetine as compared to sleep-deprived rats. (p<0.01).Combination of CoQ10 and Ramipril showed significant increase in percentage time spent. [Table 3]
Weight of animal
As compared to day 1, a significant decrease (p<0.05) in weight was seen in the sleep-deprived group, sleep-deprived (SD) with Corn oil compared to control. SD with CoQ10 and its combination with ramipril showed a significant increase in weight.[Table 4]
Table 2: Effect of CoQ10 and Ramipril on REM sleep deprivation induced alteration in latency in MWM.
|
Groups |
Acquisition trials-Latency in secs Mean±SEM |
|||
|
Day1 |
Day2 |
Day3 |
Day4 |
|
|
Control |
11.45±0.47 |
11.95±0.44 |
8.52±0.42 |
6.61±0.25 |
|
Sleep deprived (SD) |
23.97±3.16 |
12.33±0.39 |
11.44±0.49 |
12.49±0.28⁎ |
|
SD + Corn oil(CO) |
12.72±0.70 |
11.70±0.43 |
5.40±0.40 |
3.23±0.34a |
|
SD+Donepezil |
16.90±0.69 |
12.41±0.52 |
6.76±0.24 |
3.17±0.17a |
|
SD+Vinpocetine |
15.69±0.51 |
11.82±0.38 |
5.42±0.40 |
2.22±0.13a |
|
SD+CoQ10 |
17.64±0.69 |
12.21±0.44 |
6.31±0.30 |
2.54±0.18a |
|
SD+Ramipril |
20.93±0.42 |
12.96±0.55 |
7.88±0.22 |
2.69±0.17 |
|
SD+CoQ10+R |
16.93±0.42 |
12.10±0.53 |
5.75±0.95 |
2.25±0.12a |
One way ANOVA followed by Tukey’s test ⁎p<0.001 vs control, ap<0.001 vs SD
Table 3: Effect of CoQ10, Ramipril, and Vinpocetine on time spent in target zone in MWM.
|
Groups |
Percentage time spent in Target zone Mean ± SEM (%) |
|
Control |
17.03 ± 1.23 |
|
Sleep deprived(SD) |
14.4 ± 2.41⁎b |
|
SD+Cornoil |
10.50 ± 1.14*b |
|
SD+Donezepil |
15.46±1.25 |
|
SD+Vinpocetine |
17.14 ± 0.43a |
|
SD+CoQ10 |
26.64 ± 3.25a |
|
SD+Ramipril |
24.93±1.34a |
|
SD+CoQ10+ Ramipril |
18.96±1.47*b |
One-way ANOVA followed by Tukey,s test
*p<0.01 vs Control, ap<0.01 vs SD, bp<0.01 vs Donepezil.
Table 4: Effect of CoQ10, Ramipril, and combination on body weight.
|
Groups |
Body weight at Day1 (Mean±SEM) |
Body weight at Day7 (Mean±SEM) |
|
Control |
239.16 ± 8.55 |
257.83 ± 5.03 |
|
SD |
245.50 ± 5.25 |
205.00 ± 5.89b |
|
SD + Corn oil |
230.00 ± 6.75 |
253.12 ± 7.59b |
|
SD+ Donepezil |
208.33 ± 11.25 |
205.66 ± 5.89* |
|
SD+Vinpocetine |
245.33 ± 8.11 |
236.33 ± 9.03 |
|
SD+CoQ10 |
239.00 ± 2.92 |
232.66 ± 3.29*a |
|
SD+Ramipril |
194.00 ± 10.74 |
186.50 ±9.27*a |
|
SD+CoQ10+Ramipril |
216.16 ± 5.78 |
210.83 ± 6.13* |
paired t- test *p<0.001 vs SD,a p<0.05 vs Control , bp<0.05 vs donepezil.
Histopathology
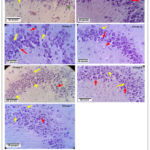 |
Figure 1: Legend: Representative photomicrographs of Cresyl violet stained hippocampal CA3 region of group 1 (control), group 2 (acute sleep deprivation), group 3 (Donepezil treated). |
Representative photomicrographs of Cresyl violet stained hippocampal CA3 region of group 1 (control), group 2 (acute sleep deprivation), group 3 (Donepezil treated) group 4 (Vinpocetine treated), group 5 (Coenzyme Q10 treated), group 6 (Ramipril treated) and group 7 (Ramipril + Coenzyme Q10 treated) [Magnification: 40×10 X]
It can be noted that the number of degenerating pyknotic neurons (indicated by red arrow) which look characteristically flame-shaped (shown in red circle) are relatively more in Group 2 when compared to those of other groups. A slight increase in the healthy neurons (indicated by the yellow arrow) can be seen in groups 4, 6, and 7 when compared to group 2. However, group 5 showed a marked reduction in the number of flame-shaped pyknotic cells and a greater number of normal healthy neurons when compared to those of group 2.
Qualitative analysis of Cresyl violet stained pyramidal neurons of hippocampal CA3 region
Cresyl violet stained pyramidal neuronal cell bodies of the hippocampal CA3 region of rats of the control group (group 1) showed normal healthy neurons (indicated by yellow arrow ) with a healthy-looking cell membrane, clear cytoplasm, and prominent nucleus. However, the Cresyl violet stained hippocampal CA3 region of the acute sleep-deprived group (Group 2) showed many degenerating, flame-shaped, pyknotic cell bodies of pyramidal neurons (indicated by red arrow). The flame-shaped cells which look deeply basophilic are indicative of karyopyknosis of the neurons of the hippocampus. It is noted that there is a marked increase in the healthy neurons and a decrease in the flame-shaped, degenerating pyramidal neuronal cell bodies in the hippocampal CA3 region of rats belonging to groups 4, 6, and 7 when compared to those of groups 2. However, group 5 showed a substantial increase in the number of healthy neurons and very few or negligible numbers of flame-shaped, degenerating pyramidal neuronal cell bodies in the hippocampal CA3 region when compared to those of group 2.
Dentate gyrus
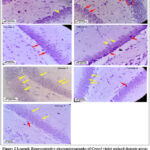 |
Figure 2 Legend: Representative photomicrographs of Cresyl violet stained dentate gyrus of group 1 (control), group 2 (acute sleep deprivation), group 3 (Donepezil treated) |
Representative photomicrographs of Cresyl violet stained dentate gyrus of group 1 (control), group 2 (acute sleep deprivation), group 3 (Donepezil treated) group 4 (Vinpocetine treated), group 5 (Coenzyme Q10 treated), group 6 (Ramipril treated) and group 7 (Ramipril + Coenzyme Q10 treated) rats showing the cell bodies of the neurons. [Magnification: 40×10 X]
It can be noted that the number of degenerating pyknotic neurons (indicated by red arrow) with characteristic flame-shape (shown in red circle) are relatively more in Group 2 when compared to those of other groups. A slight increase in the healthy neurons (indicated by the yellow arrow) can be seen in Groups 4, 6, and 7 when compared to Group 2. The number of flame-shaped pyknotic cells is markedly reduced in group 5 when compared to those of group 2.
Qualitative histopathological evaluation of Cresyl violet stained neurons of the dentate gyrus:
Cresyl violet stained dentate gyrus neurons of rats belonging to the control group (group 1) showed normal healthy cell bodies (indicated by yellow arrow ). Their plasma membrane looked healthy with distinct edges. Cytoplasm was clear and the nucleus was easily recognizable. The general arrangement of neurons also appeared normal. However, Cresyl violet stained dentate gyrus neurons of the acute sleep-deprived group (Group 2) showed many pyknotic cell bodies of neurons with characteristic flame‑shapes. (encircled and indicated by the red arrow in the figure 2). Deeply basophilic flame-shaped cells indicate the degenerating nature of these neurons which could be due to karyopyknosis. It is noted that there is a marked increase in the healthy neurons and a decrease in the flame-shaped, degenerating neuronal cells of the dentate gyrus of rats belonging to groups 4, 6, and 7 when compared to that of group 2. But, group 5 showed a substantial increase in the number of healthy neurons and very few or negligible numbers of flame-shaped, degenerating neurons of dentate gyrus when compared to those of group 2.
Table 5: Biochemical Analysis
|
Groups |
GSH (µM/mg/tissue) |
MDA (nM/g/tissue) |
AChE (µM/min/g tissue) |
|
Control |
0.027±0.01 |
0.01367±0.01 |
2.346±0.01 |
|
SD |
0.01333±0.01a |
0.02817±0.01a |
3.848±0.02*a |
|
SD + Corn oil |
0.01416±0.01a |
0.02513±0.01a |
3.446±0.01*a |
|
SD+ Donepezil |
0.01567±0.02*,** |
0.01283±0.01** |
2.351±0.02** |
|
SD+Vinpocetine |
0.01817±0.01a |
0.01533±0.04*,** |
3.861±0.03** |
|
SD+CoQ10 |
0.02517±0.01a |
0.01285±0.01 |
2.835±0.02** |
|
SD+Ramipril |
0.01417±0.01a |
0.01156±0.01** |
2.836±0.01** |
|
SD+CoQ10+Ramipril |
0.025±0.03a |
0.01455±0.01 |
2.834±0.03** |
One-way ANOVA followed by Tukey,s test
*vs control,**vs SD, a vs Donepezil
There was a significant decrease in GSH levels in the REM sleep-deprived donepezil group in comparison to the control (p˂0.05) and a significant increase (p˂0.05) in levels was seen in the REM sleep-deprived group alone. (Table 5)
There was a significant decrease in MDA levels in the REM sleep deprived -donepezil group, vinpocetine group, and ramipril group in comparison to the REM sleep-deprived group alone (p˂0.05). (Table 5)
The brain acetylcholinesterase level is significantly increased (p˂0.05) in REM sleep deprived alone and along with the corn oil group in comparison to the control. Rats REM sleep deprived treated with donepezil, vinpocetine, coenzyme Q10, ramipril, and coenzyme Q10 + ramipril groups showed a significant decrease in acetylcholinesterase level in comparison to REM sleep deprived alone. (Table 5)
Discussion
In this study, coenzyme Q10, ramipril, and its combination improve the memory impairment caused by acute sleep deprivation (total sleep deprivation -7 days) with everyday dosing. Spatial learning and memory were assessed using the Morris water maze test. The data showed that acute REM sleep deprivation for 7 days impairs learning and memory.
In the current study, sleep deprivation was induced using a modified multiple platform model 10. It has the same principle as the flower pot model i.e. loss of muscle tone during REM sleep. The data from the current study shows that REM sleep deprivation for 7 days impairs learning and memory. From various experiments performed by different researchers earlier, we can conclude that REM sleep deprivation is associated with cognition and that it causes cognitive impairment. From previous studies, it has been seen that sleep deprivation using multiple platform models impairs the acquisition rate as well as the ability to remember the position in the probe trial 11.
The multiple platform model still has some drawbacks like it can be affected by stress and anxiety12.
Studies have shown that RAS is involved in memory and cognition 13. All mammalian brains, there is a role of RAAS with Angiotensin II plays an important neuromodulator role. Studies have shown ACEI enhancing retention performance in both short-term and long-term memory behavioral tasks 14. Treatment with ACEI prevented impairment of memory, oxidative stress, and cholinergic dysfunction in rats.
ACEI has been proven to improve memory and cognition in previous studies15,16. The role of Ramipril on learning and memory is evaluated in this study. Ramipril 10mg/kg significantly ameliorates cognition impairment caused by sleep deprivation. The antioxidant activity with ramipril, an ACE inhibitor can be explained by the blockade of reactive oxygen species by a RAS blocker involving the angiotensin II signaling pathways, which results in the inhibition of reactive oxygen species 17.
Due to oxidative stress in the brain, superoxide anion, hydroxyl radical and hydrogen peroxide are generated which act on PUFA & cause lipid peroxidation. Oxidative free radical scavenging enzymes like GSH, SOD, and catalase reduce oxidative stress in the brain. The current study displays an increase in MDA and a decrease in total GSH following REM sleep deprivation suggesting free radical generation
Alzheimer’s disease patients there is an increase in cerebral ACE and angiotensin II and they promote neuroinflammatory cytokines, reduce acetylcholine release and decrease cerebral blood flow 18.
Coenzyme Q10 is a powerful antioxidant, protecting cell membranes from free radical induced oxidative damage. It also affects the expression of genes involved in the inflammatory process. Since Coenzyme Q10 is an electron acceptor in ETC, produces ATP and hence is related to cognition and cognition decline. In this study, Coenzyme Q10 10mg /kg significantly repairs cognition impairment. In Alzheimer’s disease, one of the important factors in pathogenesis is oxidative stress. So, Coenzyme Q10 offers some protection due to its antioxidant effect 19 and increasing Coenzyme Q10 concentrations in the mitochondria of brain cells and facilitating the memory and learning processes in rats. In aging mitochondrial impairment and oxidative stress are important, they are also involved in the pathogenesis of Alzheimer’s disease-inflammation is caused by excessive production of ROS and contributes to the pathogenesis of Alzheimer’s disease. Mitochondrial dysfunction has been associated with the onset and development of neurodegenerative disease 20. Beneficial therapeutic effects of coenzyme Q10 have been seen in oxidative stress-mediated neurodegenerative disorders 21.
A previous study by Sandhir et al presented that rats treated with Coenzyme 10 were able to locate the hidden platform in the Morris water maze test, hence an improvement in cognitive function 22.
Vinpocetine can cross the blood-brain barrier and enter the brain after oral or intravenous administration. It improves blood flow by cerebral vasodilator action and also increases cerebral metabolism by increasing oxygen and glucose uptake and stimulating neuronal ATP production. Also acts as an antioxidant and prevents the neurotoxic increase in sodium and calcium levels 23. Vinpocetine enhances spatial memory by modulating the cholinergic system 24. Gupta et al. found that treatment of vinpocetine reduced chronic cerebral hypoperfusion (CCH).ROS have been known to play the role in ischemic injury and neurodegenerative disorders and their findings indicate that vinpocetine enhances spatial memory through an antioxidant mechanism, the modulation of cholinergic functions, and the prevention of neuronal cell damage 25.
The cognitive enhancement is due to PDE type 1 inhibition, which increases CAMP and CGMP levels. These cyclic nucleotides in turn activate a series of kinases that phosphorylate the transcription factors (CREB) and SRF, leading to the expression of plasticity-related genes.
Accumulation of the amyloid-β protein leads to an up-regulation of interleukins and TNF α. Vinpocetine’s anti-inflammatory properties may have a protective role in Alzheimer’s disease.
Conclusion
In this study, Coenzyme Q10, ramipril, and its combination are seen to improve learning and memory in acute sleep deprivation induced cognition impairment by behavioral, histopathological, and biochemical tests performed.
Acknowledgement
I am thankful to the faculty of Central Animal Research Facility for helping in the conduct of the project ,Department of Biochemistry for chemicals for analysis and Ms. Shravya C for helping in the statistical analysis.
Conflict of Interest
There is no conflict of interest.
Funding Source
Received Postgraduate thesis grant from our Institute bearing number PGR557.
References
- Premack D. Human and animal cognition: Continuity and discontinuity. Proc Natl Acad Sci. 2007;104(35):13861-7.
CrossRef - Thamaraiselvi K, Mathangi DC, Subhashini AS. Effect of increase in duration of REM sleep deprivation on lipid peroxidation. Int J Biol Med Res. 2012;3(2):1754-9.
- Reimund E. The free radical flux theory of sleep. Med hypotheses. 1994 ;43(4):231-3.
CrossRef - Stickgold R, Malia A, Maguire D, Roddenberry D, O’Connor M. Replaying the game: hypnagogic images in normals and amnesics. Science. 2000 ;290(5490):350-3.
CrossRef - Aleisa AM, Alzoubi KH, Alkadhi KA. Post-learning REM sleep deprivation impairs long-term memory: reversal by acute nicotine treatment. Neurosci Lett. 2011 ;499(1):28-31.
CrossRef - Sherwood L. Human physiology: from cells to systems. Cengage learning; 2015.
- Laverman GD, Remuzzi G, Ruggenenti P. ACE inhibition versus angiotensin receptor blockade: which is better for renal and cardiovascular protection?. J Am Soc Nephrol. 2004 ;15(1 suppl):S64-70.
CrossRef - Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q 10 as a potential treatment. J Bioenerg Biomembr. 2004;36(4 SPEC.ISS.):381-386.
CrossRef - Morris R. (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods;11(1):47-60.
CrossRef - Wright JW, Reichert JR, Davis CJ, Harding JW. Neural plasticity and the brain renin–angiotensin system. Neurosci Biobehav Rev. 2002 ;26(5):529-52.
CrossRef - Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC. Angiotensin- converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009 ;169(13):1195-202.
CrossRef - Jenkins TA, Mendelsohn FA, Chai SY. Angiotensin‐converting enzyme modulates dopamine turnover in the striatum. J Neurochem. 1997 ;68(3):1304-11.
CrossRef - Porkka-Heiskanen, Tarja; Tuomisto, Leena; Ylinen, Markku;Stenberg,Dag . The effect of REM sleep deprivation on histamine concentrations in different brain areas. Life Sciences. 1994 ;54(22): 1719–1726.
CrossRef - Ababei DC, Bild V, Ciobica A, Lefter RM, Rusu RN, and Bild W. A Comparative Study on the Memory-Enhancing Actions of Oral Renin-Angiotensin System Altering Drugs in Scopolamine-Treated Mice. American Journal of Alzheimer’s Disease & Other Dementias® 2019;34(5) :329-336
CrossRef - Hicks RA, Okuda A, Thomsen D. Depriving rats of REM sleep: the identification of a methodological problem. Am J Psychol. 1977 ;90(1):95-102
CrossRef - Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974 ;2(4):553-6.
CrossRef - Nade VS, Kawale LA, Valte KD, Shendye N V. Cognitive enhancing effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on learning and memory. Indian J Pharmacol. 2015;47(3):263-269.
CrossRef - Ryan DK, Karhunen V, Su B, Traylor M, Richardson TG, Burgess S, Tzoulaki I, and Gill D. Genetic Evidence for Protective Effects of Angiotensin Converting Enzyme Against Alzheimer Disease But Not Other Neurodegenerative Diseases in European Populations. Neurol Genet 2022;8:e200014. doi:10.1212/NXG.0000000000200014
CrossRef - Mantle D, Heaton RA, Hargreaves IP. Coenzyme Q10, Ageing and the Nervous System: An Overview. Antioxidants 2022, 11, 2. https://doi.org/10.3390/antiox11010002
CrossRef - Gandigawad P, Radhika MS, Sor RK. Learning and memory enhancing properties of coenzyme Q10 in amnestic albino Wistar rats. Natl J Physiol Pharm Pharmacol 2020;10(11):950-54
CrossRef - Dumont M, Kipiani K, Yu F, et al. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27(1):211-223.
CrossRef - Sandhir R, Sethi N, Aggarwal A, Khera A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem Int 2014;74:16-23
CrossRef - Zhang YS, Li JD and Yan C. An update on Vinpocetine: New discoveries and clinical implications. Eur J Pharmacol. 2018 ; 819: 30–34. doi:10.1016/j.ejphar.2017.11.041
CrossRef - Shang Y, Wang L, Li Y and Pei-fei Gu. Vinpocetine Improves Scopolamine Induced Learning and Memory Dysfunction in C57 BL/6J Mice. Biol. Pharm. Bull.2016; 39(9):1412–1418
CrossRef - Gupta S, Singh P, Sharma BM, Sharma B. Neuroprotective Effects of Agomelatine and Vinpocetine Against Chronic Cerebral Hypoperfusion Induced Vascular Dementia. Curr Neurovasc Res. 2015;12(3):240-252.
CrossRef
Abbreviations
Gr.-group
REM-Rapid eye movement
NREM-Non-rapid eye movement
ACE I-Angiotensin converting enzyme inhibitor
ETC-Electron transport chain
RAS-Renin angiotensin system
RAAS-Renin angiotensin aldosterone system
PUFA-Polyunsaturated fatty acid
GSH-Glutathione
SOD-Superoxide dismutase
MDA-Malondialdehyde
ATP-Adenosine triphosphate







