Manuscript accepted on :01-03-2023
Published online on: 05-01-2024
Plagiarism Check: Yes
Reviewed by: Dr. Susmita
Second Review by: Dr. Fatma Çavuş Yonar
Final Approval by: Dr. H Fai Poon
Abha Shukla1, Supriya Dubey1*, Rishi Kumar Shukla2, Ajay Kumar3, Swati Vats1 and Priyanka Pokhriyal1
1Department of Chemistry, Kanya Gurukul Campus, Gurukula Kangri (Deemed to be University), Haridwar India
2Department of Chemistry, Gurukula Kangri (Deemed to be University), Haridwar India
3Department of Applied Science, Faculty of Engineering and Technology, Gurukula Kangri (Deemed to be University), Haridwar India.
Corresponding Author E-mail:supriyadubey2998@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2780
Abstract
Our study aims to investigate the extraction of fixed oil from the fruit of Heterospathe elata, using traditional petroleum-based solvent hexane and green solvent dimethyl carbonate (DMC), evaluation of physicochemical parameters, chemical composition, and biological activities of extracted oil. The resulting oil components were subjected to GC-FID analysis. The evaluation of different biological activities like antioxidant, anti-inflammatory, and photoprotective activities has been done by the spectrophotometric method. The results obtained from GC-FID analysis proved the presence of fatty acids, of which myristic acid was the most prevalent component in the DMC and hexane fractions, respectively. Additionally, DMC-extracted oil possess stronger antioxidant, anti-inflammatory, and photoprotective activities than hexane-extracted oil. The findings showed that DMC is effective in replacing potentially hazardous solvents to extract oil from the Heterospathe elata fruit, rich in components relevant to the human diet, including vital polyunsaturated fatty acids and phenolic compounds, with better biological activity.
Keywords
Antioxidant; Anti-inflammatory; GC-FID; Heterospathe elata; Photoprotective activity
Download this article as:| Copy the following to cite this article: Shukla A, Dubey S, Shukla R. K. Kumar A; Vats S, Pokhriyal P. A Comparative Study and Characterization of Green Solvent and Hexane Extracted Bioactive lipids from Heterospathe elata Fruit. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Shukla A, Dubey S, Shukla R. K. Kumar A; Vats S, Pokhriyal P. A Comparative Study and Characterization of Green Solvent and Hexane Extracted Bioactive lipids from Heterospathe elata Fruit. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3H5gulo |
Introduction
Hexane has been widely used for oil and fat extraction. From petroleum and other hydrocarbons, hexane is produced. Hexane reacts with pollutants when it is discharged into the atmosphere, creating ozone and photochemicals1. The highly lipophilic nature of hexane makes it soluble in neural lipids. So, it became toxic for neural system when inhaled by humans. According to the REACH regulation (European Directives and Registration, Evaluation, Authorization, and Restriction of Chemicals), hexane is a category 2 compound that is prohibited from being used in cosmetic items and is both aquatic chronic toxic and reprotoxic2. Finding solvents other than those derived from petroleum is now a major concern for chemists because of the advent of green chemistry and the increased focus on environmental and safety concerns. Green solvents are made from renewable materials such as wood, starch, fruit, and oils. Green solvents are biodegradable, non-toxic, and non-flammable, and they have a strong salvaging power2. Consumer interest in foods with health benefits and foods with physiological activity has grown recently. Essential fatty acids are required for good health but cannot be produced by the body; they can only be obtained through dietary sources. Vital nutrients for humans, fats and oils supply both calories and essential fatty acids3. These fatty acids are widely employed in sectors like pharmaceuticals, cosmetics, and medicine. The amount and type of fatty acids in a particular diet have a significant impact on its lipid profile. The food business has been heavily influenced by consumers’ concerns about the ability of the diet to maintain the ratio of saturated to unsaturated fatty acids and identify good fats that are crucial for nutraceutical purposes. It is believed that polyunsaturated fatty acids are crucial adaptive mediators for enhancing and preserving human health. Omega-3 fatty acids are helpful in human pathologies such as diabetes, stroke, atherosclerosis, and cardiovascular disorders4. For the structural elements of cells, tissues, and organs as well as for the creation of several biologically active chemicals, fatty acids are regarded as a vital building block. Fatty acids are viewed as a crucial building block for the structural components of cells, tissues, and organs as well as for the creation of some physiologically important substances5,6.While few studies have also been reported on the characterization of fixed oils (the non-volatile fraction) from these Arecaceae family species like Caryota mitis, Borassus flabellifer, Areca catechu nut, and Cocus nucifera7. To the best of our knowledge, there has been no publication on fixed oils from Heterospathe elata fruit.
The present study investigated and evaluated GC-FID of fixed oil extracted from Heterospathe elata fruit using petroleum–based solvent hexane and green solvent dimethyl carbonate (DMC), physicochemical parameters, and biological activities using antioxidant, anti-inflammatory, and photoprotective activities.
Material and Methods
Sample Collection and Authentication
Samples were collected in July 2021 from the northern Indian state of Uttar Pradesh. The sample authentication was carried out, and the identification of the plant material based on the morphological criteria was confirmed at the Department of Botany, Banaras Hindu University (voucher specimen no. 1/7A, 22/07/21). The fruit of Heterospathe elata was washed under distilled water to remove dust. The fruit samples were dried in the air for 20 days. Then, the fruit was ground using electrical grinders (Pulverizer, HR 1500) and then sieved (0.25 mm) to obtain a uniform particle size. The sample was stored in a refrigerator for further examination.
Chemicals and Reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulpfonic acid (ABTS), 2,4,6-tri(2-pyridyl)-s- triazine (TPTZ), ascorbic acid, phenolic standards namely gallic acid, quercetin, were procured from Sigma-Aldrich. Folin-ciocalteu reagent, ammonium acetate, sodium carbonate, potassium persulphate, sodium acetate trihydrate, manganese chloride (II) and iron (III) chloride hexahydrate, and Benzophenone were purchased from Himedia, India. Bovine albumin serum (CDH), Hexane and Dimethyl carbonate from SRL Pvt. Ltd. All other chemicals used were analytical grades.
Extraction of Fixed Oil
The Soxhlet apparatus was used to extract the oil according to standard method4. To prevent solvent loss by evaporation, extraction was done close to the dimethyl carbonate (DMC) solvent boiling point. When the first drop of the extraction solvent was recycled back into the thimble, the extraction period began. The solvent was collected and reused in the subsequent extraction batch in a rotary evaporator operating under a vacuum. After cooling, the oil was weighed. Oil was extracted into amber-colored vials, sealed with Teflon tops, and kept in the refrigerator at 4°C for later analysis. Similarly, oil was extracted from the same fruit sample using n-hexane The following expression was used to compute the oil yield:
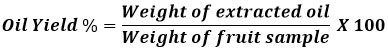
Determination of Physical Properties
Physical properties color, odour, peroxide value, acid value, refractive index, Iodine value, ester value, saponification value were estimated using standard reported methods8.
Total phenolic content
Using the Folin-Ciocalteu reagent, the total phenolic contents of the extracted oil were calculated with slight modification9. Briefly, 250μl of freshly made Folin-Ciocalteu reagent, 0.75 ml of 20% sodium carbonate, and 3 ml of pure water made up the reaction mixture, along with 50μl of each plant extract. The absorbance at 765 nm was measured after 2 hours of reaction at room temperature, and the phenolic content was calculated using gallic acid as a reference.
Chemical Composition of Fixed Oil by GC-FID
Fatty acids composition investigation was carried out by means of GC-FID (gas chromatography-flame ionization detection after derivatization of fatty acid methyl esters (FAMEs). TQ8040 Shimadzu GC, HS20 headspace sampler, mobile phase He at 1 ml/min, SH-Rxi-5Sil MS capillary column (stationary phase: 5% diphenyl-95% dimethyl polysiloxane, length: 30 m, inner diameter ID: 0.25 mm, film thickness df: 0.25 mm), split ratio 1:10, injection temperature 280, column oven temperature increased from 80 to 280 at a rate of 5 /min. The bioactive compounds were identified based on retention time with those of certified FAME mix. The results were expressed as the percentage of each fatty aci in the total10.
Antioxidant Assay
1-Diphenyl-2-picryl-hydrazyl (DPPH) assay
The radial scavenging activity of the Heterospathe elata oil was assessed using DPPH with some modifications11. Different test tubes were filled with ascorbic acid extracts at various concentrations. Petroleum ether was added to bring the volume to 100 mL. These tubes were filled with five mL of a 0.1 mM petroleum ether solution of DPPH and vigorously shaken. For 30 minutes, the tubes were left to stand at 27 0C. Without any extract, the control was made in the same manner. At 512 nm, the produced samples variations in absorbance were measured. The inhibition percentage was used to estimate the amount of radical scavenging activity, and the following formula was used to do so:
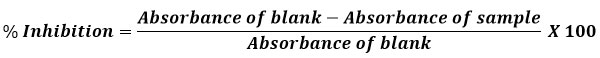
2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assay
The ABTS radical cation scavenging assay was used to calculate the total antioxidant activity with slight modification12. In an ABTS (stable radical) solution with 2.4 mM potassium persulfate, the ABTS radical cation was produced in the absence of light for 12 to 16 hours. The ABTS solution was diluted in petroleum ether (1:89 v/v) prior to the experiment to produce an absorbance of 0.70 ±0.02 at 734 nm. Ascorbic acid was added to 1 mL of diluted ABTS solution along with triplicates of the 10 µL samples. After 30 minutes of incubation at 30 0C, the reaction mixtures absorbance was measured at 734 nm in comparison to petroleum ether (blank).
Anti-inflammatory assay by bovine albumin serum denaturation
The ability of extracted oil to induce protein denaturation was determined according to the method described with some modification13. The reaction mixture consists of 2.8 mL of phosphate buffered saline (pH 6.4), 0.2 mL of fresh bovine albumin serum, and 2 mL of various extracts at variable concentrations. The reaction mixtures were then incubated in the BOD incubator for 15 minutes at ambient temperature before being heated for 5 minutes at 72 0C. Their absorbance at 660 nm (Systronic 118, UV-VIS) was measured using a blank after cooling. In order to determine absorbance, sodium diclofenac was used as a reference medication and handled similarly to plant extracts. The following equation was used to determine the percent inhibition of protein denaturation:
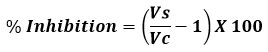
Where, Vs = absorbance of test sample, Vc = absorbance of control.
Photoprotective Activity
Determination of the sun protection factor (SPF)
Using a UV-VIS spectrophotometer, the absorbance values of each extract dilution were calculated at 5-nm intervals between 290 and 320 nm, using MeOH/H2O (80:20, v/v) as a blank. SPF was calculated using an equation with slight modification14.
SPF spectrophotometric = C F × Σ EE(λ) × I(λ) × Abs(λ)
Where, EE (λ) indicates erythemal effect spectrum;
I (λ) indicates the solar intensity spectrum;
Abs (λ) indicates absorbance of the sunscreen product
CF stands for the correction factor (=10).
The values of EE × I are constant.
Statistical Analysis
All the experiments were done in triplicates & the results were expressed as Mean± SD. The data were statistically analyzed using one way ANOVA followed by Duncan’s test. Mean values were considered statistically significant when p>0.05.
Results and Discussion
Oil Extraction Yield
Hexane and the environmentally friendly solvent dimethyl carbonate (DMC) were used to produce Heterospathe elata fruit oil. 18% oil yield was obtained with hexane extraction and 24% with DMC extraction (Fig. 1). In the extraction of DMC oil, the yield is greater. The most popular method for extracting lipids from petroleum using petroleum solvents like hexane is known as solvent extraction. Due to their extreme volatility, petroleum solvents are a major threat to both human health and the environment. Humans that breathe in hexane suffer neurological harm. A less toxic and environmentally friendly alternative, known as a “green solvent,” has begun to be sought due to environmental, health, and safety concerns. According to recent laboratory testing, many green solvents have the potential to replace petroleum solvent in the extraction process12,13. Dimethyl carbonate (DMC) is one of the environmentally friendly solvents that could eventually take the place of hexane. Green solvents disposal would be less expensive than petro-based solvent disposal. We can say that DMC is a good alternative solvent for the extraction of lipids because our findings showed that it has a higher ability to extract oil than hexane. When Farid Chemat et al. evaluated various green solvents for oil extraction, they discovered that they were more effective than hexane12,14.
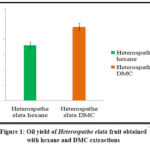 |
Figure 1: Oil yield of Heterospathe elata fruit obtained with hexane and DMC extractions |
Physicochemical Properties
The refractive index is the degree of deflection of a beam of light that occurs when it passes from one transparent medium to another. The refractive indices of DMC and hexane-extracted oil were 0.98 and 1.53, respectively. The extracted oil was also subjected to evaluation of its acid value. The acid values for DMC and hexane-extracted oil were found to be 0.72 mg KOH/g and 0.93 mg KOH/g, respectively. Acidity is used as an indicator of the edibility of oil. It was found that the measured iodine value for DMC-extracted oil was 72.59 g/100 g and 78.12 g/100 g for hexane-extracted oil. The low iodine values indicated that this oil sample has greater oxidative storage stability. The oil sample was also subjected to evaluation of its saponification value. Saponification values of DMC-extracted oil were found at 345.12 mg KOH/g and 223.48 for hexane-extracted oil. The saponification value indicates the average molecular weight of a fat or oil. An ester value of DMC and hexane-extracted oil was found to be 344.4 and 222.5, respectively. Peroxide value is a very important characteristic of lipid quantity. The assessment of hydroperoxide provides an estimate of the overall oxidation status of lipids and lipid-containing foods, especially in the primary phase of oxidation, generally known as the induction period. Fruit DMC-extracted oil indicated a low peroxide value. 0.84 g/kg and a lower number of peroxide values indicate a good quality of oil and a good preservation status15,16.
Table 1: Physicochemical Properties of Fixed oil from Heterospathe elata fruit
|
Characteristics |
Oil ( extracted with DMC) |
Oil (extracted with Hexane ) |
|
|
Yield |
24% |
18% |
|
|
Color |
Light pink |
Light pink |
|
|
Odour |
Characteristic smell |
Characteristic smell |
|
|
Refractive Index |
0.98 |
1.53 |
|
|
Iodine Value |
72.59 |
78.12 |
|
|
Acid Value(mg KOH/g) |
0.72 |
0.93 |
|
|
Saponification value (mgKOH/g) |
345.12 |
223.48 |
|
|
Ester Value |
344.4 |
222.55 |
|
|
Peroxide Value(g/kg) |
0.84 |
1.83 |
|
Total Phenolic Content
Phenolic molecules have a significant impact on the sensory and nutritional quality of oil and guard against lipid oxidation by quenching radical reactions. To prevent heart diseases, the potential for phenol-containing oils to promote health exists. The overall amount of polyphenol content is known to be influenced by the polarity of the extraction solvent as well as other elements such as plant cultivar, seed maturity level, environmental changes, and growing location17. Heterospathe elata fruit oil extracted using the green solvent DMC showed a higher total phenolic content of 12 mg GAE/100 g DW. As a result, the DMC suggested in this work enabled the extraction of Heterospathe elata fruit oil with the highest possible polyphenol content. Based on these findings, it appears that greenly extracted oils contain a significant amount of phenolic compounds, which may contribute to the stability of the oil under accelerated oxidative stress9,17.
Table 2: Total Phenolic Content of Heterospathe elata fruit oil
|
Extracts |
Total Phenolic Content (mgGAE/100gm dw) |
|
Oil extracted with DMC |
12 |
|
Oil extracted with hexane |
5 |
Fatty Acid Composition
Essential fatty acids are necessary for healthy health. Since the human body is unable to synthesize these fatty acids, they must be obtained through diet. Table 3 provides a summary of the fatty acid profile (GC-FID) of the oil derived from the Heterospathe elata fruit using hexane and the green solvent DMC. Both DMC and hexane-extracted oil contain Lauric acid, Myristic acid, Palmitic acid, Stearic acid, Oleic acid, and Linoleic acid in significant amounts. Butyric acid (3.515%), caproic acid (3.389%), and caprylic acid (1.692%) were also identified only from the oil extracted from DMC. Butyric acid can reduce arterial blood pressure and be used to treat or prevent cancer, irritable bowel syndrome, diverticulosis, and diarrhea18. A medium-chain fatty acid known as caprylic acid is thought to have strong antibacterial, antifungal, and anti-inflammatory activities. According to some previous research, it may help treat high cholesterol, skin diseases, digestive problems, and yeast infections. The likelihood of antibiotic resistance may be reduced by using it as a disinfectant. Capric acid has direct uses as antimicrobials, plant growth stimulants, and feed additives. Additionally, it can be used as a base for several products, such as lubricants, perfumes, paint additives, and medications19. The lipid membrane of bacteria can be destroyed by saturated fatty acids. Linoleic acid is an Omega-3 fatty acid that has anti-inflammatory properties and lowers the risk of chronic disease. Linoleic acid (6.176%), which is present in Heterospathe elata oil, confers anti-inflammatory characteristics to the substance20,21. Oleic acid is a member of the monounsaturated omega-9 fatty acid family. The development of deadly brain disorders is slowed by oleic acid. Oleic acid is a helpful source for improving memory. Additionally, oleic acid can lower blood pressure19,21. Heterospathe elata fruit oil can be employed as a source of an ingredient in oleochemicals, soap, cosmetics, and medicine due to the presence of these fatty acids. The ester isopropyl myristate of myristic acid is frequently used as a component in soaps and shaving creams. The most prevalent saturated fatty acids are lauric acid, myristic acid, stearic acid, and palmitic acid21.
Table 3: Fatty acid composition of fixed oil of Heterospathe elata Fruit
|
Fatty acids |
Oil extracted using DMC (%) |
Oil extracted using Hexane (%) |
|
Butyric acid |
3.515 |
– |
|
Caproic acid |
3.389 |
– |
|
Caprylic acid |
1.692 |
– |
|
Lauric acid |
15.131 |
16.37 |
|
Myristic acid |
45.063 |
47.98 |
|
Palmitic acid |
12.526 |
13.55 |
|
Stearic acid |
1.046 |
1.34 |
|
Oleic acid |
11.462 |
12.85 |
|
Linoleic acid |
6.176 |
7.91 |
 |
Figure 2: GC-FID chromatogram of Hexane extracted fixed oil from Heterospathe elata Fruit |
 |
Figure 3: GC-FID chromatogram of DMC extracted fixed oil from Heterospathe elata Fruit |
Biological Activities
Antioxidant activity
The antioxidant properties of Heterospathe elata fruit oil produced using conventional and environmentally friendly solvents were examined in this work. Oil extracted using DMC green solvent had an IC50 of 227.14 ±0.89 µg/mL) when tested using the DPPH assay and (139.25±0.47 µg/mL ) when tested using the ABTS assay. Hexane-extracted oil has an IC50 of (457.20±1.34µg/mL and 263.33±0.25µg/mL) when tested with DPPH and ABTS, respectively. A lower IC50 value and much better antioxidant activity were observed in Heterospathe elata oil extracted with DMC compared to oil extracted with hexane, which is an interesting finding. Linoleic and oleic acids can be found in Heterospathe elata fruit oil, according to the GC-FID. The antioxidant action is caused by these fatty acids. Fixed oils, compared to ascorbic acid used as a reference, showed significant antioxidant activity. According to our research, DMC green extracted oil has a greater overall phenolic content value. Phenolic compounds are powerful antioxidants and efficient intercellular ROS scavengers17. Overall, our findings imply that DMC might be a great hexane replacement for recovering Heterospathe elata with increased antioxidant activity. Therefore, Heterospathe elata improved bioactivity may help to reduce lipid peroxidation at both low and high temperatures, and it may be employed as a functional oil in dietary supplements22.
Table 4: Antioxidant activity of Heterospathe elata Fruit Fixed Oil
|
Sample |
DPPH IC50 µg/mL |
ABTS IC50 µg/mL |
|
Fixed oil ( extracted with hexane) |
475.34 |
273.24 |
|
Fixed oil (extracted with DMC) |
275.30 |
166.60 |
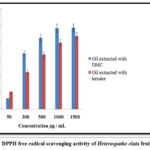 |
Figure 4: DPPH free radical scavenging activity of Heterospathe elata fruit fixed oil. |
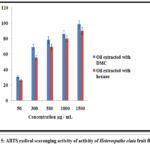 |
Figure 5: ABTS radical scavenging activity of activity of Heterospathe elata fruit fixed oil |
Anti-inflammatory activity
Fixed oils of Heterospathe elata fruit were evaluated against denaturation of bovine albumin. This study represents a concentration-dependent inhibition of protein (albumin) denaturation by fixed oil throughout the concentration range of 100 to 1000µg/mL. Sodium diclofenac, which is used as a standard drug, also exhibited concentration-dependent inhibition of protein denaturation; however, fixed oil of Heterospathe elata showed significant inhibition (Fig. 6). Denaturation of tissue proteins is one of the authenticated reason of diseases like inflammatory and arthritic23. The formation of antibodies in some arthritic diseases may be due to denaturation of proteins. The increments in absorbances of test samples with respect to control indicated stabilisation of protein, i.e., inhibition of heat-induced protein (albumin) denaturation by fixed oil and the reference drug sodium diclofenac. From the IC50 values, it becomes evident that fixed oil was amply active after sodium diclofenac, being effective at lower concentrations. It is reported that many non-steroidal anti-inflammatory drugs have the ability to stabilise (forbid denaturation) heat-treated egg albumin at pH 6.2–6.5. Hence, from this experiment, it can be concluded that the fixed oil of Heterospathe elata fruit showed a significant in vitro anti-inflammatory effect against the denaturation of protein. The IC50 of oil extracted with DMC green solvent was 336.84 ±1.09) and hexane-extracted oil has an IC50 value of 496.80±0.37. The anti-inflammatory efficacy of Heterospathe elata oil extracted with DMC was remarkably higher than that of oil extracted with hexane, which is interesting. Linoleic acid (6.176%) and other polyunsaturated fatty acids are present, according to fatty acid composition (GC-FID). The anti-inflammatory effects are caused by polyunsaturated fatty acids.
 |
Figure 6: Anti-inflammatory activity of Heterospathe elata fruit fixed oil |
Photoprotective Activity
The fixed oil of Heterospathe elata had the photoprotective activity evaluated with the method developed by Mansur 198613. The results of the determinations of in vitro SPF values are shown in Table 5. According to Brazilian law, only SPF greater than or equal to 6 is suitable for use in cosmetic products with photoprotective activity13. This activity can be attributed to the flavonoids and phenolic compounds found in the oil of Heterospathe elata fruit which is efficient in absorbing ultraviolet light, and usually show two maximum peaks of ultraviolet absorption, one between 240-280 nm and another 300-550 nm Bobin 1995. Several classes of natural compounds were examined for their antioxidant and photoprotective activities. Previous studies reviewed the importance of natural sunscreens in the compositions of commercial sunscreens and therefore analyzed their role in the prevention of skin cancer. Photoprotective properties have been evaluated in many varieties of plants; oil proved to be good at reducing agent-induced erythema as compared to benzophenone. The calculated values for a sun protection factor of hexane and DMC-extracted oil were 20.38 ± 0.11 and 44.14±0.19, respectively. DMC-extracted oil from Heterospathe elata fruit oil has a greater SPF rating than hexane-extracted oil. Palmitic acid offers strong skin protection, whereas linoleic acid works well as an emollient, so Heterospathe elata fruit oil can be used in the cosmetic industry.
Table 5 Photoprotective activity of Heterospathe elata fruit fixed oil
|
Λ (nm) |
EE × I |
Absorbance of hexane extracted oil |
Absorbance of DMC extracted oil |
|
290 |
0.0150 |
0.601 |
0.901 |
|
295 |
0.0817 |
0.498 |
0.812 |
|
300 |
0.2874 |
0.377 |
0.788 |
|
305 |
0.2780 |
0.269 |
0.621 |
|
310 |
0.1864 |
0.127 |
0.536 |
|
315 |
0.0837 |
0.099 |
0.401 |
|
320 |
0.0180 |
0.063 |
0.347 |
|
Total |
1 |
2.034 |
4.406 |
|
SPF |
|
20.38±0.11 |
44.14±0.19 |
Conclusion
The findings indicate that fixed oil extracted from Heterospathe elata fruit had good qualities for industrial use based on physicochemical parameters. Green solvent DMC is a promising alternative to hexane for recovering enhanced functional oils. The Heterospathe elata oil yield extracted using green solvent was increased compared to conventional solvent hexane. The oil extracted with DMC had the highest total phenolic content, antioxidant, and anti-inflammatory activity. DMC-extracted oils possess potent photoprotective activity also due to the presence of active constituents in the composition of fixed oil. These results showed that using a substitute solvent to extract oil from Heterospathe elata would be helpful to obtain oils with enhanced bioactive components. The fixed oil of Heterospathe elata fruit could be a potential natural source and could have greater importance as a therapeutic agent in preventing or slowing oxidative stress and inflammation-related disorders.
Acknowledgement
None
Conflict of interest
There is no conflict of interest
Funding Sources
There are no funding source.
References
- Byrne F.P, Jin S, Paggiola G, Petchey T.H.M, Clark J.H, Farmer T.J, Hunt A.J, Robert McElroy C, and Sherwood J. Tools and techniques for solvent selection: green solvent selection guides. Sustain. Chem. Process. 2016; 4:1–24. https://doi.org/10.1186/s40508-016-0051-z
- Welton T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015;471. https://doi.org/10.1098/rspa.2015.0502
- Tarczykowska A. Green solvents Green solvents Katedra i Zakład Chemii Leków , Wydział Farmaceutyczny , Collegium Medicum w Bydgoszcz Bydgoszczy , Uniwersytet Mikołaja Kopernika w Toruniu , ul . dr .A .Jurasza 2017;2 :85-089. https://doi.org/10.5281/zenodo.893346
- Muna H. A, Reem O.H, Dhulfiqar S.S, Majed A,and Shah A. K.Gas chromatography-mass spectrometry analysis and in vitrobiological studies on fixed oil isolated from the waste pits of twovarieties of Oleaeuropaea L. OCL.2019; 26,28.https://doi.org/10.1051/ocl/2019022
- Bourgou S,Bettaieb R. I, Ben K. S, Hammami M, Dakhlaoui S, Sawsen S,Msaada K, Isoda H, Ksouri R and Fauconnier M.L.Green Solvent to Substitute Hexanefor Bioactive Lipids Extraction fromBlack Cumin and Basil Seeds. Foods. 2021 ; 10: 1493. https://doi.org/10.3390/foods10071493.
- Durkin L.A, Childs C.E, and Calder P.C.Omega-3 polyunsaturated fatty acids and the intestinal epithelium-A Review. Foods. 2021; 10: 199.
- Quílez M, Ferreres F,López-Miranda S, Salazar E, andJordánM.J.Seed oil from mediterranean aromatic and medicinal plants ofthe Lamiaceae family as a source of bioactive components with nutritional. Antioxidants.2020; 9: 510.
- AOCS. Official Methods and Recommended Practices of theAmerican Oil Chemists’ Society, AOCS Press, Champaign, Ill,USA, 4th edition, 2003
- Zengin G, Uysal S, Ceylan R, and Aktumsek A. Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalumnarbonense L. from Turkey: A phytochemical study. Ind. Crops Prod. 2015;70: 1–6. https://doi.org/10.1016/j.indcrop.2015.03.012
- Naik R.R.“GC-FID analysis of fatty acids and biologicalactivity of Zanthoxylumrhetsaseed oil,” Oriental Journal ofChemistry. 2015;31( 4): 1929–1935.
- Barapatre A, Meena A.S, Mekala S, Das A, and Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol.2016;86: 443–453. https://doi.org/10.1016/j.ijbiomac.2016.01.109
- Huang S.S, Su S.Y, Chang J.S, Lin H.J, Wu W.T, Deng J.S, and Huang G.J. Antioxidants, anti-inflammatory, and antidiabetic effects of the aqueous extracts from Glycine species and its bioactive compounds. Bot. Stud. 2016; 57. https://doi.org/10.1186/s40529-016-0153-7
- Chaima M, Soumia M, Kafia O, Sara M, and Hamada H.In vitro photoprotective, hemostatic, anti-inflammatory and antioxidantactivities of the species LinariascariosaDesf. South African Journal of Botany .2020;130: 383-388.
- Dick F.D. Solvent neurotoxicity. Occup Environ Med. 2006; 63(3):221–6.
- Silva A.C and Jorge N. “Bioactive compounds of the lipidfractions of agro-industrial waste,” Food Research International . 2014; 66:493–500.
- Kimbonguila A, Nzikou J.M and Matos L.“Proximatecomposition and physicochemical properties on the seeds andoil of Annonamuricatagrown in Congo-Brazzaville,” ResearchJournal of Environmental and Earth Sciences. 2010;2:13–18,
- Shi Y.X, Xu Y.K, Hu H, Bin Na. Z and Wang W.H. Preliminary assessment of antioxidant activity of young edible leaves of seven Ficus species in the ethnic diet in Xishuangbanna, Southwest China. Food Chem. 2011;128: 889-894.https://doi.org/10.1016/j.foodchem.2011.03.113
- Tasioula M. M and Okogeri O. Simultaneous determination of phenolic compounds and tocopherols in virgin olive oil using HPLC and UV detection. Food. Chem. 2001; 74: 377–383.
- Angers P, Morales M.R and Simon J.E. Fatty acid variation in seed oil among Ocimum species. J. Am. Oil Chemists’ Soc. 1996; 73: 393–395.
- CheikhR.S, Besbes S, Hentati B, Blecker C, Deroanne C and Attia H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food. Chem. 2007; 101: 673–681.
- HamrouniS.I, Kchouk M.E and Marzou B.Lipid and aroma composition of black cumin (Nigella sativa L.) seeds from Tunisia. J. Food. Biochem. 2008;32:335–352.
- Azadfar M, Gao A.H and Chen S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015; 75:58–66. https://doi.org/10.1016/j.ijbiomac.2014.12.049
- Mahmoud A. E, Ragaa A,Hamoudaab, Salah G,Alic E.A. Sedeekcand Elsayed A. Antibacterial, antioxidant and anticancer of fermentation by Bacillus subtilis on bagasse and wheat bran. Current Chemistry Letters. 2022;11: 383–392.







