Surgical Oncology Division, Department of Surgery, Medical Faculty Universitas Udayana, Bali, Indonesia.
Corresponding Author E-mail: hendry_irawan@rocketmail.com
DOI : https://dx.doi.org/10.13005/bpj/2788
Abstract
Background: Squamous cell carcinoma (SCC) of the oral cavity can grow quickly and the response to therapy in each patient is different. The immune system plays a major role in the development of cancer cells, especially in SCC of the oral cavity. Natural killer cells (NK cells) play a major role in both innate immunity and adaptive immunity. Objective: To evaluation CD56 expression on NK cells and the correlation with clinical outcomes in oral squamous cell carcinoma. Methods: We collect the literature from Journal, Pubmed, Web of Science, Scopus, Crossref, and Google Scholar. CD56 expression was examined by immunohistochemical staining in cancer tissue. On each slide, the number of positive stained cells was read on 5 high power fields (HPF) 400x, then the number of cells stained on each HPF was added up and section 5. Cells with positive CD56 showed brown staining in the intracytoplasm regardless of the intensity of the staining. Results: High CD56 expression was associated with good overall survival (OS) (HR 0.27; 95%CI 0.12-0.60; p=0.001), good progression-free survival (PFS). (HR 0.35; 95%CI 0.17-0.74; p=0.005), and good distant metastasis-free survival (DMFS) (HR 0.27; 95%CI 0.13-0.55; p=0.0004) in head and neck SCC. Other study in oropharyngeal SCC, that high CD56 expression had good OS (HR 0.32; 95%CI 0.10-0.96; p=0.04). The meta-analysis study showed that the CD56 marker on NK cell was a good prognostic marker for OS in head and neck SCC (HR 0.19, 95%CI 0.11-0.35, p<0.00001). Conclusion: NK cells with CD56 expression will increase cytotoxic function. The presence of CD56 can increase infiltration into tumor tissue. Based on these data, CD56 on NK cells can be used as an indicator of cancer prognosis in patients with SCC of the oral cavity. Apart from that, it can be used as an immunotherapy target which functions to restore the immune response against cancer cells.
Keywords
CD56; Cytotoxicity; NK cell; Immune Response; Oral Cavity Cancer; Prognostic Marker; Squamous Cell Carcinoma; Tumor Infiltration
Download this article as:| Copy the following to cite this article: Irawan H. CD56 Expression on Natural Killer Cells and its Correlation with Clinical Outcomes in Oral Squamous Cell Carcinoma Patients. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Irawan H. CD56 Expression on Natural Killer Cells and its Correlation with Clinical Outcomes in Oral Squamous Cell Carcinoma Patients. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3RCBpBb |
Introduction
Squamous cell carcinoma (SCC) of the oral cavity is a problem in the world, especially in developing countries. More than 90% of the histopathological types of oral cavity cancer found are SCC.1 This cancer can grow quickly and the response to therapy in each patient is different. This will have an impact on the prognosis of SCC patients.
Oral cavity cancer is one of the head and neck cancers. In the United States in 2014 there were 54,000 new cases of head and neck cancer, with an incidence of 15 per 100,000 people with deaths reaching 12,000 people.2 Based on GLOBOCAN 2018, the most common head and neck cancer based on location is oral cavity cancer with an incidence of oral cavity cancer of 2% (354,864 cases) with mortality reaching 1.9% (177,384 cases).3
In 2018, the incidence of oral cavity cancer in men was 0.66% (246,420 cases) and in women it was 0.26% (108,444 cases). The death rate in men is 0.32% (119,693 cases) and in women is 0.14% (57,691 cases).3 Oral cavity cancer occurs more often in men after the 5th decade of life.1
The formation of head and neck SCC is caused by various genetic events that cause the inactivation of tumor suppressor genes or the activation of protooncogenes. Molecular techniques are able to identify genetic and epigenetic changes in premalignant and invasive lesions, thereby describing the hypothetical carcinogenic progression of oral cavity cancers.4
Malignant cells have mechanisms to evade immune-mediated destruction, not only by evading the immune system but also by directly inhibiting antitumor immune defenses. Patients with head and neck SCC show reduced blood concentrations of CD3+, CD4+, and CD8+ T cells, which may persist even years after curative surgery.5 Many mechanisms for immune system evasion have been proposed, including avoidance of immune recognition and elimination caused by tumor factors, disruption of T lymphocyte activity, immunosuppressive cell activity, and cytokines that mediate local and systemic effects.4
Further research into the development of cancer relates to changes in genetics and the microenvironment. The microenvironment or more commonly referred to as tumor stroma is needed to provide nutrition and to eliminate waste products, which consists of connective tissue, blood vessels, innate immune cells, and adaptive immune cells.6
The immune system plays a major role in the development of cancer cells, especially in SCC of the oral cavity, which is a type of immunogenic cancer that has a mechanism to evade the immune system. Immunological markers may indicate a superior prognosis compared to cancer staging systems.7
Innate immune cells consist of macrophages, dendritic cells, granulocytes, natural killer cells (NK cells), and mast cells. NK cell function in innate immunity and adaptive immunity by secreting cytokines or through direct interaction with dendritic cells. NK cell is an important component in the initial defense against tumor cells, so they can be used as a prognostic marker in SCC of the oral cavity.6,7 Membrane protein expression is used to differentiate cells in the immune system and cluster of differentiation (CD) is the nomenclature for cell surface molecules.8
Besides that, effect of cetuximab is enhanced by binding to Fcγ receptors on NK cells, so it can induce antibody-dependent cell-mediated cytotoxicity (ADCC). NK cell can be evaluated by looking at the expression of a marker on the cell surface, namely CD56, which is a marker for the specific phenotype of NK cell.7,9,10 Based on it, CD56 expression on NK cells can be a marker to evaluation the clinical outcomes of oral SCC.
Role CD56 and NK Cell
NK cell is usefull as an initial defense against pathogens, cell damage, and destroying cells. NK cell has a cytotoxic function that resembles the CD8 cytotoxic cells circulating in the blood and lymphoid tissues. NK cell originates from common lymphoid progenitors (Figure 1). Lymphoid progenitors are also precursors for T cells, B cells, and innate lymphoid cells.8
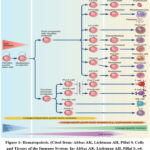 |
Figure 1: Hematopoiesis. (Cited from: Abbas AK, Lichtman AH, Pillai S. Cells and Tissues of the Immune System. In: Abbas AK, Lichtman AH, Pillai S, ed. Cellular and Molecular Immunology, 9th Edition. Elsevier; 2018. p.13–37.)8 |
NK cell (Figure 2) can work directly to kill infected cells and can also produce IFN-γ (interferon gamma) due to the presence of IL-12 (interleukin 12) from macrophages, thus activating macrophages to destroy cells that have been phagocytosed.11 NK cells, which are the innate immune system, can activate the adaptive immunity system (ADCC) by binding CD16 on NK cells with Fc in Immunoglobulin (Ig) G. This will produce lytic granules which will destroy target cells.12
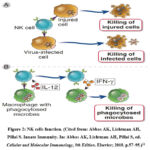 |
Figure 2: NK cells function. (Cited from: Abbas AK, Lichtman AH, Pillai S. Innate Immunity. In: Abbas AK, Lichtman AH, Pillai S, ed. Cellular and Molecular Immunology, 9th Edition. Elsevier; 2018. p.57–95.)11 |
CD56 examination is an immunohistochemical examination method that uses mouse CD56 antibodies; Clone 123C3 (ScyTek A00121-IFU-IVD), which is stained on the surface of NK cell in cancer tissue. This antibody identifies two proteins (185kDa and 145kDa), as two isoforms of neural cell adhesion molecule (NCAM/CD56).13 On each slide, the number of positive stained cells was read on 5 high power fields (HPF) 400x, then the number of cells stained on each HPF was added up and section 5.6,14 Cells with positive CD56 (Figure 3) showed brown staining in the intracytoplasm regardless of the intensity of the staining.6,13,14
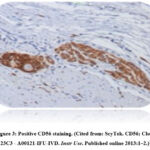 |
Figure 3: Positive CD56 staining. (Cited from: ScyTek. CD56; Clone 123C3 – A00121-IFU-IVD. Instr Use. Published online 2013:1–2.)13 |
A study conducted by Stangl showed that high CD56 expression was associated with good overall survival (OS) (HR 0.27; 95%CI 0.12-0.60; p=0.001), good progression-free survival (PFS). (HR 0.35; 95%CI 0.17-0.74; p=0.005), and good distant metastasis-free survival (DMFS) (HR 0.27; 95%CI 0.13-0.55; p=0.0004) in head and neck SCC.15
Wagner’s study also showed similar results in oropharyngeal SCC, that high CD56 expression had good OS (HR 0.32; 95%CI 0.10-0.96; p=0.04). This study also shows that the presence of CD56 expression in tumor and stromal tissue has better survival, while the prognosis is poor if there is no CD56 expression.14
A meta-analysis study conducted by Bisheshar showed that the CD56 marker on NK cell was a good prognostic marker for OS in head and neck SCC (HR 0.19, 95%CI 0.11-0.35, p<0.00001).7
Other study got relationship CD56 with human papillomavirus (HPV) infection at oropharyngeal SCC. NK cell can be a defense to HPV related oral SCC. CD56 cell found higher at HPV-positive in tumor and stomal compared to HPV-negative. The percentage of CD56 staining per HPF of HPV-positive were significant higher compare to HPV-negative, such as 74% vs. 45% in tumor (p=0.004), 62% vs. 34% in stroma (p=0.004), and 82% vs. 57% in both compartements (p=0.007). This study found that HPV-positive were better survival than HPV negative patients (p<0.001).14
CD56 cells are also fewer found in tonsil cancers, so it could be a mechanism for escaping from the immune system. Meanwhile, HPV-positive tumors are more common in tonsil cancer and can attract immune cells to increase CD56 cells. The corelation of HPV16 infection and cancer was strongest in tonsil (OR 15.1), intermediate in oropharynx (OR 4.3), and weakest in oral (OR 2.0) and larynx (OR 2.0).16
A study was found interesting risk factors that significant related of alcohol and smoking to CD56 cells. Patients with more alcohol intake get lower CD4 cells in tumor (62% vs. 36%, p=0.009), stroma (67% vs. 47%, p=0.039), and in both compartements (48% vs. 28%, p=0.03). Smoker patients also get lower CD4 cells in stroma compared with non smoker patients (64% vs. 36%, p=0.01).14
The presence of specific prognostic markers is necessary to evaluate the treatment of head and neck SCC. NK cell originates from the innate immune system, but also perform functions in the adaptive immune system. The immune system influences tumor growth and control.
NK cell can be a biomarkers, because the ability to lyse tumor cells that lack major histocompatibility complex (MHC) class 1. The presence of high CD56 markers on NK cell is associated with better OS, due to the response to killing cancer cells by the cells. NK can be done directly without the need for sensitization. Apart from these markers, NK cell can activate Fcγ receptors to bind to IgG, thereby activating antibody-dependent cell-mediated cytotoxicity (ADCC).7
NK cell has surface receptors that play a role in triggering cytotoxic functions, namely killer activation receptor (KAR) and killer inhibitory receptor (KIR). These two receptors have opposite signaling functions. These NK cell can recognize between normal cells and abnormal cells based on class 1 MHC molecules. In normal cells, KIR activation occurs to prevent cell lysis due to NK cell activation. In cancer cells, class 1 MHC is damaged, resulting in disruption of the signal to activate NK cell.17 In addition, NK cell plays a role in modulating other immune cells and play a role in eliminating tumor cells. Human NK cell is broadly divided into three (Figure 4), namely regulatory NK cell CD56bright CD16– for greater cytokine production, cytotoxic NK cell CD56dim CD16+ for greater cytotoxic anti-tumor/anti-virus functions, and memory NK cell CD56bright or CD56dim and CD16+ or CD16–.10
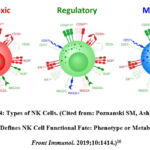 |
Figure 4: Types of NK Cells. (Cited from: Poznanski SM, Ashkar AA. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front Immunol. 2019;10:1414.)10 |
In blood, spleen, and lung, most NK cells are characterized by low expression of CD56 (CD56dim) and high CD16 (CD16bright), whereas NK cells present in non-reactive lymph nodes, tonsil, gut, and peripheral tissues express CD16 (CD16–) poorly and are positive for CD56 (CD56bright).18–20 CD56bright NK cells are less cytolytic and produce large amounts of cytokines interferon-γ, and are considered to be immunoregulatory, whereas CD56dim NK cells have high cytotoxic activity.19,20
Immunohistochemical staining was not able differentiate CD56 dim and bright, so there are immunofluorescence staining to evaluation CD56 with granular Granzyme B (GZMB). GZMB was a marker of cytotoxic activity of NK cells. If the NK cells were lower GZMB, it can be categorized as regulatory NK cells. An increase in cytotoxic lymphocytes and tumor-infiltrating NK cells will improve the prognosis of cancer patients.14,21
In conditions where there is no activation signal, NK cell will be silent, while in inflammatory conditions, cytotoxic NK cell will be active by increasing proliferation, synthesis of pro-inflammatory cytokines and strong cytotoxicity. NK cells with CD56 expression will increase cytotoxic function and increase IFN-γ production, especially CD56superbright and CD56dim which are less toxic. In hypoxic conditions it can suppress the function of cytotoxic NK cells.10
Conclusion
The presence of CD56 on NK cells can increase infiltration into tumor tissue thereby improving good clinical outcomes. The correlation of OS and high NK cell counts suggests the hypothesis that the innate immune system also plays a role in the clinical outcomes of head and neck SCC patients.15
NK cells can promote target cell death by increasing tumor necrosis factor and death receptor/ligand interactions or by exocytosis of perforin and granzymes with granules. The presence of cytotoxic lymphocytes and tumor-filtrating NK cells correlates with an improved prognosis of cancer patients. Therefore, it can be concluded that the cells with CD56 found are cytotoxic NK cells.14
NK cell is capable of performing a very diverse set of functions, ranging from anti-tumor and anti-viral cytotoxic effector functions, to regulatory roles in controlling inflammatory immune responses and promoting tissue growth.10
Based on these data, CD56 on NK cells can be used as an indicator of cancer prognosis in patients with SCC of the oral cavity. Apart from that, it can be used as an immunotherapy target which functions to restore the immune response against cancer cells.
Acknowledgement
None.
Conflict of Interest
Author do not have any conflict of interest.
Funding sources
No funding
References
- Montero PH, Patel SG. Cancer of the Oral Cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. doi:10.1016/j.soc.2015.03.006
CrossRef - Rettig EM, D’Souza G. Epidemiology of Head and Neck Cancer. Surg Oncol Clin N Am. 2015;24(3):379–396. doi:10.1016/j.soc.2015.03.001
CrossRef - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
CrossRef - Argiris A, Karamouzis M V, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. doi:10.1016/S0140-6736(08)60728-X.
CrossRef - Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(11):3755–3762. doi:10.1158/1078-0432.CCR-04-0054
CrossRef - Agarwal R, Chaudhary M, Bohra S, Bajaj S. Evaluation of natural killer cell ( CD57 ) as a prognostic marker in oral squamous cell carcinoma : An immunohistochemistry study. J Oral Maxillofac Pathol. 2016;20(2):173–177. doi:10.4103/0973-029X.185933
CrossRef - Bisheshar SK, De Ruiter EJ, Devriese LA, Willems SM. The prognostic role of NK cells and their ligands in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology. 2020;9(1):e1747345. doi:10.1080/2162402X.2020.1747345
CrossRef - Abbas AK, Lichtman AH, Pillai S. Cells and Tissues of the Immune System. In: Abbas AK, Lichtman AH, Pillai S, ed. Cellular and Molecular Immunology, 9th Edition. Elsevier; 2018:13–37.
- Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front Immunol. 2017;8:892. doi:10.3389/fimmu.2017.00892
CrossRef - Poznanski SM, Ashkar AA. What Defines NK Cell Functional Fate: Phenotype or Metabolism? Front Immunol. 2019;10:1414. doi:10.3389/fimmu.2019.01414
CrossRef - Abbas AK, Lichtman AH, Pillai S. Innate Immunity. In: Abbas AK, Lichtman AH, Pillai S, ed. Cellular and Molecular Immunology, 9th Edition. Elsevier; 2018:57–95.
- Clarke S, Li BT. Components of the immune system. In: Clarke S, Li BT, ed. Fast Facts: Immuno-Oncology. Health Press Limited; 2017:11–23.
CrossRef - ScyTek. CD56; Clone 123C3 – A00121-IFU-IVD. Instr Use. Published online 2013:1–2.
- Wagner S, Wittekindt C, Reuschenbach M, et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;138(9):2263–2273. doi:10.1002/ijc.29962
CrossRef - Stangl S, Tontcheva N, Sievert W, et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Gro. Int J Cancer. 2018;142(9):1911–1925. doi:10.1002/ijc.31213
CrossRef - Hobbs CGL, Sterne JAC, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31(4):259–266. doi:10.1111/j.1749-4486.2006.01246.x
CrossRef - Konjević G, Vuletić A, Martinović KM, Džodić R. The Role of Activating and Inhibitory NK Cell Receptors in Antitumor Immune Response. In: Aribi M, ed. Natural Killer Cells. IntechOpen Limited; 2017:49–65.
CrossRef - Quatrini L, Della Chiesa M, Sivori S, Mingari MC, Pende D, Moretta L. Human NK cells, their receptors and function. Eur J Immunol. 2021;51(7):1566–1579. doi:10.1002/eji.202049028
CrossRef - Bellora F, Castriconi R, Dondero A, et al. Human NK cells and NK receptors. Immunol Lett. 2014;161(2):168–173. doi:https://doi.org/10.1016/j.imlet.2013.12.009
CrossRef - Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi:10.1111/j.1365-2567.2008.03027.x
CrossRef - Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol Immunother. 2014;63(3):297–303. doi:10.1007/s00262-013-1511-x
CrossRef









