Fraction K. Dzinjalamala*, Daniellah Lwanda, Getrude Pelusi, Maisha Mphanga, Felix Mbalule, Paul Makocho, Wilson Mandala, Mwaiwawo Madanitsa, Petros Chigwechokha and Gama Bandawe
Academy of Medical Sciences, Malawi University of Science and Technology, Limbe, Malawi.
Corresponding Author E-mail: fdzinjalamala@must.ac.mw
DOI : https://dx.doi.org/10.13005/bpj/2773
Abstract
Background: The global challenge of antimicrobial resistance has spurred scientific research efforts to find alternative sources of new antibiotics. The ethnopharmacological importance of Tithonia diversifolia is well-known. Objective: The present study’s aim was to evaluate the in vitro activity of crude aqueous leaf, stem and root extracts of locally growing Tithonia diversifolia against the clinical bacterial isolates: E. coli, K. pneumoniae, and P. mirabilis. Methods: To obtain antibacterial activity data, the Broth macrodilution testing and Zone of inhibition Kirby-Bauer approaches were used. Results: Estimated diameters of zone of inhibition showed leaf extracts of Tithonia diversifolia had significantly greater antibacterial activity (19.5 ± 3.9 mm, 95% CI: 15.4 - 23.6 mm) than stem (15.2 ± 2.0 mm, 95% CI: 13.0 - 17.3 mm, p = 0.021) or root extracts (15.0 ± 2.1 mm, 95% CI: 12.8 - 17.2 mm, p=0.019). K. pneumoniae was the most susceptible isolate to growth inhibition by extracts from all plant parts. In broth macrodilution testing, leaf extracts exhibited greater potency on all study isolates compared to stem and root extracts. Conclusion: These findings support the traditional use of Tithonia diversifolia decoctions and infusions in infectious processes that are due to these pathogens and further strengthens recommendations for additional work to isolate and characterize the bioactive chemical compounds responsible for the observed antibacterial properties of the plant.
Keywords
Broth macrodilution assay; Ethnopharmacology; Kirby-Bauer disk diffusion assay; Tithonia diversifolia; Zone of inhibition
Download this article as:| Copy the following to cite this article: Dzinjalamala F. K, Lwanda D, Pelusi G, Mphanga M, Mbalule F, Makocho P, Mandala W, Madanitsa M, Chigwechokha P, Bandawe G. Antibacterial Activity of Crude Aqueous Extracts of Tithonia Diversifolia from Chichiri Area in Blantyre District, Malawi. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Dzinjalamala F. K, Lwanda D, Pelusi G, Mphanga M, Mbalule F, Makocho P, Mandala W, Madanitsa M, Chigwechokha P, Bandawe G. Antibacterial Activity of Crude Aqueous Extracts of Tithonia Diversifolia from Chichiri Area in Blantyre District, Malawi. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3H2ZPyU |
Introduction
Various studies have reported alarming trends in antimicrobial resistance in blood stream infection isolates in Malawi1, 2, 3 and an ongoing large scale study is characterizing the morbidity, mortality and economic cost of third-generation cephalosporin resistant bloodstream infection 4.
The combination of restricted access to the few effective antibiotics in resource-poor settings such as Malawi and the global burden of AMR has spurred scientific investigation of phytochemicals as an alternative source of new antimicrobial drugs.
Medicinal plants in most developing countries, including Malawi, are well recognized as alternative therapeutic agents for the maintenance of good health5. In an ethnobotanical study of traditional medicinal plants used for the treatment of infectious diseases by local communities in Mzimba district of the northern region of Malawi, Chisamile recorded eighty medicinal plants belonging to 43 families and 77 genera6. Similarly, in an ethnomedicinal survey by Chikowi in Zomba district of the southern region of Malawi, fifty-nine medicinal plant species belonging to 38 families were reported to be in use as prophylaxis and treatment for 27 communicable and non-communicable diseases/conditions7. The afore-cited research findings underscore the observation that Malawi has a rich biodiversity of medicinal plant species that represent an exploitable resource in discovery research for lead bioactive compound development.
There is, however, a paucity of published data from Malawi on the in vitro antibacterial activity of native medicinal plants. Pterocarpus angolensis (locally known as Mlombwa tree) grows in many parts of Malawi. In a study by Chipinga, the aqueous, dichloromethane and methanolic extracts of the leaves, stem-bark, fruits and roots of Pterocarpus angolensis were shown to be effective against Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae and Candida krusei by the macrotube dilution method.
Tithonia diversifolia (Hemsl) A. Gray is a member of the sunflower family, Asteraceae. Whereas this plant is native to Central America and the West Indies, it has become naturalized in Malawi, growing around agricultural fields, in waste places, along river banks and in many other ecological environments. Decoctions or infusions of T. diversifolia have been widely reported as being of medicinal use in many countries: demonstrating antibacterial, antiplasmodial activities of various parts of the plant8-11. External use on wounds has also been reported12. The ethnopharmacological importance of this plant is comprehensively reviewed by Ajao and Moteetee13.
It is recognized that Tithonia diversifolia can grow in many different environmental conditions14 where it does not require a large amount of nutrients because it is able to increase the amount of essential nutrients in the soil on its own15 and exhibits seasonal as well as geographical variation in phytochemical composition16,17. Geographical variation in phytochemical composition has been observed in other plant species18,19. In the present study the purpose was, therefore, to investigate and validate the in vitro antibacterial activity of aqueous extracts of T. diversifolia growing in Blantyre city, Malawi.
Materials and Methods
Nutrient agar (ATICO India, India), Mueller-Hinton agar (Oxoid, UK), Blood Agar Base (Mast Group Ltd, UK), Bacterial isolates (E. coli, Proteus mirabilis, K. pneumoniae) from Malawi-Liverpool Wellcome Trust Program, drugs (Malawi Medicines Regulatory Authority).
Source of Samples, Identification, and Preparation
This study was conducted from the 16TH of November, 2022 to the 6TH of December, 2022. T. diversifolia (Figure 1) samples were collected from Chichiri near Polytechnic hostels and transported in a cooler box to the Biological Sciences Laboratories at The Malawi University of Science and Technology (MUST). T. diversifolia specimens underwent identification and authentication by the National Herbarium and Botanic Gardens of Malawi and the voucher specimens (Accession number 84191) were deposited at the herbarium. Once at MUST, plant samples (the leaves, roots and stems) were washed with distilled water and left to air dry separately for 4 days away from sunlight as shown in the setup in Figure 2A.
 |
Figure 1: Photograph of T. diversifolia from Chichiri area, |
Plant Extraction Procedure
The air-dried leaves, stems and roots of T. diversifolia were crushed separately using traditional mortar and pestle typically used by ordinary Malawians when preparing these plants for medicinal use (Figure 2B). This resulted in a fine powder of each plant part as shown in Figure 2C.
 |
Figure 2: Photographs showing preparation of T. diversifolia. (A) Air-drying of plant. (B) Powdering of dry plant material using traditional mortar and pestle. (C) Tithonia powder by plant part. |
Thirty grams (30 g) of each powder obtained from the leaves, stems and roots was separately suspended in 225mls of sterile distilled water in three conical flasks which were cupped to be airtight. The suspension of the roots, leaves and stem were then left to soak in the airtight conical flasks for 2 days before filtration into a Petri dish using a cheese-cloth to obtain the desired aqueous filtrate for each plant part. The filtrate was then transferred from the Petri dish to an evaporation flask using a sterile funnel. Gentle evaporation of the filtrate was done in a Pyrex glass beaker by using a heating mantle sent at 450C until a residue was obtained.
Test Organism
The clinical isolates of bacteria (Klebsiella pneumoniae, Escherichia coli and Proteus spp.) used in this studywere provided by The Malawi Liverpool Welcome Trust laboratory based at Queen Elizabeth Central Hospital in Blantyre and were stored in the freezer at -800C.
Preparation of Nutrient Agar
Nutrient Agar was prepared according to the manufacturer’s recommendation which was to suspend 14g of the nutrient agar powder in 500mls of distilled water. The suspension was left on a heating mantle at 1000C for 6 minutes to completely dissolve the powder which was later autoclaved at 1210C and a pressure of 15psi for 15 minutes. The prepared media was then poured into 17 sterilized petri dishes aseptically and left to solidify for 20 minutes. The prepared media was left in the fridge for storage.
Preparation of Mueller-Hinton Agar
Mueller Hinton Agar was prepared according to the manufacturer’s recommendation which was suspending 38g of the Mueller Hinton agar powder in 1000mls of distilled water. The suspension was left on a heating mantle at 1000C for 6 minutes to completely dissolve the powder which was later autoclaved at 1210C and a pressure of 15psi for 15 minutes. The prepared media was then poured into 30 sterilized petri dishes aseptically and left to solidify for 20 minutes. The prepared media was left in the fridge for storage.
Preparation of Blood Agar (BA)
Blood Agar was prepared according to the manufacturer’s recommendation which was suspending 9.375g of the nutrient agar powder in 250mls of distilled water. The suspension was left on a heating mantle at 1000C for 6 minutes to completely dissolve the powder which was later autoclaved at 1210C and a pressure of 15psi for 15 minutes. After autoclaving, the suspension was left to cool to 450C were 5% of human blood was added. The prepared media was then poured into 4 sterilized petri dishes aseptically and left to solidify for 20 minutes. The prepared media was left in the fridge for storage.
Bacterial culture
E. coli and K. pneumoniae isolates were resuscitated by streaking on separate petri dishes of nutrient agar and incubating at 370C for 24 hours. Proteus was resuscitated on blood agar at 370C for 24 hours. Stock cultures of the resuscitated E. coli, K. pneumonia and Proteus isolates were then maintained at 4°C on slopes of nutrient agar and blood agar respectively.
Preparation of inoculum
Bacterial (Klebsiella pneumoniae, Escherichia coli and Proteus spp) inoculums were prepared with Nutrient agar and Blood Agar and standardized to 0.5 McFarland solution. 1 in 100 dilutions of the standardized Nutrient and Blood agar preparations brought the cell count to 5×106 CFU/ml which represented inoculum stocks.
Antimicrobial Susceptibility Testing
Antibacterial activity by disc diffusion assay
The Kirby-Bauer disc diffusion assay was carried out based on the Clinical Laboratory Standard Institute (CLSI) guidelines. To evaluate the drug susceptibility of study bacterial isolates, drug-impregnated disks of common antibiotics (Chloramphenicol, Gentamicin, Sulfamethoxazole Trimethoprim, Amoxicillin and Erythromycin) were placed on 3 separate agar plates inoculated with either E. coli or K. pneumoniae or P. mirabilis. In these tests blood agar plates were used for P. mirabilis while Mueller Hinton agar plates were used for E. coli and K. pneumoniae.
To evaluate the activity of the plant extracts against the bacterial isolates, disks (6 mm in diameter) impregnated by different concentrations of the plant extracts (0.625g/ml, 0.333g/ml, 0.165 g/ml, 0.083g/ml, 0.041g/ml and 0.021 g/ml), Gentamicin (10µg as positive control) and a blank disk (negative control) were placed on each of the agar plate that had been inoculated with a test isolate. All the plates were then incubated for 24 hours at 370C.
Antimicrobial activity was evaluated by measuring the zones of inhibition, against the tested microorganism in millimeter (mm). Each assay was carried out in duplicates.
Antibacterial Activity by Broth Macrodilution Assay
For each serial dilution of the plant extracts (concentrations: 62.5 mg/ml, 33.3 mg/ml, 16.7 mg/ml, 8.33 mg/ml, 4.17 mg/ml, 2.08 mg/ml, 1.04 mg/ml, 0.52 mg/ml, 0.26 mg/ml, 0.13 mg/ml) a final bacterial cell count of about 5 x 105 CFU/mL was achieved by transferring 1 ml of the prepared inoculum for each microorganism into the appropriate volume of serial dilution of each extract, representing 1 in 10 dilution of the respective inoculum stock. After 24 hours of incubation at 370C, bacterial cells were enumerated by direct microscopic count method.
Data management and analysis
After 24 hrs of incubation some Typical photos of agar plates were taken to demonstrate typical inhibition zones against the bacterial strains. Estimates of zones of inhibition are tabulated in MS Word based on plant extract concentration level versus bacterial strain. For each plant extract (i.e. for root extract or stem extract or leaf extract) and commercial antibiotic disks, inhibition zone diameters are reported in the tables. Inhibition zone data at 0.625 g/ml of extract or Gentamicin was pooled from all the tabulated data and used to calculate Mean (+/-SD)[a1] and 95% Confidence Interval for the inhibition zone estimates and reported within the text of the results section. The Mean(+/-SD) calculations were done using Stata SE version 17.0 (Stata Corp, College Station, TX, USA).
Broth macrodilution assay data were analyzed in GraphPad Prism 8.0 (https://www.graphpad.com) and results are reported as graphs of percentage of surviving bacterial cells versus concentration of crude T. diversifolia extract. From these graphs the minimum concentration (of plant extract) that inhibits 50% of the bacterial strain (MIC50) can be read off.
Results
Growth Inhibition Zone
Tables 1-3 show measured (after 24 hours of incubation) clear zones of inhibition around the drug-impregnated disks. Based on the EUCAST breakpoint interpretation for the antibiotic’s zone of inhibition all the isolates, K. Pneumoniae, P. mirabiris and E. coli were sensitive to Gentamicin with mean inhibition zone ranging from 22 – 25 mm (23± 1.5 mm). For this reason, Gentamicin was chosen for use as the positive control antibiotic in both the Kirby-Bauer disc diffusion and broth macrodilution assays for the assessment of Tithonia diversifolia antibacterial potency.
Tables 4-6 show inhibition zone data that demonstrate antibacterial activity of crude aqueous extracts (leaf, stem and roots) of Tithonia diversifolia by the Kirby-Bauer disc diffusion assay. At a concentration of 0.625g/ml, the mean inhibition zone for the leaves, stems and root extracts (mean = 18.0 ± 0.9 mm, 95%CI: 16.0 – 20.0 mm, n=12) were comparable to inhibition zone diameters for Gentamicin (Mean = 17.3 ± 0.9 mm, 95%CI: 15.9 – 18.6 mm, n=12) action against E. coli and K. pneumoniae only. In the same concentration, Tithonia diversifolia leaf, stem and root extracts appeared significantly less inhibitory towards P. mirabilis when compared to Gentamicin (mean zone diameter = 13.7 ± 1.5 mm, 95% CI: 12.1 – 15.2 mm, n=6 versus zone diameter =23.3 ± 3.7 mm, 95% CI: 19.5 – 27.2 mm, n=6 respectively; p=0.0003). Figures 3 – 5 show typical agar plate pictures of the Kirby-Bauer disc diffusion assay results.
Generally, based on estimated diameters of inhibition, leaf extracts of Tithonia diversifolia had significantly greater antibacterial activity (19.5 ± 3.9 mm, 95% CI: 15.4 – 23.6 mm) than stem (15.2 ± 2.0 mm, 95% CI: 13.0 – 17.3 mm, p = 0.021) or root extracts (15.0 ± 2.1 mm, 95% CI: 12.8 – 17.2 mm, p=0.019).
When assayed against P. mirabilis, the leaf extract mean inhibition zone diameter (15.5 ± 0.5 mm [95%CI: 9.1 – 21.9 mm]) was significantly wider than the stem extract zone diameter (13.0 ± 0.0 mm [95% CI: 13.0 – 13.0 mm], p=0.02) whereas when compared to the root extract inhibition zone it tended towards being wider without achieving statistical significance (12.5 ± 0.5 mm [95% CI: 6.1 – 18.9 mm], p=0.063).
Similarly, when assayed against K. pneumoniae, the leaf extract mean inhibition zone diameter (24 ± 0.0 mm [95%CI: 24 – 24 mm]) was significantly wider than the stem or root extract zone diameters (17.5 ± 0.5 mm [95% CI: 11.1 – 23.9 mm], p=0.024; and 17.0 ± 0.0 mm [95% CI: 17.0 – 17.0 mm], p= cannot be estimated, respectively).
Finally, when assayed against E. coli, the leaf extract mean inhibition zone diameter (19.0 ± 1.4 mm [95%CI: 6.3 – 31.7 mm]) tended to be wider than the stem or root extract zone diameters (15.0 ± 0.0 mm [95% CI: 15.0 – 15.0 mm], p=0.078; and 15.5 ± 0.5 mm [95% CI: 9.1 – 21.9 mm], p=0.066, respectively).
Table 1: E. coli growth inhibition zone diameters from bioassays that used Commercial antibiotics. Trimethoprim-Sulfa =Trimethoprim-Sulfamethoxazole.
|
Commercial Antibiotic |
Inhibition Zone Diameter (mm) |
Interpretation based on the EUCAST Breakpoint |
|
Gentamicin |
22 |
Sensitive |
|
Trimethoprim-Sulfa |
27 |
Sensitive |
|
Amoxicillin |
13 |
Resistant |
|
Erythromycin |
10 |
Resistant |
|
Chloramphenicol |
19 |
Resistant |
Table 2: K. Pneumoniae growth inhibition zone diameters from bioassays that used commercial antibiotics. Trimethoprim-Sulfa =Trimethoprim-Sulfamethoxazole.
|
Commercial Antibiotic |
Inhibition Zone Diameter (mm) |
Interpretation based on the EUCAST Breakpoint |
|
Gentamicin |
25 |
Sensitive |
|
Trimethropim-Sulfa |
6 |
Resistant |
|
Amoxicillin |
6 |
Resistant |
|
Erythromycin |
29 |
Sensitive |
|
Chloramphenicol |
14 |
Resistant |
Table 3: P. mirabiris growth inhibition zone diameters from bioassays that used commercial antibiotics. Trimethoprim-Sulfa =Trimethoprim-Sulfamethoxazole.
|
Commercial Antibiotic |
Inhibition Zone Diameter (mm) |
Interpretation based on the EUCAST Breakpoint |
|
Gentamicin |
24 |
Sensitive |
|
Trimethropim-Sulfa |
6 |
Resistant |
|
Amoxicillin |
6 |
Resistant |
|
Erythromycin |
6 |
Resistant |
|
Chloramphenicol |
16 |
Resistant |
Table 4: Antibacterial activity of leaf aqueous extracts of Tithonia diversifolia by the Kirby-Bauer disc diffusion assay
|
Bacterial isolate |
Zone of Inhibition (mm) |
|||||||
|
Concentration of leaf extract (g/ml) |
|
|
||||||
|
0.625 |
0.333 |
0.165 |
0.083 |
0.041 |
0.021 |
(positive control) Gentamicin |
(negative control) Blank disk |
|
|
E. coli |
20 |
15 |
11 |
6 |
6 |
6 |
16 |
6 |
|
18 |
14 |
10 |
6 |
6 |
6 |
17 |
6 |
|
|
K. pneumoniae |
24 |
18 |
13 |
6 |
6 |
6 |
21 |
6 |
|
24 |
16 |
12 |
6 |
6 |
6 |
19 |
6 |
|
|
P. mirabilis |
16 |
13 |
11 |
6 |
6 |
6 |
22 |
6 |
|
15 |
12 |
10 |
6 |
6 |
6 |
21 |
6 |
|
Table 5: Antibacterial activity of stem aqueous extracts of Tithonia diversifolia by the Kirby-Bauer disc diffusion assay.
|
Bacterial isolate |
Zone of Inhibition (mm) |
|||||||
|
Concentration of stem extract (g/ml) |
|
|
||||||
|
0.625 |
0.333 |
0.165 |
0.083 |
0.041 |
0.021 |
(positive control) Gentamicin |
(negative control) Blank disk |
|
|
E. coli |
15 |
8 |
6 |
6 |
6 |
6 |
16 |
6 |
|
15 |
9 |
6 |
6 |
6 |
6 |
17 |
6 |
|
|
K. pneumoniae |
17 |
12 |
6 |
6 |
6 |
6 |
17 |
6 |
|
18 |
12 |
6 |
6 |
6 |
6 |
17 |
6 |
|
|
P. mirabilis |
13 |
11 |
6 |
6 |
6 |
6 |
20 |
6 |
|
13 |
10 |
6 |
6 |
6 |
6 |
21 |
6 |
|
Table 6: Antibacterial activity of root extracts of Tithonia diversifolia by the Kirby-Bauer disc diffusion assay
|
Bacterial isolate |
Zone of Inhibition (mm) |
|||||||
|
Concentration of root extract (g/ml) |
|
|
||||||
|
0.625 |
0.333 |
0.165 |
0.083 |
0.041 |
0.021 |
(positive control) Gentamicin |
(negative control) Blank disk |
|
|
E. coli |
16 |
12 |
6 |
6 |
6 |
6 |
15 |
6 |
|
15 |
12 |
6 |
6 |
6 |
6 |
15 |
6 |
|
|
K. pneumoniae |
17 |
11 |
6 |
6 |
6 |
6 |
21 |
6 |
|
17 |
10 |
6 |
6 |
6 |
6 |
16 |
6 |
|
|
P. mirabilis |
12 |
10 |
8 |
6 |
6 |
6 |
28 |
6 |
|
13 |
9 |
8 |
6 |
6 |
6 |
28 |
6 |
|
 |
Figure 3: Photograph of typical zones of inhibition of E. coli growth by aqueous root extracts of T. diversifolia. |
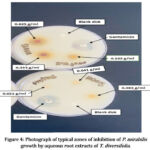 |
Figure 4: Photograph of typical zones of inhibition of P. mirabilis growth by aqueous root extracts of T. diversifolia. |
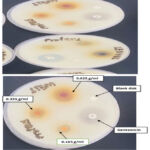 |
Figure 5: Photograph of typical zones of inhibition of P. mirabilis growth by aqueous stem extracts of T. diversifolia. |
Minimum Inhibitory Concentration
Graphs 1-3 show the proportion of E. coli, P. mirabilis and K. pneumoniae cells remaining alive at different T. diversifolia extract concentrations. In general, leaf extracts exhibited greater potency on all study isolates compared to stem and root extracts.
The MIC50 of leaf extract was 1.58 g/ml on E. coli whereas the MIC50 for stem and root extracts on the same organism was 7.76 g/ml and 16.22 g/ml respectively.
The MIC50 of leaf extract was 0.912 g/ml on P.mirabilis whereas the MIC50 for stem and root extracts on the same organism was 10.12g/ml and 6.76 g/ml respectively.
The MIC50 of leaf extract was 3.27 g/ml on K. pneumoniae whereas the MIC50 for stem and root extracts on the same organism was 10.23g/ml and 10.72 g/ml respectively.
 |
Graph 1: Proportion of E. coli cells remaining alive versus T. diversifolia extract concentration. |
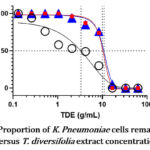 |
Graph 2: Proportion of K. Pneumoniae cells remaining alive |
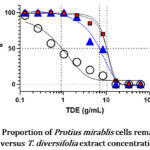 |
Graph 3: Proportion of Protius mirablis cells remaining alive versus T. diversifolia extract concentration |
Discussion
The present study is the first to show the in vitro antimicrobial activity of aqueous extracts of Malawian T. diversifolia against the following laboratory-adapted bacterial isolates from patients: E. coli, K. pneumoniae and P. mirabilis. The antibacterial effect of T. diversifolia leaf and stem extracts was similar to that of Gentamicin, a prescription drug which was used as a control antibiotic. Intriguingly, leaf extracts were observed to possess greater potency than the other plant parts.
These findings are in broad agreement with those obtained in previous studies. John-Dewole et al. (2013) observed that aqueous extracts of T. diversifolia produced a 10mm zone of inhibition against E. coli20. Similarly, in a study by Liasu and Ayandele (2008), it was observed that at a concentration of 10mg/ml, aqueous extracts of T. diversifolia leaves exhibited a zone of inhibition of 17 mm against E. coli21. Compared with the potency of the afore-cited aqueous leaf extracts, the current study reports lesser potency for the same plant part. This may be due to a difference in phytomedicine (glycosides, flavonoid, tannins, terpenoids, steroids, glycosides, carbohydrates, proteins and phenols) content that comes with geographical location of the plant. One limitation of the current study is that no phytochemical screening has been done yet at the time of writing this paper.
Previous phytochemical analyses of T. diversifolia revealed the presence of glycosides, flavonoid, tannins, terpenoids, steroids, glycosides, carbohydrates, proteins and phenols in aqueous extracts22,23. These phytochemicals have been proved to harbor pharmacological effects and are among raw materials used in chemical synthesis of some drugs used in orthodox medicine. Tannins, for example, have demonstrated antibacterial, anticancer and anti-inflammatory effects20, 24, 25. Notably, a number of phytochemical analyses reported the concentration of these bioactive compounds to be the greatest in the leaf extracts when compared to the stem or root extracts of T. diversifolia13,20,26. These findings of greater concentration of the aforementioned known bioactive phytochemicals in leaves other than in the stems or roots, may explain the present study’s observation of greater antibacterial activity/potency in T. diversifolia leaf extracts compared to the stem or root extracts.
Conclusion
Crude aqueous extracts of Tithonia diversifolia leaves showed remarkable growth inhibitory activity against E. coli, K. pneumoniae and P. mirabilis isolated from patients. These findings support the traditional use of Tithonia diversifolia in infectious processes due to these pathogens and further strengthens recommendations for additional work to isolate and characterize the bioactive chemical compounds responsible for the observed antibacterial properties of the plant.
The examination of antibacterial activity in only aqueous extracts of Tithonia represents a major limitation of the current study. However, organic solvents were excluded because this study aimed to investigate activity in extracts that represent traditional methods of herbal medicine preparation in the social settings of the study. Intriguingly, in contrast to the findings in the current study, other authors previously demonstrated insignificant antibacterial activity in aqueous leaf and root extracts of Tithonia compared to various organic solvents27.
Antimicrobial resistance (AMR) poses a critical threat to global public health and modern health care systems. In 2019 a systematic paper by the Institute for Health Metrics and Evaluation revealed that at least 1.27 million deaths were linked to AMR in 2019 and that an estimated 4.95 million people who died in 2019 suffered from drug-resistant bacterial infections. It was further reported that the largest of this mortality burden occurred in the sub-Saharan Africa region28. This scenario represents a big challenge to the realization of sustainable development goals for the region29. The present study is among many that have demonstrated that medicinal plant species from Africa’s rich forests represent a largely an unexploited resource for the discovery of new antibiotics to overcome the menacing scourge of AMR in the world today.
Acknowledgement
Authors would like to thank Dr Baxter Kachingwe of Kamuzu University of Health Sciences, Malawi, for helping with sourcing some of the antibiotics used in this study. A word of gratitude is also extended to Mr. Jeverson Mwale for his assistance in the Microbiology laboratory.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Funding Sources
The Malawi University of Science and Technology supported the research with funds drawn from NORHED II project on maternal and neonatal health at AMS. The project no. QZA-21/0159.
References
- Musicha P, Cornick JE, Bar-Zeev N, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis 2017 Oct; 17(10):1042-1052.
CrossRef - Haigh K, Dube Q, Kasambara W, et al. Cephalosporin resistance in Malawi. Lancet Infect Dis 2020 Mar; 20(3): 285-286.
CrossRef - Robinson LM, Sclar DA, Skaer TL. Medicinal Plants used by traditional healers in Malawi: Focus on Neem,Tephrosia, Moringa, Jatropha, Marula, and Natal Mahogany. Malawi Agroforestry Extension Project 2002; 8-10.
- Lester R, Maheswaran H, Jewell CP, et al. Estimating the burden of antimicrobial resistance in Malawi: protocol for a prospective observational study of the morbidity, mortality and economic cost of third-generation cephalosporin resistant bloodstream infection. Lancet Public Health 2022 Nov; 7(11): E897-913.
- UNESCO (1996) Culture and Health, Orientation texts- World Decade for cultural Development Documents CLT/DEC. PRO-1996, Paris, France, pp.29.
- Chisamile WA, Sonibare MA, Kamanula JF. Ethnobotanical Study of Traditional Medicinal Plants Used for the Treatment of Infectious Diseases by Local Communities in Traditional Authority (T/A) Mbelwa, Mzimba District, Northern Region, Malawi. J 2023; 6:115–139.
CrossRef - Chikowe I, Mnyenyembe M, Jere S, et al. An Ethnomedicinal Survey of Indigenous Knowledge on Medicinal Plants in the Traditional Authority Chikowi in Zomba, Malawi. Current Traditional Medicine 2020;6 (3):225-241(17).
CrossRef - Elufioye TO, Agbedahunsi JM. Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J Ethnopharmacol 2004; 93 (2-3):167-171.
CrossRef - Bork M, Schmitz MC, Weimann C, et al. Nahua Indian medicinal plants (Mexico): Inhibitory activity on NF-kb as an anti-inflammatory model and antibacterial effects. Phytomed 1996; 3:263–269.
CrossRef - Madureira MC, Martins AP, Gomes M, et al. Antimalarial activity of medicinal plants used in traditional medicine in S. Tome and Principe islands. J Ethnopharmacol 2002;81:23–29.
CrossRef - Obafemi CA, Sulaimon TO, Akinpelu DA, et al. Antimicrobial activity of extracts and a germacranolide-type sesquiterpene lactone from Tithonia diversifolia leaf extract. Afr J Biotechnol 2006;5(12):1254-1258.
- Kuo YH, Chen CH. Diversifolol, a novel rearranged Eudesmane sesquiterpenes from the leaves of Tithonia diversifolia. Chem Pharm Bull 1997;45:1223-1224.
CrossRef - Ajao AA, Moteetee AN. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. South Afr J Botany 2017;113:396-403.
CrossRef - Orwa C, Mutua A, Kindt R, et al. Agroforestree Database: a tree reference and selection guide version 4.0. 2009.
- Olabode OS, Sola O, Akanbi WB, et al. Evaluation of Tithonia diversifolia(Hemsl.) A Gray for soil improvement. World J Agric Sci 2007;3(4):503-507.
- Oluwasola TA, Dairo FAS. Proximate composition, amino acid profile and some anti-nutrients of Tithonia diversifolia cut at two different times. Afr J Agric Res 2016;11(38):3659–3663.
CrossRef - Lamaty G, Menut C, Zollo P-HA, et al. Aromatic plants of tropical Central Africa. III. Constituents of the essential oil of the leaves of Tithonia diversifolia (Hemsl.) A. Gray from Cameroon. J Essent Oil Res 1991;3(6):399–402.
CrossRef - Liu Y, Chen P, Zhou M, et al. Geographic Variation in the Chemical Composition and Antioxidant Properties of Phenolic Compounds from Cyclocarya paliurus (Batal) Iljinskaja Leaves. Molecules 2018 Oct;23(10):2440.
CrossRef - Khattak KF, Rahman TR. Effect of geographical distributions on the nutrient composition, phytochemical profile and antioxidant activity of Morus nigra. Pak J Pharm Sci 2015 Sep;28(5):1671-8.
- John-Dewole O and Oni S. Phytochemical and antimicrobial studies of extracts from the leaves of T. diversifolia for pharmaceutical importance. IOSR Journal of Pharmacy and Biological Sciences 2013;6(4):21-25.
CrossRef - Liasu MO, and Ayandele AA. Antimicrobial activity of aqueous and ethanolic extracts from Tithonia diversifolia and Bryum coronatum collected from Ogbomoso, Oyo State, Nigeria. Advances in Natural and Applied Sciences 2008;2(1):31-34.
- Otusanya O and Ilori O. Phytochemical Screening and the Phytotoxic Effects of Aqueous Extracts of Tithonia diversifolia (Hemsl) A. Gray. International Journal of Biology 2012;4(3):97-101.
CrossRef - Ahmed SO, Samson OB. Preliminary phytochemical screening of bioactive chemicals in sunflower (Tithonia diversifolia) roots. International Journal on Human Computing Studies 2020;2(6):12-18.
CrossRef - Abkhoo J and Jahani S. Antibacterial Effects of Aqueous and Ethanolic Extracts of Medicinal Plants Against Pathogenic Strains. Int J Infect 2017 April;4(2):e42624.
CrossRef - Misrahanum M, Safarah Z and Ismail YS. Antibacterial activity of Mexican sunflower leaf Tithonia diversifolia (Hemsl.) A. Gray Aqueous extract against methicillin-resistant Staphylococcus aureus. Pharmaciana 2022; 12(1): 128-135.
CrossRef - Umar OB, Alex RD and Obukohwo EE. Phytochemical and proximate composition of Tithonia diversifolia (HEMSL.) A GRAY (2015). Annals Food Science and Technology 2015;16:195-200.
- Odeyemi AT, Agidigbi TS, Adefemi SO, et al. Antibacterial activities of crude extracts of Tithonia diversifolia against common environmental pathogenic bacteria. The Experiment 2014;20(4):1421-1426.
- Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022; 399: 629-655.
CrossRef - Gajdács M, Urbán E, Stájer A, et al. Antimicrobial resistance in the context of the Sustainable Development Goals: a brief review. Eur J Investig Health Psychol Educ 2021;11:71-82.
CrossRef







