Norhaida Che Azmi** , Anis Athiqah Suhimi
, Anis Athiqah Suhimi , Tuan Syaripah Atiqah Najwa Tuan Yahya1
, Tuan Syaripah Atiqah Najwa Tuan Yahya1
Universiti Kuala Lumpur, Institute of Medical Science and Technology, Taman Kajang Sentral, Kajang Malaysia.
Corresponding Author E-mail: norhaida@unikl.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2797
Abstract
Thrombotic diseases such as strokes, deep vein thrombosis (DVT), pulmonary embolism are serious consequences of the thrombus formed in blood vessels. The coagulation cascade is a complicated system that involves clotting factor of extrinsic, intrinsic, and common pathways. Imbalance between the process can cause excessive clotting that led to thrombosis. Although several anticoagulants drugs have been developed over the decades, most are accompanied by undesirable side effects such as mild or severe bleeding. Therefore, this study has made use of the natural resources in the search for complementary and alternative therapies. The methanolic extract of Momordica charantia (MC) (bitter gourd) fruit flesh is utilized to identify their effects through in vitro anticoagulant and thrombolytic activity. The anticoagulant activity is tested using prothrombin time (PT) and activated partial prothrombin time (APTT) test with 10, 20 and 30 mg/ml of MC extraction, while the thrombolytic activity is carried out by using clot lysis assay. Momordica charantia fruit flesh methanolic extract was proven to have significant anticoagulant properties at concentration 20 and 30 mg/ml compared to normal and negative control. The extract however was not significant at 10 mg/ml but still demonstrated some anticoagulation effect. It also revealed a substantial thrombolytic activity at 100 mg/ml, when compared to negative and positive control indicating ability to lyse blood clot. It was discovered by our analysis that Momordica charantia fruit flesh possesses significant anticoagulant and thrombolytic activity which can be further exploited in the treatment of blood coagulation disorder.
Keywords
Anticoagulants; aPTT; methanolic; Momordica charantia; PT; thrombolytic
Download this article as:| Copy the following to cite this article: Azmi N. C, Suhimi A. A, Yahya T. S. A. N. T. Anticoagulant Evaluation of Momordica charantia Fruit Flesh Extract on Prothrombin Time and Activated Partial Prothrombin Time Test. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Azmi N. C, Suhimi A. A, Yahya T. S. A. N. T. Anticoagulant Evaluation of Momordica charantia Fruit Flesh Extract on Prothrombin Time and Activated Partial Prothrombin Time Test. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3MGEE9d |
Introduction
Thrombotic diseases such as myocardial or cerebral infarction are serious consequences of the thrombus formed in blood vessels 1. Thrombolysis is process of lysing blood clots using thrombolytic therapy. The thrombolytic agents such as tissue plasminogen activator, urokinase, streptokinase (SK), etc are used to dissolve the already formed clots in the blood vessels 2. These drugs do, however, come with significant limitations that might have harmful, occasionally deadly, effects. Examples include haemorrhage, a severe allergic reaction, a lack of specificity, etc.
The coagulation cascade is a complicated system that involves many clotting factors which must act in an exact sequence to produce clot formation. The procedure is rapid and efficient but needs to be regulated as excessive clotting can cause thrombosis if it is not under control. There are two pathways involved which are the extrinsic (tissue factor) and intrinsic (contact factor) pathways. At the end of this pathways, a solid hemostatic clot is formed. A shift in the balance between blood coagulation and inhibition of coagulation may result in life-threatening thromboembolism or hemorrhage 3. Anticoagulants are chemical agents that interact with the body’s natural blood coagulation system and typically used for treating thrombotic disorders such as deep vein thrombosis (DVT), pulmonary embolism (PE), Venous thromboembolism (VTE), and Atrial fibrillation. Anticoagulant drugs are widely used to control blood coagulation in both healthy and diseased conditions such as cardiovascular disease, diabetes mellitus, and cancer. Although a number of these drugs have been developed over the decades, most are usually accompanied by undesirable side effects such as mild or severe bleeding. Therefore, there is a rise in interest in research into the discovery of natural anticoagulant drugs with less toxicity and fewer side effects.
Medicinal plants have been found to be relevant sources of novel therapeutic agents. Momordica charantia, a part Cucurbitaceae family, grows in tropical areas and is also named as bitter melon, bitter gourd, karela, pare and balsam pear. The white, or green unripe version of the fruit has bitter taste that intensifies as it ripens. The fruit consists of extensive variation of bioactive chemicals such as alkaloids, saponins, triterpens, proteins, steroids, flavonoids, and acids. Momordica charantia chemical constituents help to enable it to be effective against fungal, bacterial, and viral infection, besides inhibiting fertility, tumour formation and even carcinogenic substances. It also has hypoglycaemic effect when it is consumed 4. Yet, to date, there are no previous studies on the effect of Momordica charantia fruit flesh on thrombolytic and blood coagulation activity. Thus, it is essential to investigate and explore the potentials of this plant by measuring their effect on in vitro clot lysis and anticoagulant properties via prothrombin time (PT) and the activated partial thromboplastin time (aPTT) test.
Materials and Methods
Preparation of plant material
1 kg of Momordica charantia fruits was purchased from a local market in Kajang, Selangor. The voucher specimen No. NCA0001 was deposited at the Institute of Medical Science Technology, Universiti Kuala Lumpur. The fruits were cleaned with running tap water, and thoroughly dried and finely powdered prior to extraction with methanol.
Preparation of plant extracts
Momordica charantia flesh methanolic extraction
Momordica charantia was prepared according to the previous study with some modification 5. 170 g worth of Momordica charantia powder was weighed using balance scale (AY220, Shimadzu, Japan). The powder was then soaked in a Scott’s bottle filled with 1 litre of 90 % methanol up to 4 days. After soaking, the mixture was filtered using Whatman No. 1 filter paper (Whatman, England). Excess powder that has been filtered out , disposed and the filtered solution later then was transferred into a beaker. Magnetic stirrer (IKA C-Mag HS7) was used to constantly stir the solution at 50 ˚C for few days until the extract became concentrated due to evaporation of methanol. The crude extract was kept at -20 ˚C until further use.
Plasma Sample
The pool of normal human plasma was obtained by purchasing the STAGO pool plasma (STAGO 00538, Diagnostica Stago, France).
Anticoagulant Activity
The two anticoagulant assays used in this study are aPTT and PT test. The experiment consists of two groups: control group and experimental group. 100 μl of sample plasma was transferred to the test tubes for both groups before other substances were added.
For control, 100 μl of saline solution was added to a test tube already containing sample plasma for negative control. Another test tube containing plasma only, serves as the normal control. In experimental group, 1 ml each of stock solution with different concentration (10. 20, and 30 mg/ml) of the plant extraction was pipetted separately into three different test tubes respectively. All tubes with the mixture were shaken gently.
Prothrombin time (PT)
PT was determined by using a STAGO Neoplastin Cl Plus reagent kit (Diagnostica Stago, France). For PT test, the mixture of both control and experimental group were incubated in water bath at 37 ˚C for 2-5 minutes. 200 μl of prewarmed PT reagent (Neoplastine) were added to the test tubes and the content was rapidly mixed. Stopwatch was started immediately, and the tubes were gently tilted at regular interval until first sign of clotting was formed. The stopwatch was stopped, and the clotting time were recorded.
Activated partial thromboplastin time (aPTT)
The aPTT test was determined using STAGO CK Prest 2 reagent kit (Diagnostica Stago, France). The mixture from both control and experimental group were incubated in water bath at 37 ˚C for three minutes only. After exactly 3 minutes, 100 μl of aPTT reagent (CK Prest 2) were added into the mixture, followed by adding of 100 μl of pre-warmed 25 mM of calcium chloride. The test tubes and the contents were promptly mixed to induce the coagulation. Stopwatch was started immediately, and the tubes were gently tilted at regular interval until first sign of clotting was formed. The stopwatch was stopped and the clotting time were recorded.
In vitro thrombolytic assay
4.5 ml venous blood was drawn from a healthy volunteer and distributed in nine different pre-weighed sterile Eppendorf tube (0.5 ml/tube). Then, the Eppendorf tubes (0.5 ml/tube) was incubated at 37 ˚C for 45 minutes. After clot formation, serum was removed completely without interrupting the clot. The tubes contain clot were weighed again and the clot weight was obtained.
[Clot weight = weight of clot containing tube – weight of tube alone]Then, 100 μl of Momordica charantia extract (100 mg/ml) was added to each of microcentrifuge tube containing pre-weighed clot. 100 μl of Heparin (Heparinol, 5000 unit) was used for positive control while 100 μl of saline solution was used as negative control. Then, all tubes were incubated at 37 ˚C for 90 minutes and breakdown of clot was observed. Subsequent of incubation, the fluid formed was disposed and weight of tubes were taken again to observe the difference in weight after clot have been lysed. The changes in weight that were obtained prior to and after clot lysis were presented in percentage of clot lysis. The experiment was repeated with the blood samples from at least ten (10) healthy volunteers 6.
Clot lysis (%) = (weight of lysis) / (weight of clot before lysis) × 100
Statistical analysis
For the anticoagulant assay, the data collected were analysed by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple comparisons using SPSS version 20.0 (SPSS for Windows, Version 20.0, IBM Corporation, New York, USA). ANOVA had identified the Prothrombin Time (PT) test and Activity Partial Thromboplastin Time (aPTT) test at three different concentrations. For the thrombolytic test, the data were expressed in mean ± standard deviation for triplicate and were expressed in percentages. The significance percentage of clot lysis (%) between positive, negative control and Momordica charantia fruit flesh extracts were analyzed by using the ANOVA, SPSS software, version 20.0. The P-values <0.05 were considered statistically significant.
Results and Discussion
The present study investigated the in vitro effects of Momordica charantia fruit flesh extract on blood coagulation and the thrombolytic activity in terms of percentage of weight loss of in vitro formed clots lysis. The PT measures clotting factors involved in common and extrinsic pathways, while aPTT measures clotting factors involved in common and intrinsic pathways. The results showed significant prolongation of PT and aPTT clotting time by the methanol extract in a concentration-dependent manner (10, 20, 30 mg/ml).
PT and aPTT Clotting time of Momordica charantia extraction
30 mg/ml Momordica charantia fruit flesh extract showed the highest Activated Partial Thromboplastin time (aPTT) with 21.33 ± 1.528 seconds, followed by 20 mg/ml extract with 21 seconds and 10 mg/ml with 19 ± 1.732 seconds as shown in Table 1.
Table 1: aPTT test (seconds) of methanolic extract of Momordica charantia fruit flesh at different concentrations.
|
Concentration |
N |
Mean (SD) |
F-value |
P-value |
|
aPTT |
|
|
5.514 |
0.013 |
|
10 mg/ml |
3 |
19 (1.732) |
||
|
20 mg/ml |
3 |
21 (0) |
||
|
30 mg/ml |
3 |
21.33 (1.528) |
||
|
Normal control |
3 |
17.33 (2.082) |
||
|
Negative Control |
3 |
16.67 (1.528) |
ANOVA test
**Significant at α < 0.05
In post hoc analysis, Dunnett’s multiple comparisons test showed that activated partial thromboplastin time test of 10 mg/ml methanolic extraction of Momordica charantia fruit flesh was not significantly higher in comparison to normal control and negative control with p > 0.05 (0.044, 0.487). However, activated partial thromboplastin time test of 20 mg/ml methanolic extraction of Momordica charantia fruit flesh was significantly higher in comparison to normal control and negative control with p < 0.05 (0, 0.001). Activated partial thromboplastin time test of 30 mg/ml methanolic extraction of Momordica charantia fruit flesh was also significantly higher in comparison to normal control and negative control (p < 0.05). Thus, the result is statistically significant for methanolic extract of Momordica charantia at concentration of 20 and 30 mg/ml (Figure 1).
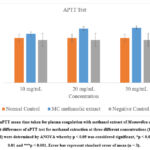 |
Figure 1: aPTT mean time taken for plasma coagulation with methanol extract of Momordica charantia. |
Significant differences of aPTT test for methanol extraction at three different concentrations (10, 20 and 30 mg/ml) were determined by ANOVA whereby p < 0.05 was considered significant, *p < 0.05, **p < 0.01 and ***p < 0.001. Error bar represent standard error of mean (n = 3).
For Prothrombin Time (PT) analysis, 30 mg/ml Momordica charantia fruit flesh extract showed the highest number with 100.67 ± 5.508 seconds, followed by 20 mg/ml extract with 55.33 ± 4.041 seconds and 10 mg/ml with 40.67 ± 7.506 seconds (Table 2).
Table 2: PT test (seconds) of methanolic extract of Momordica charantia fruit flesh at different concentrations.
|
Concentration |
N |
Mean (SD) |
F-value |
P-value |
|
PT |
|
|
97.090 |
< 0.05 |
|
10 mg/ml |
3 |
40.67 (7.506) |
||
|
20 mg/ml |
3 |
55.33 (4.041) |
||
|
30 mg/ml |
3 |
100.67 (5.508) |
||
|
Normal control |
3 |
28 (2.646) |
||
|
Negative Control |
3 |
36 (4.359) |
ANOVA test
**Significant at α < 0.05
In post hoc analysis, Dunnett’s multiple comparisons test showed that prothrombin time test of 10 mg/ml methanolic extraction of Momordica charantia fruit flesh was not significantly higher compared to normal control and negative control with p > 0.05 (0.463, 0.265). Still, prothrombin time test of 20 mg/ml methanolic extraction of Momordica charantia fruit flesh was significantly higher in comparison to normal control and negative control with p < 0.05 (0.041, 0.021). Prothrombin time test of 30 mg/ml methanolic extraction of Momordica charantia fruit flesh was also significantly higher in comparison to normal control and negative control with p < 0.05 (0.052, 0.029).
Statistically, f-value was 97.090 and p < 0.05. Thus, the result is statistically significant for methanolic extract of Momordica charantia at concentration of 20 and 30 mg/ml (Figure 2).
 |
Figure 2: PT mean time taken for plasma coagulation with methanol extract of Momordica charantia. |
Significant differences of aPTT test for methanol extraction at three different concentrations (10, 20 and 30 mg/ml) were determined by ANOVA whereby p < 0.05 was considered significant, *p < 0.05, **p < 0.01 and ***p < 0.001. Error bar represent standard error of mean (n = 3).
Thrombolytic assay of Momordica charantia extraction
100 mg/mL Momordica charantia fruit flesh extract showed the highest clot lysis percentage with 49.916 ± 18.00662 percent, followed by the negative control with normal saline at 37.8203 ± 17.28720 percent and positive control with heparin at 34.2379 ± 21.79589 percent.
The mean difference in clot lysis percentage between 100 mg/mL methanolic extraction of Momordica charantia fruit flesh, positive and negative controls was very significant (p < 0.005). Percentages of clot lysis obtained after treating the clots with Momordica charantia fruit flesh and appropriate controls are shown in Table 3 and their comparison was presented in Figure 3.
Table 3: Clot lysis (percentage) of methanolic extract of Momordica charantia fruit flesh at 100 mg/ml.
|
Concentration |
N |
Mean (SD) |
F-value |
P-value |
|
Clot Lysis |
|
|
|
|
|
Negative Control |
30 |
37.8203 (17.28720) |
5.531 |
0.005 |
|
100 mg/ml |
30 |
49.9160 (18.00662) |
||
|
Positive Control |
30 |
34.2379 (21.79589) |
ANOVA test
**Significant at α < 0.05
 |
Figure 3: Mean percentage of clot lysis in Thrombolytic Assay of Momordica charantia fruit flesh methanolic extract. |
Significant differences of aPTT test for methanol extraction at three different concentrations (10, 20 and 30 mg/ml) were determined by ANOVA wherebyp < 0.05 was considered significant, *p < 0.05, **p < 0.01 and ***p < 0.001. Error bar represent standard error of mean (n = 3).
As noted in the results, Momordica charantia fruit flesh methanolic extract was proven to have significant anticoagulant properties at concentration 20 and 30 mg/ml compared to normal and negative control. The extract anticoagulant activity however was not significant at 10 mg/ml but was still having some anticoagulation effect. For both aPTT and PT test, the anticoagulation effect was prolonged significantly with dose-dependent increase. This shows that the extract was capable to inhibit the clotting factors of intrinsic (Factor VII) and extrinsic pathway (clotting factors XII, XI, IX, VIII ). In addition, this prolonged time could also affect the inhibition in the common pathway factors (X, V, II, and I). It is recommended to evaluate the mechanism of action of these plant extracts on the coagulation cascade.
It also showed significant thrombolytic activity at 100 mg/ml, when compared to negative and positive control indicating ability to lyse blood clot. This indicates that MC extract has potential as thrombolytic agents in thrombolytic therapy. Conflicts occurred when it comes to thrombolytic medication are that, although they are considered mostly safe, some complications may arise in term of bleeding. 1.3 % of patients on thrombolytic therapy had experienced intercranial haemorrhage and 11 % of them have had significant bleeding issues. Some of the adverse effects from using thrombolytic drugs consists of vascular lesions, severe hypertension, brain tumour, ischemic stroke and active bleeding 7.
Previous study on Momordica charantia seed extract (MCSE) has exhibited strong anticoagulant effect with a mechanism that interrupts the intrinsic pathway of the plasma coagulation cascade. The plasma recalcification time and in-vivo bleeding time were analysed to investigate the finding. Both in-vitro and in-vivo anticoagulant activity showed constant proof of presence of anticoagulant activity by MCSE. MCSE also demonstrated thrombolytic activity, specifically by fibrinolytic activity by hydrolyzing fibrinogen and fibrin clot, through degrading all the chains of partially cross-linked fibrin clot. The extract did not cause hemolysis, hemorrhage and edema on the tested mice 8.
Anticoagulant properties of the Momordica charantia fruit flesh extract may be due to the compound linolenic acid that is present in the bitter gourd fruit flesh. It was described that Ocimum sanctum fixed oil was capable to prolong the time taken for blood coagulation with mechanism that was comparable to the anticoagulation action of aspirin, specifically through the antiplatelet mechanism 9.
Besides linolenic acid, other compound in the extract may also have caused anticoagulation and thrombolytic effect. It has also reported on the anticoagulation activity of tarragon leaf extract, due to the presence of the compound, coumarin, by acting as an antiplatelet agent, inhibiting platelet segregation, and decreasing protein secretion up to 50 % 10. Methanol extraction of coumarin compound from the tarragon leaves had the most satisfactory result, with highest increase of PT time. However, decoction, the most frequently used pharmaceutical herb preparation technique, was not able to extract the coumarin compound afterward.
Crinumin isolated from Crinum asiaticum latex has also been demonstrated to have thrombolytic effect, mainly with fibrinolytic mechanism that dissolves human blood clot in a dose-dependent and time-dependent manner. It also showed a rapid onset of action which is highly beneficial for the treatment of medical emergencies like stroke and myocardial infarction 11. Further study on Momordica charantia fruit flesh molecule build-up can determine exact inhibition that occurs during anticoagulant and thrombolytic activity.
Momordica charantia have previously been proved of its antidiabetic properties. It has reported that patients that are on anticoagulant therapy shall avoid Cyamopsis tetragonoloba after it was found that it exhibits anticoagulant properties thus it may enhance the anticoagulation potential of anticoagulant agent 12. Therefore, patients that are on anticoagulant drugs that are consuming Momordica charantia for its antidiabetic or other benefits shall also take precaution due to possibility of enhancing the anticoagulant effect of the medication. However, for a long time, diabetes has been recognized as a risk factor for acute myocardial infarction 13. Globally, diabetic patients have been proved to have greater risk for myocardial infarction and heart failure 14. Myocardial infarction and stroke are typically caused by arterial thrombosis. Further research can enable Momordica charantia to serve as multipurpose drugs, treating or controlling both diabetes and thrombotic disorder simultaneously.
Conclusion
Although the beneficial effects of anticoagulation and thrombolytic therapy are well established in the market, the search for alternative and complimentary therapy is continuing due to some reasons including availability and diversity of natural resources. The study showed that Momordica charantia fruit flesh methanolic extract has significant anticoagulant and thrombolytic activity which can be further developed in the treatment of blood coagulation disorder. Nevertheless, as this is merely a preliminary analysis, it is premature to draw any firm conclusions regarding the probability of these Momordica charantia as anticoagulant and thrombolytic drugs, in vivo study are yet to be investigated. For future work, it is also recommended to discover the active compound and elucidate their exact mechanism of action that contribute to the anticoagulation and clot lysis properties.
Acknowledgement
The authors thank the Clinical and Biomedical Science Section, University Kuala Lumpur Institute of Medical Science Technology (UniKL MesTech), Kajang, Selangor.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Source
There are no funding sources.
References
- .R, Amrin N, Begum J and Mazid M.A. Thrombolytic activity of some spices and plants available in Bangladesh. The Thai Journal of Pharmaceutical Sciences., 2012; Apr 1;36:72-7.
- Mahmud S, Akhter S, Rahman M.D, Aklima J, Akhter S, Merry S.R, Jubair SM, Dash R and Emran TB. Antithrombotic effects of five organic extracts of Bangladeshi plants in vitro and mechanisms in in silico models. Evidence-Based Complementary and Alternative Medicine., 2015 Jan 1; 2015.
CrossRef - Ayodele O.O, Onajobi F.D and Osoniyi O. In vitro anticoagulant effect of Crassocephalum crepidioides leaf methanol extract and fractions on human blood. Journal of experimental pharmacology., 2019;11:99.
CrossRef - Ahmad N, Hasan N, Ahmad Z, Zishan M and Zohrameena S. Momordica Charantia: for Traditional Uses and Pharmacological Actions. Journal of Drug Delivery and Therapeutics., 2016; 6(2): 40-44.
CrossRef - Shahik S.M, Sikder M.O, Patwary N.I, Sohel M, Islam M.S, Nishi T.F, Sultana T and Barua R. In vitro thrombolytic and cytotoxic evaluation of Mentha arvensis L., Mentha spicata L. and Mentha viridis L. Journal of Pharmaceutical and Biological Sciences., 2014; 9: 97-102.
CrossRef - and Mahmood A.A. Study on in-vitro thrombolytic activity of methanolic extract of Mesua ferrea leaves. 2015; 2(3):52–55.
- and Mousa F.M. The prophylactic and therapeutic effects of Momordica charantia methanol extract through controlling different hallmarks of the hepatocarcinogenesis. Biomedicine and Pharmacotherapy., 2018; 98 (December 2017): 491–498.
CrossRef - and Sannaningaiah D. Momordica charantia seed extract exhibits strong anticoagulant effect by specifically interfering in intrinsic pathway of blood coagulation and dissolves fibrin clot. Blood Coagulation and Fibrinolysis, 2015; 26(2): 191–199.
CrossRef - and Dash D. Thrombolytic along with anti-platelet activity of crinumin, a protein constituent of Crinum asiaticum. Blood Cells, Molecules, and Diseases., 2011; 47(2): 129–132.
CrossRef - and Sofic E. Anticoagulant activity of some artemisia dracunculus leaf extracts. Bosnian Journal of Basic Medical Sciences., 2015;15(2): 9–14.
CrossRef - and Rashid A. Anti-coagulant activity of plants: mini review. Journal of Thrombosis and Thrombolysis., 2017; 44(3): 406–411. https://doi.org/10.1007/s11239-017-1546-5
CrossRef - and Cosentino F. Diabetes: Prevalence, prognosis and management of a potent cardiovascular risk factor. European Journal of Preventive Cardiology., 2017; 24(3): 52–60.
CrossRef - Bharti SK, Krishnan S, Kumar A, Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Therapeutic Advances in Endocrinology and metabolism., 2018; Mar; 9(3):81-100.
CrossRef - and Freedman, J. E. Thrombosis and platelets: An update. European Heart Journal., 2017; 38(11): 785–791.







