S M Seeni Mohamed Aliar Maraikkayar1* , R Tamilselvi2
, R Tamilselvi2 and M Parisa Beham3
and M Parisa Beham3
ECE, Sethu Institute of Technology, Kariapatti, Virudhunagar, Tamil Nadu, India.
Corresponding Author E-mail: tamilselvi@sethu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2796
Abstract
Pregnancy is a time of change. Stress during pregnancy can increase the chances of having a premature baby or a low birth weight baby. Any unusual changes in the heart rate of the baby in the uterus can be examined using Electronic fetal monitoring (EFM). Over the past decades, enormous devices and methods have been developed for EFM. Few devices are invasive and few are non-invasive in nature, larger in size, and high in cost. The main parameter needed to be analyzed during pregnancy is the stress in the fetus as well as the mother. For fetal stress monitoring, two main tests have been used such as CTG (Cardiotocography) and BPP (Biophysical Profile). The BPP test is very important in determining whether the fetus is in a suspicious or normal condition based on a specific BPP score. However, BPP is still used as a visualization method to assess the condition of the fetus. As a result, a quantitative analysis of the BPP parameters is required. But still, now there is no specific BPP database for evaluating the condition of the fetus. So, a novel database for Biophysical profile is proposed named “BIOPHYSIT”, which is used to analyze the health condition of the fetus using various BPP parameters.
Keywords
Biophysical profile; Electronic fetal monitoring; Fetal stress; Fetal Movement; Fetal Heart Rate; Uterine Contraction
Download this article as:| Copy the following to cite this article: Maraikkayar S. M. S. M. A, Tamilselvi R, Beham M. P. BIOPHYSIT: A Novel Biophysical Profile Database for Fetal Stress Measurement in High-Risk Pregnancies. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Maraikkayar S. M. S. M. A, Tamilselvi R, Beham M. P. BIOPHYSIT: A Novel Biophysical Profile Database for Fetal Stress Measurement in High-Risk Pregnancies. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3u2VbOi |
Introduction
Fetal monitoring is commonly used to measure fetal surveillance, but it is essentially a winding path to determining fetal well-being or fetal oxygenation sufficiency1,2,3. The primary goal of fetal monitoring during pregnancy is to ensure a healthy baby is delivered. Any unusual changes in the baby’s heart rate in the uterus can be detected using electronic fetal monitoring (EFM). Initially, direct monitoring of fetal heart sounds can be examined with a stethoscope which is placed on the maternal abdomen.EFM was used for complex pregnancies in the early stages of 1965. However, as technology and ease of use improved, this EFM4 began to be used in all pregnancies, including low-risk pregnancies. Some of the complex events in the uterus can have a negative neonatal outcome. Inadequate fetal monitoring can result in fetal death. The risk of neonatal death in developing countries, particularly India5, is six times that of developed countries, and more than eight times that of less-developed countries. Figure 1 depicts data on the fetal mortality rate in India from 2009 to 2019. If it goes undetected, it has a significant impact on neonatal mortality.
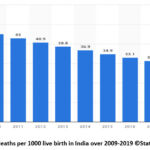 |
Figure 1: Fetal deaths per 1000 live birth in India over 2009-2019 ©Statista 2021 [1]. |
Due to advancements in medical technology, few devices are available for providing reasonable and Information about the fetus during its intrauterine life that is reliable. There are several important parameters for monitoring the status of the fetus. Among those, Fetal Heart Rate (FHR)6 gives the most important information and becomes a commonly used parameter.FHR and other parameters can now be monitored using a variety of measuring, recording, and analyzing instruments. Ultrasound, Doppler ultrasound, fetal cardiotocography (FCTG), fetal electrocardiography (FECG)7, and fetal magnetocardiography are some of the tests available. Many methods and devices for monitoring fetal health during the first trimester and during labor were reviewed in this study. Monitoring the baby’s health condition in the uterus during pregnancy is often undertaken using a cardiotocography (CTG)8 machine.
Monitoring fetal movements has thus been proposed as a useful addition to predicting fetal abnormality. This is because a decrease in fetal movement can result in the death of the baby. Fetal movement is thought to be less frequent if the oxygen supply to the baby through the placenta is insufficient. To view fetal movements, the biophysical profile (BPP)9 and the modified biophysical profile (MBPP) have been introduced. The ultrasound test is used in the Biophysical Profile to assess 1) fetal body movement, 2) fetal tone movement, 3) fetal breathing movement, and 4) amniotic fluid volume that surrounds the baby. A modified BPP is sometimes used first, involving only the CTG trace and the amniotic fluid volume. If there is a chance that abnormality conditions will occur, the full BPP is used. The BPP and MBPP10 were compared to conventional CTG monitoring on pregnancy in high-risk pregnancies in this review of trials. CTG and BPP tests are used to monitor fetal conditions during the non-stress test. This paper is organized as follows: Section I explains the details about fetal stress, its occurrence, and the methods for measuring fetal stress. The subsequent section explains the Analysis of Fetal stress using CTG and BPP. Section III detailed the Overview of the Existing Fetal Monitoring Device. Section IV explains the proposed fetal stress monitoring device. Section V summarizes the review and gives a comparative analysis.
Material and Methods
Analysis of Fetal stress using CTG and BPP
CTG Test
The Cardiotocography (CTG) machine11 will measure the heart rate of the baby. It also monitors uterine contractions at the same time. It is used to observe the baby for any signs of stressful conditions both before and during birth (antenatal). Doctors can see how the baby is coping by examining various features of the baby’s heart rate. The equipment used to perform a CTG is depicted in Figure 2. It is usually placed beside your bed while you are being monitored. External CTG tests are most commonly performed. The equipment used to monitor the baby’s heartbeat using an electrode placed on the mother’s abdomen is referred to as this. The mother’s abdomen is wrapped in an elastic belt.
 |
Figure 2: Cardiotocography (CTG) machine [2]. |
The midwife may apply jelly to the mother’s abdomen to help get a strong signal. The CTG belt is connected to a machine that decodes the FHR and UC signals12 produced by the plates. The foetal heart rate is represented by a beating or pulsing sound produced by the CTG machine. The machine also prints out the FHR over a specified time period. If you have CTG during labour, you may be asked to press a button on the machine every time the baby moves. The CTG will only monitor the baby’s heart rate because you will not be having contractions at this time. Internal monitoring can be used if an FHR signal cannot be found using the external monitor during labour. Internal monitoring makes use of the electrode. After being inserted through the vagina, this is placed on the baby’s scalp. If you are pregnant with twins (or multiples), the electrode can only be used on the baby closest to the womb’s neck. Furthermore, internal monitoring can only be used if the baby is to be delivered head first. Internal monitoring will not function in the case of a breech (bottom or foot first) presentation. CTG monitoring employs a type of ultrasound known as a Doppler probe. Because this type of ultrasound can measure moving structures, it is useful for monitoring foetal heart rate.
Bio Physical Profile Test
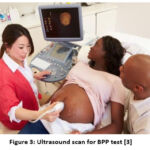 |
Figure 3: Ultrasound scan for BPP test [3]. |
Figure 3 depicts the Biophysical Profile test, which is used to assess your baby’s health. It detects foetal muscle tone and movement. It is also used to determine how quickly the FHR accelerates during foetal movement and the amount of amniotic fluid that protects your baby in the womb. The BPP13 is both non-hazardous and non-invasive. The procedure takes approximately 30 minutes. An ultrasound is included as part of the BPP. This monitors foetal movement and measures amniotic fluid levels in the womb. The mother is asked to lie on her back while an ultrasound technician holds the wand against her belly. A non-stress test that monitors FHR for about 20 minutes is also included in the BPP. The results of the BPP test are added together to form a total BPP score, which includes the heart rate, breathing movement, body movement, muscle tone, and amount of amniotic fluid. A score of 8 to 10 indicates that your baby is healthy. If your Score is less than eight, you should retest. If the score is less than 5, it may indicate that your baby is under stress. As a result of the above, your doctor may recommend early delivery. Some women have BPP testing earlier or later in their pregnancy.
Table 1: Comparative analysis of CTG and BPP test.
|
S. No |
Non-Stress Test |
Parameters |
Time Duration |
Advantages |
Disadvantages |
|
1. |
Cardiotocography (CTG) test |
· Fetal Heart Rate(FHR) · Uterine Contractio ns(UC) |
· Most commonly at Third trimester of the pregnancy. · CTG records the FHR and UC for about 30 minutes. |
· FHR and UC can be monitored at the same time. · Smooth HR in the timeline. · Reduce rates of seizure in newborn. · Rather robust and reliable. |
· Prevent Mother from Moving. · Unable to change position. · Ultrasound Radiation. · No information about BTB (Beat-to- Beat) variability. |
|
2. |
Bio Physical Profile test(BPP) |
· Fetal Heart Rate · Fetal Breathing Movement · Fetal Tone Movement · Fetal Body Movement · |
· About 30 min · ≥1 episode lasting · >30 seconds ≥1 episode of extensions (limb or trunk) with return of flexion. · ≥3 discrete body/limb movement s · ≥1 pocket measuring 2 cm in two perpendicu lar planes |
· This is Non- invasive and helps to evaluate the fetal conditions · The false negative rate of a normal BPP is less than 0.1%. |
· Ultrasound Radiation. It is a long procedure and takes more time to evaluate the fetal condition. · BPP test is not cost effective. |
From Table 1, it’s known that the CTG and BPP test helps to measure the baby’s health condition throughout the gestational period. For fetal stress monitoring, various existing devices are available. Few devices are invasive and few are non-invasive in nature, larger in size, and high in cost. Medical devices such as fetal Monitor – LPM 703 (Life Plus), BPL FM9852, Bistros BT350 Fetal Monitor CTG Machine, etc., measure FHR, Fetal Movements, and Uterine Contraction only. By analyzing these parameters, the fetal conditions are classified as Normal/ Suspicious/ Pathological conditions. By taking this into consideration, a new device must be proposed to analyze the fetal health condition using various parameters like a uterine contraction, Fetal Heart Rate, Maternal Heart Rate, and BPP parameters. In the existing devices, only a few parameters have been calculated, it is high in cost and has poor accuracy. To achieve high accuracy in the proposed device, we need a bunch of Biophysical profile databases for the analysis of fetal health conditions. For that, a benchmark Biophysical profile database is proposed and named as BIOPHYSIT database. With the help of this BIOPHYSIT database, a new fetal stress monitoring device can be proposed in the future.
Bio Physical Profile
A biophysical profile (BPP) test14 assesses the fetus’s health during pregnancy. A non-stress test with electronic fetal heart monitoring and fetal ultrasound may be included in a BPP test. The baby’s heart rate, muscle tone, movement, breathing, and amniotic fluid level in the womb are all assessed by the BPP. A BPP is typically performed in the third trimester of pregnancy. If there is a chance that the baby will have problems during the pregnancy, a BPP may be performed as early as 32 to 34 weeks (high-risk pregnancy). Some women with high-risk pregnancies may have a BPP test every week or twice a week during the third trimester. It is considered normal to have a normal fluid index15 score of 10/10 or 8/10.
In the Fetal body movement16, fetal movements have their own time-of-day and sleep- activity rhythm. The baby kicks the most during light sleep in the last month or so of pregnancy. The fetus may move less during its sleep cycles. Similarly, in healthy mammalian fetuses, Fetal breathing movement occurs episodically. FBMs are rhythmic diaphragmatic contractions that are centrally organized; similar to postnatal breathing, but they may also involve other skeletal muscles such as those of the chest wall and upper respiratory tract. In Amniotic Fluid Level, AFI between 8 and 18 is considered normal. From week 20 to week 35, when amniotic fluid begins to decrease in preparation for birth, the median AFI level is around 14. Oligohydramnios is defined as an AFI of 5-6. It refers to an ultrasound grading system of the placenta based on its maturity in Placental Grading17. This has the greatest impact on the extent of calcifications. Placental grading has been phased out of obstetric practice in some countries due to a weak correlation with adverse prenatal outcomes. Similarly, the ability to flex and extend an arm or leg is measured in the Fetal Tone Movement by counting quick, jerky movements.
BIOPHYSIT Database
The lack of a suitable database for further research and development in the biomedical field and in healthcare applications inspired us to create a new and uncommon database, which we named the BIOPHYSIT database. This BIOPHYSIT database was designed primarily to serve as a resource for fetal stress measurements. BIOPHYSIT was created using ultrasound fetal movement images collected from a popular and standard scan center in Chennai. The fetal movement video is recorded for 2-5 minutes and used to evaluate the health of the fetus. Ultrasound is used to record the sequences.
This database consists of two ultrasound video sequences, which are then converted into planes. The plane has 300 ultrasound scan images, which are collected from 50 female subjects. A fetal biophysical profile is a prenatal test used to assess the health of a baby. The test combines fetal heart rate monitoring and fetal ultrasound to determine a baby’s heart rate, breathing, fetal movements, muscle tone, and amniotic fluid level. A sample BIOPHYSIT AF (Amniotic fluid), is shown in Figure 4. Similarly, the sample BIOPHYSIT FBM (Fetal Body movement) is shown in Figure 5. Then, the sample BIOPHYSIT FBRM (Fetal breathing movement) is discussed in Figure 6. The sample BIOPHYSIT PG (Placental Grading) is shown in Figure 7. The sample BIOPHYSIT FT (Fetal tone movement) is shown in Figure 8.
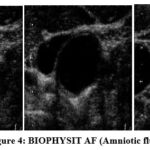 |
Figure 4: BIOPHYSIT AF (Amniotic fluid). |
 |
Figure 5: BIOPHYSIT FBM (Fetal Body movement). |
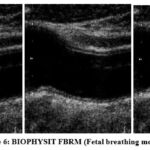 |
Figure 6: BIOPHYSIT FBRM (Fetal breathing movement). |
 |
Figure 7: BIOPHYSIT PG (Placental Grading). |
 |
Figure 8: BIOPHYSIT FT (Fetal tone movement). |
Basic structure of BIOPHYSIT database:
 |
Chart 1: Structure of BIOPHYSIT database. |
Details of Construction and Composition of the database
The BIOPHYSIT database is built from ultrasound scan images of Indians. A total of 300 ultrasound images were collected from the 50 female subjects. All of the images are manually cropped and it is saved as image files in ‘PNG’ format. The BPP measurement plots for each subject’s ultrasound scan images have been provided in the same png format. The database is divided into five categories: BIOPHYSIT AF, BIOPHYSIT FBM, BIOPHYSIT FBRM, BIOPHYSIT PG, and BIOPHYSIT FT. The main database, BIOPHYSIT-DB, contains all of the scan images, and the sample BIOPHYSIT AF contains Amniotic fluid images. Similarly, images of Fetal Body movement can be found in the sample BIOPHYSIT FBM. The sample BIOPHYSIT FBRM then contains fetal breathing movement images, while the sample BIOPHYSIT PG contains Placental Grading images. Fetal tone movement images are included in the sample BIOPHYSIT FT.
Labeling the BIOPHYSIT dataset
All of the images in the BIOPHYSIT database are perfectly labeled for the accessibility of researchers. The subject ID, BPP, and gender of the subject can be easily identified from the label. Images of ultrasound scans are obtained for each subject. The label for an ultrasound scan image, for example, is BIOPHYSIT AF 010.png and BIOPHYSIT PG 023. png. In the first module, BIOPHYSIT is an image of a fetal ultrasound scan, AF is an image of amniotic fluid in a female subject, and 010 is the subject ID. BIOPHYSIT PG denotes an ultrasound image, PG denotes a female subject’s placental grading image, and 023 is the subject ID in the latter case. Similarly, BIOPHYSIT FBM 033.png and BIOPHYSIT FBRM 002.png are ultrasound images of female subjects’ fetal body movements with subject IDs 033 and 002, respectively.
Annotation
BIOPHYSIT provides detailed annotation by carefully analyzing each ultrasound scan image. We manually annotated the following attributes for each fetal image.
Biological data of the subject:
Name of the subject
Age
Gender
Height (cm)
Weight (kg)
CTG Recordings
UC recordings
Amniotic fluid level
Fetal Body Movement
Fetal Breathing Movement
Placental Grading
Fetal Tone Movement
Performance Measure
Table 2 details the Bio physical profile score values.
Table 2. Bio Physical Profile score
|
Variable |
Score |
||
|
2 |
1 |
0 |
|
|
Fetal breathing (FBM) |
One episode lasting at least 60 sec within 30 min |
One episode lasting 30- 60 sec within 30 min |
No breathing movement or breathing movements less than 30 sec within 30 min |
|
Fetal reactivity (FR) |
Definite reactivity (over 2 acceleration in 30 min) |
Equivocal reactivity (1 or 2 accelerations in 30 min) |
No reactivity (no acceleration in 30 min) |
|
Qualitative evaluation of amniotic fluid volume (AFV) |
2 cm square or more |
Between 1 cm and 2 cm |
Less than 1 cm |
|
Fetal tone |
At least one episode involving both extremities and spine |
At least one episode involving either extremities or spine |
Extension movement of extremities not followed by return or flexion |
|
Fetal Movement (FM) |
3 or more in 30 min |
1 or 2 in 30 min |
No movement in 30 min |
Non-conclusive studies should prompt a medical investigation into the cause and preparation of the mother and fetus for delivery. The interdisciplinary medical team must reach an agreement on the urgency of delivery, which must consider all factors such as gestational age, medical conditions, and hospital capabilities.BPP considers a variety of fetal well-being factors and is an effective tool for guiding management in high-risk obstetric cases. It is easily reproducible and can be performed in a wide range of settings, from the most remote to the most prestigious hospitals.
Clinical Interpretation
The aim of the proposed database was to inspire the research community to identify risk factors associated with pregnancy. The proposed database has been validated by radiologists and obstetricians using the BPP score. The pregnant women undergo an ultrasound test and the radiologist will generate an ultrasound video sequence. The videos are converted into frames once they are received. The images will be validated by the obstetrician using the BPP score. If any of the BPP parameters is found to be 0/2, the fetus’s condition is confirmed to be critical. Similarly, if any of the values is 1/2, the fetus’s condition is suspicious. If all of the values are 2/2, the condition is considered normal. To test the proposed database, the various subjects are evaluated by the obstetrician with a BPP score, and the condition of the fetus is also given in table 3.
Table 3. Interpretation of clinical results for subjects based on BPP score.
|
Subject Id |
Age |
Weight (Kg) |
Height (Cm) |
Bio Physical Profile- parameters |
Fetal Risk Condition |
||||
|
Amniotic Fluid Level |
Fetal Body |
Fetal Breathing Movement |
Placental Grading |
Fetal Tone |
|||||
|
001 |
35 |
68 |
156 |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
002 |
28 |
69 |
166 |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
003 |
35 |
76 |
163 |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
004 |
26 |
82 |
158 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
Pathological |
|
005 |
25 |
62 |
159 |
1/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
006 |
30 |
78 |
170 |
1/2 |
1/2 |
1/2 |
1/2 |
1/2 |
Suspicious |
|
007 |
27 |
66 |
150 |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
008 |
32 |
78 |
168 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
Pathological |
|
009 |
22 |
65 |
169 |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
Normal |
|
010 |
25 |
53 |
158 |
1/2 |
1/2 |
0/2 |
1/2 |
1/2 |
Suspicious |
Table 3 shows the clinical result of fetal ultrasound images for the 10 selected subjects and their respective risk status. The biophysical profile uses ultrasound and cardiotocography (CTG), also known as electronic fetal heart rate monitoring, to examine the fetus. Five components are measured during the biophysical exam. Each component that meets the criteria in Table 3 receives a two-point score. The tests are repeated until either all criteria are met or 30 minutes have elapsed. The points are then added up for a total possible score of 2. The Normal amniotic fluid is defined as a total score of 2 points or 1 out of 2 points. A score of 0 indicates that there may be issues that need to be evaluated or monitored further. Based on Table 3, it is concluded that subjects 006 and 010 are suspicious, and subjects 004 and 008 are classified as having pathological conditions due to their low BPP score. The remaining subjects were discovered to be in Normal condition, with a good BPP score.
 |
Figure 9: Sample annotated image of a subject. |
Figure 9 shows the sample annotated image of a subject. The obstetrician has evaluated subject 001, with the Biophysical profile score. In this all five BPP parameters show a 2/2 BPP score, so it’s confirmed that the fetus is in Normal condition only. Likewise, all the subjects will be evaluated by the obstetricians with the help of the Biophysical profile score. If the fetus is found to be in a pathological condition, immediate actions will be taken by the doctors. Through proper analysis of the ultrasound scan maternal abdominal images and their clinical results, we can prevent the fetus from risk conditions during the gestational period.
Conclusion
The unusual changes in the heart rate of the baby in the uterus can be examined using CTG and BPP tests. In this paper, we have introduced a new medical image database called BIOPHYSIT, a collection of ultrasound scan images for biomedical and healthcare application research. It is developed with the intention of providing a common benchmark for fetal stress condition research and related development. The main characteristics of this BIOPHYSIT database are: a) 300 ultrasound images have been collected from 50 female subjects b)explains the construction and composition of the database c) Annotation of the entire subject’s biological data d) Clinical report with suggestions by the obstetrician for all the subjects. By providing this database available to the biomedical research community, we hope to promote the analysis of many uncertain problems. The BIOPHYSIT database along with all clinical measures will be publically made available for research.
Acknowledgement
The authors thank Dr. Rajkumar, Radiologist, at Government Hospital, and Ramnad, India for his help and suggestions in building this database. The authors also immensely thank Dr. Ananthi Babu, Obstetrician, and Madurai, India for providing ultrasound scan images with all clinical interpretations to create this BIOPHYSIT database.
Conflict of Interest
There are no conflict of interest.
Funding Sources
This work is funded by the DST-SEED project titled, “Non-invasive fetal stress monitoring device” in the year 2021. The seed number is SEED/WS/2019/327.
References
- Freeman R K, Garite T J, Nageotte M P and Miller L A Fetal Heart Rate Monitoring 2012 (Philadelphia, PA: Lippincott Williams & Wilkins).
- Freeman RK, Garite TJ, Nageotte MP Fetal heart rate monitoring. Philadelphia: Lippincott, Williams &Wilkins, 2003,pp. 1–4.
- D. Ayres de Campos, C. Y. Spong, and E. Chandraharan FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography Int. J. Gynaecol. Obstet., 2015, vol. 131, pp. 13-24.
CrossRef - Raymond G Kennedy MRCOG Electronic fetal heart rate monitoring: retrospective reflections on a twentieth-century technology Journal of the Royal Society of Medicine, 1998, Volume 91.
CrossRef - Neilson, J.P Fetal electrocardiogram (ECG) for fetal monitoring during labor Cochrane Database Syst. Rev. 2006, 3.
CrossRef - Freeman, R.K.; Garite, T.J.; Nageotte, M.P.; Miller, L.A. Fetal Heart Rate Monitoring Lippincott Williams & Wilkins: Philadelphia, A, USA, 2012.
- Karvounis E. C., Tsipouras M. G., Fotiadis D. I An automated methodology for fetal heart rate extraction from the abdominal electrocardiogram IEEE Trans InfTechnol Biomed, 2007, Vol.11, pp.628–638.
CrossRef - Ungureanu, M., Bergmans, J.W. M.,Mischi, M. Improved method for fetal heart rate monitoring 27th annual international conference of the engineering in medicine and biology society, IEEE-EMBS, Shanghai, China,2005, pp.5916–5919.
CrossRef - Christina S. Han, Lawrence D. Platt Fetal Diagnosis and Care (Second Edition)in Obstetric Imaging: 2018.
- Pavitra Reddy Nalamaru, Modified biophysical profile in the role of predicting fetal outcome in high risk pregnancies, 2020, Volume: 7, Issue: 3.
CrossRef - R. Swarnalatha and D. V. Prasad A novel technique for extraction of FECG using multi stage adaptive filtering 2010, J. Appl. Sci., vol. 10, no. 4, pp. 319–324.
CrossRef - Jimmy Espinoza A simple Bio physical profile American journal of Obstetrics and Gynecology, 2018, vol.218, issue.01.
CrossRef - Neil S. Seligman Optimizing and validating an EMG-based Fetal monitor to identify true preterm labor Rochester, NY 14642. 2012.
- https://my.clevelandclinic.org/health/diagnostics/21013-biophysical-profile
- Megan Lord; Sarah Marino; Martha Kole Amniotic fluid index National library of Medicine, March 22, 2022.
- Lesley Carmichael, Karen Campbell and John Patrick Fetal breathing, gross fetal body movements, and maternal and fetal heart rates before spontaneous labor at term American journal of Obstetrics and Gynecology,1984, pp.675-679.
CrossRef - D Deopa, C S Ramesh Babu, Comparison of placental grading by ultrasonographic study in normal and high risk pregnancy in north Indian population Journal of anatomical society of India, 2011, Issue no.60, pp no.31-36.
CrossRef







