Manuscript accepted on :04-05-2023
Published online on: 14-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Mohammed Najim Abed and Dr. Sonali Dahat
Second Review by: Dr. Amit Panaskar
Final Approval by: Dr. Patorn Promchai
Suvadra Das1 , Joyeeta Bhattacharya2
, Joyeeta Bhattacharya2 , Srija Sur3
, Srija Sur3 , Subhasis Chakraborty2
, Subhasis Chakraborty2 , Aparna Lakshmi4
, Aparna Lakshmi4 , Tanay Pramanik1
, Tanay Pramanik1 and Partha Roy5*
and Partha Roy5*
1Basic Science and Humanities Department, University of Engineering and Management, Kolkata, India.
2Department of Pharmaceutical Technology, DmbH Institute of Medical Science, Dadpur, Hooghly, West Bengal, Inida.
3Department of Pharmaceutical Technology, Adamas University, Kolkata, India.
4Raghu College of Pharmacy, Visakhapatnam, Dakamarri, Bheemunipatnam, Andhra Pradesh, India.
5 Gitam School of Pharmacy, GITAM (Deemed to be University), Vishakhapatnam, India.
Corresponding Author E-mail:partharoy2502@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2715
Abstract
Vitis pedata is a woody climber of the Vitaceae family with a multitude of pharmacological effects like anti-inflammatory, antibacterial, anti-nociceptive, anti-arthritic, anti-oxidant, astringent, and hemostatic qualities. The research targets to assess the anti-diabetic efficacy of Vitis pedata aqueous extract nanoformulation in alloxan-induced diabetes rats. Biocompatible polycationic polymer chitosan is used for nanoformulation development. The aqueous extract loaded chitosan nanoparticle formulation displayed a particle size of 186± 5.37 nm with a polydispersity index of 0.226 and zeta potential value of 23 ± 1.93 mV. The nanoformulations were assessed for its anti-diabetic properties in comparison to aqueous extracts for the first time by in- vivo methodologies. Several parameters like body weight, blood glucose level, blood and serum biomarkers, were examined. Changes in tissue histoarchitecture, liver glycogen content, oxidative stress response and antioxidant status were also studied. In diabetic rats, the nano-scale formulation significantly reduced blood glucose levels, glucose tolerance, lipid profiles, and serum biomarkers, comparable to the oral hypoglycemic medication glibenclamide. The restoration of antioxidant status and pancreatic histoarchitecture through by size assisted delivery may be related to the improved control of diabetes by the nanoformulation than the extract alone. The work marks the first report of Vitis pedata nanoformulation for diabetes management and can serve as a prelude for further preclinical/clinical evaluations.
Keywords
Antihyperglycemic; Diabetes; Vitis pedata; extract; nanoformulation
Download this article as:| Copy the following to cite this article: Das S, Bhattacharya J, Sur S, Chakraborty S, Lakshmi A, Pramanik T, Roy P. Vitis Pedata Nanoformulation in the Management of Alloxan Induced Experimental Diabetes. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Das S, Bhattacharya J, Sur S, Chakraborty S, Lakshmi A, Pramanik T, Roy P. Vitis Pedata Nanoformulation in the Management of Alloxan Induced Experimental Diabetes. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3sZiRlP |
Introduction
The journey of a new bioactive from laboratory to market involves huge research and development investment both in terms of money as well as time. The complexity enhances further as the new product seeks to ventilate through stern regulatory requirements which vary with diverse geographical frontiers. Additionally the burden of patent expiry further aggravates the issue. Keeping this scenario in backdrop scientific interest in 21st century has shifted from new drug discovery to advanced drug delivery options for existing 1. Invasion of nano-dimensional drug carriers in drug delivery arena have offered several advantages like accessibility to various routes of administration, a decrease in dose as well as dosing frequency, targeted delivery, controlled drug delivery, enhancement of drug stability and minimization of side effects 2. Among diverse nanocarriers prevalent in drug delivery like liposomes, niosomes, solid lipid nanoparticles, metal nanoparticles etc. polymeric ones have mostly captivated scientific interest as they have facile synthesis techniques, ease of loading both hydrophilic and lipophilic drugs, excellent stability, bio-compatibility, biodegradability, non-toxicity, site specific delivery and extended in-vivo circulation with possibility of imparting stealth characteristics 3. Lee et.al reported that polymeric nanocarriers occupied more than 30% of approved nano-scale drug products 4. Among the different polymers in healthcare applications chitosan is a natural poly-saccharide, biocompatible in nature extracted from crustacean shells and is approved by U.S. FDA for nano-scale drug delivery. The cationic polymer is widely explored because of its suitability for application through diverse administration routes and possibility of fac-ile functionalization to meet specific delivery requirements by chemical modification of the polymer functional groups 5. Additionally chitosan is a permeation enhancer which enhances both paracellular and transcellular drug transport by widening of epithelium tight junctions which makes it more attractive as a delivery device. Chitosan nanoparticles are attractive delivery options and often explored in diabetic management 6,7 due to their excellent biodegradability, non-toxicity, sustained release capacity and mu-co-adhesive nature. As per International Diabetes Federation presently nearly 463 million adults are infected with diabetes which is anticipated to shoot up to 700 million diabetic patients by 2045. Type 2 diabetes is invading most countries and interestingly 79% of adults located in economically developing countries are suffering with the disease wheth-er diagnosed or undiagnosed 8. World Health Organization confirms diabetes as a major stimulus for the genesis of several clinical complications such as sightlessness, hepatotoxicity, myocardial infarctions, stroke and amputation of limbs 9. Age, obesity, desk-bound lifestyle and dietary issues are the root causes linked to the high spread of the disease 10. Though lifestyle modulations remains a pivotal pillar in diabetes manage-ment several classes of antidiabetic agents like biguanides, sulfonylureas, thiazolidinedi-one, dipeptidyl peptidase 4 inhibitors and α-glucosidase inhibitors are used clinically for this purpose. Existing therapeutics in diabetes are associated with several reported adverse effects like weight gain, cardiovascular risk, pancreatitis, cardiac failure, bladder cancer, hepatic disorders, nephrological dysfunctions, pedal edema, nausea, vomiting etc 11. Even long term diabetes management with insulin products have presented newer complexities like resistance development 12. Therefore scientific quest to search for a safer antidiabetic alternative is always warranted clinically.
The medicinal values of plants and their derived products and their therapeutic ap-plications against various ailments including diabetes have progressively improved with mankind civilization. Even nowadays 4/5th of the global population are dependent on such products as their primary healthcare option 13. According to ethnobotanical literature, a wide variety of plants and their by-products are effective in the management of diabetes 14. Positive outcomes of these greener products have aided in their progression through the clinical evaluation stages 15. Additionally plant derived products like glycosides alkaloids, steroids, terpenoids, flavonoids etc, are also reported by various re-searchers of exhibiting a specific mechanism of action against diabetes which can be suitably explored in herbal formulations.
Woody climber Vitis pedata (Cayratia pedata) 16 belonging to family Vitaceae expresses diverse pharmacology including anti-inflammatory 17, diarrhea, hemorrhage, astringent, hemostatic and wound healing properties 18 Pleotropic plant extract have also been reported to be antimicrobial 19, anti-nociceptive 20, anti-arthritic and anti-oxidant properties 21. Diabetes mellitus is associated with enhanced free radical formation and compromised antioxidant status resulting in oxidative damage of cellular organelles 22. This laid the foundation of our previous report where aqueous extract of the plant emerged as a better antidiabetic option than its alcoholic extract. Chitosan nanoparticles of Physalis alkekengi-L extract have enhanced the stability and antioxidant property 23. Our earlier work with silybin nanoparticles was found to ameliorate the diabetic condition and restore the oxidative damage better than the free phytochemical 24. Several plant bioactives like Curcumin, Resveratrol, Naringenin, Quercetin nano-formulations has reported by researchers to overcome their biopharmaceutical limitations and improve their therapeutic efficacy including diabetes 25.
So keeping the scientific evidences in backdrop the present work is envisaged to explore the similar strategy with aqueous extract of Vitis pedata and evaluate its impact in experimental diabetes. Present work is focused on the nanoformulation design for Vitis pedata aqueous extract with biopolymer chitosan and its characterization. The antidiabetic activity of chitosan nanoparticles loaded with the ex-tract, aqueous extract alone and clinically approved antidiabetic agent glibenclamide was investigated in Alloxan induced experimental diabetes. This investigation marks the first report of Vitis pedata extract loaded nanoformulation in diabetic management.
Material and Methods
Materials
The company Spectrachem Pvt. Ltd. provided the alloxan. Chitosan, sodium tripolyphosphate (TPP), water of HPLC grade, and acetic acid were purchased from Sigma-Aldrich (MO, USA) and Spectrochem, respectively (Mumbai, India). The estimate kits for blood and serum came from Span Diagnostics. The study employed glibenclamide that was purchased from Darwin Formulations. The Glycosylated hemoglobin assay kit was purchased from EAGLE Diagnostics in the United States. For data analysis, statistical SPSS (USA) software was employed.
Formulation of aqueous extract of Vitis pedata and extract loaded chitosan nanoparticles
The aqueous extract of VP was prepared as per our earlier report 26. Nanoprecipitation method was explored to prepare aqueous extract loaded chitosan nanoparticles (CNP) with tripolyphosphate. Firstly 100 ml chitosan aqueous solution containing (1% v/v acetone) was prepared to achieve a concentration of 10 mg/ml of the polymer. The polymer solution was added drop-wise at a controlled rate to AEVP solution (50 ml) containing 0.5% (w/v) tripolyphosphate under stirring 120 rpm for 45 min. This was followed by centrifugation at 14000 rpm which accumulated the nanoparticles which was then rinsed with HPLC grade water and preserved for further analysis. The nanoparticles were characterized based on their size, polydispersity index and zeta potential. Morphology of the chitosan nanoparticles was traced in TEM 27, 28.
Diabetes Induction and In-vivo Study design
Albino Wister rats weighing 150–200 grams were purchased from Mahaveer Enterprise in Hyderabad (REF No. 1656/PO/BT/S/12/CPCSEA), and they were housed there for experimental purposes in the animal housing facility of Raghu College of Pharmacy in Visakhapatnam. For a week, the animals were acclimated at a temperature of 27° 2°C with 12-hour light-dark cycles. The bedding for the animals was constructed from paddy husks, and their cages were built of polypropylene. Rat pellets were used to feed the animals, and water was available at all times as long as strict hygienic standards were followed. The Institutional Animal Ethical Committee (IAEC) of Raghu College of Pharmacy, Visakhapatnam, approved the experimental procedures in accordance with the standards established by the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (REF-1549/PO/Re/S/2011/CPCSEA).
A single intraperitoneal (i.p.) injection of Alloxan (120 mg/kg by weight in 0.3% sodium CMC) is administered to wistar rats. Prior to the experiment, the animals were made to fast for 24 hours. To prevent an early hypoglycemia phase, the animals were fed 5% monitored dextrose solution for a day and free access to food after an hour of alloxan administration. Following the administration of alloxan, blood was taken from the tail vein every 24 hours till 72 hours in order to measure the blood glucose levels. Animals included for the remaining portion of the investigation were those with diabetes levels more than 300 mg/dl. The following groupings were created from the animals:
Group-I: administered with saline and served as the standard control (C).
Group II: served as a diabetic control and received saline with alloxan (120 mg/kg b.w). (D).
Group-III: administered with a dose of 5 mg/kg b.w. of Glibenclamide (p.o.) and 120 mg/kg b.w. of Alloxan (GBT)
Group IV: administered with a dose of 400 mg/kg b.w., p.o. of AEVP plus 120 mg/kg b.w. of Alloxan. (AEVPT) (26)
Group-V: administered with a CNP 100 mg/kg b.w., p.o. plus Alloxan 120 mg/kg b.w. (CNPT)
Diabetic rats received the treatment mentioned for a span of 21 days orally and then subjected to different tests for evaluation of their anti-diabetic activity 27. Blood samples were collected from animals under light anesthesia and were preserved in vials with or without anticoagulant for plasma and serum analysis, maintaining the temperature at -20℃ for further studies. Animals were sacrificed by cervical dislocation immediately after collection of blood and the tissues were isolated for further analysis.
Oral glucose tolerance test (OGTT)
Before undergoing an oral glucose tolerance test, animals were permitted to fast for a total of 16 hours 29. After the final treatment, which lasted for two hours, rats were given an intragastric gavage containing glucose solution (2g/kg). The blood was drawn from the tail vein of treated animals at intervals of 0, 30, 60, 90, and 120 minutes to estimate glucose levels.
CNP’s effect on body weight
According to our prior research [26], the animals’ body weights were recorded at 0, 7, 14, and 21 days into the experiment for each group.
Serum Analysis
After being coagulated for 30 minutes at room temperature, the blood samples were centrifuged for 10 minutes at 3000 rpm. The extracted serum was used to assess the concentrations of serum insulin, triglycerides, and cholesterol. The method described by Lowry et al. involved measuring the total protein in the serum and liver tissue using bovine serum albumin (BSA) 30.
Evaluation of glycogen content of liver
The liver’s glycogen content was evaluated using the techniques described by Murat and Serfaty 31. Homogenization of the liver tissues was carried out using an ice-cold buffer (0.1 M, pH 4.2) at a ratio of 1:9 (w/v). Centrifugation was then performed at 10,600 g for 30 minutes at 40℃. The free glucose content of the supernatant was then measured using the glucose oxidase peroxidase (GOD/POD) method. 2 mg of amyloglucosidase was added to 500 ml of homogenate. Additionally, the components were incubated at 37°C for an additional 4 hours. The glucose content was reported in its entirety. The difference between total and free glucose was used to estimate the quantity of hepatic glycogen31.
Determination of glycohemoglobin (HbA1c) content
According to our prior article24, the ion exchange resin method was used to assess the HbA1c in a blood sample. The whole blood was mixed once the lysing agent had been applied, and the hemolysate was then placed into a cation exchange column. The HbA1c is the percent that eluted. The HbA1c percentage is calculated by dividing the hemolysate’s total hemoglobin fraction by the HbA1c fraction’s absorbance at 415 nm.
Serum fructosamine
Based on the method presented by Johnson et al. 33,34, the examination of serum fructosamine was carried out. 100 l of serum were added to 1 ml of nitroblue tetrazolium (NBT), and the mixture was then incubated at 37°C for one hour. At 530 nm, the absorbance was measured. Fructosamine concentration was determined in relation to 1-deoxy-1-morpholino-fructose (1-DMF).
Measuring the serum lipid peroxidation level
Yagi et al. compute serum lipid peroxidation using malondialdehyde (MDA) levels in the serum. The serum was diluted and mixed with trichloroacetic acid solution (TCA), which was then left at for fifteen minutes at room temperature. This solution was then given 1.5 ml of 76% thiobarbituric acid (TBA) that contained 0.05 (M) NaOH, and it was incubated at a boiling water bath for 30 minutes. 4 mL of n-butanol was added to the mixture after it had cooled. The final chromophore’s fluorescence emission intensity was measured at 553 nm35 after the butanol phase was eliminated.
Estimation of superoxide dismutase (SOD), Catalase and GSH in liver tissue
The liver tissues were dissected into minute pieces and homogenized in 10mM potassium phosphate buffer for the measurement of SOD. At 6000 g for 10 minutes at 4°C, the homogenate was centrifuged. The leftover supernatant was recovered to measure the levels of SOD, catalase, and GSH.
According to Koyu et al approach36, the SOD and catalase were estimated. The procedure is determined by the amount of enzyme required to prevent pyrogallol’s self-oxidation in the tissue homogenate’s supernatant. Units per gramme of tissue were used to express the enzyme activity. On the other hand, the quantity of catalase needed to break down 1 mM of hydrogen peroxide (H2O2) per minute at 25°C serves as evidence of the enzyme’s activity. After 1 minute, the optical density at 240 nm decreased in comparison to the blank, which is a sign that H2O2 is disintegrating.
The GSH content of liver tissues was assessed using Ellman’s technique. The supernatant was centrifuged with sodium EDTA solution (20 mM) and 5% TCA. Following centrifugation, 50 mL of 5,5-dithiobis-2-nitrobenzoic acid and 500 mL of Tris buffer (200 mM, pH 8.4) were added to the supernatant (DTNB, 100 mM). At 412 nm, the optical density was computed in relation to deionized water. The real GSH level in mM/mg of liver tissue was determined by subtracting the blank from the sample reading 37.
Histopathological studies
The pancreatic tissues were kept intact, treated with 10% formalin, and dehydrated for 12 hours using progressively greater concentrations (70 %, 80 % and 90 %) of isopropyl alcohol. Absolute alcohol was used during the final stage of dehydration. After that, the tissues were cleaned with chloroform. The tissues were subjected to paraffin infiltration in the automated tissue processing machine. The tissues were stained with hematoxylin-eosin, mounted on neutral DPX media, and photographed using a camera light microscope assembly.
Statistical analysis
The data has been presented in the form of the mean and standard deviation. Statistical differences in mean values were examined using the Student’s t-test. Differences were taken into consideration if P < 0.05, which served as the threshold for statistical significance. Statistical analysis was done using SPSS Statistics 29 (SPSS, USA).
Results and Discussion
Characterization of Nanoparticle
Nanotechnology guided delivery solutions for therapeutics should be biocompatible and also exhibit high degree of biodegradability with no toxicity towards the living system. Considering these properties, we considered chitosan as the perfect candidate as a nano-scale delivery device for the present study. Chitosan, a natural polysaccharide, is acquired from the outer skeleton of crustaceans and has been included in FDA list of Generally Recognized as Safe (GRAS) candidate which enhances its acceptability in clinical applications like drug delivery application38. Chitosan is derived from partial deacetylation (65-70%) of chitin which is the most abandoned natural biopolymer next to cellulose. In addition to easy availability, lower toxicity and cellular organelle mediated easy degradation further increase the application window of chitosan as drug delivery vehicle 39. At slightly acidic pH, chitosan carries a net positive surface charge owing to the presence of glucosamine residue. Positively charged chitosan efficiently interact with the anionic mucin protein present in the mucus layer imparting high drug influx in the gastrointestinal tract confirming its suitability for oral delivery.
Among the various synthetic routes for formulating chitosan nanoparticles ionic cross-linking with tripolyphosphate have been widely used owing to its high drug entrapment efficacy, facile nature, usage of non-toxic solvents and inexpensive instruments 40. In the present study, the chitosan nanoparticle formed, displayed a particle size of 186± 5.37 nm with a polydispersity index of 0.226 (Figure 1) when examined by dynamic light scattering. The extract loaded nanoparticles exhibited the characteristics of a stable formulation as evident from zeta potential value of 23 ± 1.93 mV. Nanoparticle morphology figured out in TEM revealed spherical to quassi-spherical surface geometry (Figure 2).
 |
Figure 1: CNP characterization (A) Particle Size (B) Zeta Potential (C) Phase Plot in DLS. |
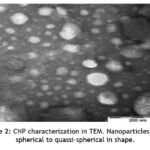 |
Figure 2: CNP characterization in TEM. Nanoparticles were spherical to quassi-spherical in shape. |
Interestingly reports confirm that average hydrodynamic diameter of chitosan nanoparticles is directly proportional to the polymer concentration but the spherical geometry remains unaffected by changes in chitosan content 41.
Effect of CNP on alloxan induced diabetes and oral glucose tolerance test
Fasting blood glucose levels were assessed in each group at regular intervals, and the findings are shown in the Figure (3A). Alloxan, a pyrimidine derivative causes a hyperglycemia as compared to that in the control group (P < 0.001). The induction of alloxan has damaged the β-cells of pancreas minimizing the endogenous insulin release which in turn resulted in the spike of blood glucose levels 42. Additionally the increased toxicity of the cellsdue to the action of alloxan is triggered by reactive oxygen species (ROS) production like extremely reactive hydroxyl radical which aggravates cellular damage. Alongside alloxan causes upsurge in cytosolic calcium which results in speedy demolition of beta cells of pancreas 43. The continuous exposure of the diabetic rats with GB, AEVP and CNP resulted in the marked decline in blood glucose levels as compared to the diabetic control. However, CNPT, displayed the most remarkable effect of normalizing blood glucose level even better than the GBT the end of 21 days of treatment. Significant hypoglycemic property of CNPT may be attributed to enhanced intracellular delivery of the extract by nanoparticulation along with restoration and stimulation of the β-cells by Vitis pedata favoring insulin release44. The OGTT challenge serves as one of the primary tools to evaluate glucose tolerance, physiological duty of β-cells and occurrence of insulin resistance. Diabetes results in the destruction of pancreatic beta-cells, which impairs the tissues’ ability to absorb glucose, resulting in decreased glucose tolerance45. Though, derivation of definite conclusion about whole body sensitivity to insulin from OGTT findings is still an arduous task 46. The figure 3 (B) depicted that the diabetic rats showcases the maximum glucose intolerant behavior that was consistent for the next two hours than the rest of the treatment groups. The rats that received treatment with AEVP returned to normal levels around 120 min however the glucose levels were higher compared to GBT treated group. CNBT treatment was able reverse the glucose tolerance significantly within 120 minutes and the final glucose levels were better compared to both the GBT and AEVPT group. Similar results were obtained with gold nanoparticles which portrayed similar control of blood glucose level in case of streptozotocin-induced diabetes47.
 |
Figure 3: (A) Blood glucose levels in different groups after treatment for 21 days (B) Oral glucose tolerance test in different study groups. |
Effect of treatment on body weight
The Figure 4 demonstrated the outcome of CNPT and extract on the body weight of the animals. Treatment with alloxan resulted in considerable decrease in of body weight. Studies have highlighted the deterioration muscles as a result of hyperglycemia 48. In this study, induction of alloxan is hypothesized to be responsible for wasting of tissue protein thereby causing a drastic decrease in body weights. Our previous study has showed a gradual improvement of body weight after the treatment with plant extract [26]. Present study reveals, that CNPT treatment displayed even better outcome than AEVPT as well as marketed drug GBT.
 |
Figure 4: Comparison of body weights of animals at 0, 7, 14, 21 days of treatment. Results expressed as mean ± SD (n=6). |
*P-value < 0.0001 highly significant difference with the diabetic group.
Ω P-value < 0.001 significant difference with the diabetic group
Serum Analysis
Alloxan induced diabetes influences partial destruction of the β cells of pancreas which drastically declines both quantity and quality of insulin produced by these cells. So as expected there was a sharp decline in insulin level in alloxan induced diabetes group compared to control group (Table 1). Diabetes also influences dysfunctions in fat metabolism due to hampering of VLDL degradation and chylomicron clearance which is mostly linked to poor performance of lipoprotein lipase49. Additionally, total cholesterol level also spikes up due to weak metabolic control as reflected in Table 1. Although moderate improvement was observed after plant extract treatment drastic normalization took place in case of nanoparticle treated group and the results sometimes was even improved than the clinically approved agent glibenclamide. Significant rise in insulin level by CNPT treatment may be linked to size dominated improved intracellular delivery of the extract which induced speedy β -cell revival. The p-values calculated depicts that similar level of significance was recorded by the GBT and CNPT treated groups in case of insulin, cholesterol and triglyceride levels compared to the diabetic group. Whereas for the extract treated group insulin levels and triglyceride metabolism exhibited moderate significant difference compared to the diabetic group50. This indicates that the nano-scale formulation has the ability to show significant effect on diabetes management with respect to insulin secretion, cholesterol and triglyceride metabolism compared to the plant extract alone.
Table 1: Serum insulin, cholesterol and triglyceride levels in different study groups.
|
Study groups |
Insulin (µg/lit) |
Cholesterol (mg/dl) |
Triglyceride (mg/dl) |
|
Control (C) |
0.65±0.04 |
74.8±5.24 |
59±6.09 |
|
Diabetic (D) |
0.14±0.01 |
148.7±8.83 |
170.77±16.13 |
|
Glibenclamide treated (GBT) |
0.54±0.02* |
85.3±6.34* |
85.9±5.63* |
|
AEVP treated (AEVPT) |
0.24±0.05 Ω |
105.2±9.12* |
123.1±18.07Ω |
|
CNP treated (CNPT) |
0.61±0.05* |
89.8±7.12* |
79.55±7.08* |
Results expressed as mean ± SD (n=6)
*P-value < 0.0001 highly significant difference with the diabetic group.
Ω P-value < 0.001 significant difference with the diabetic group
Estimation of glycogen content of liver and glycohemoglobin (HbA1c) content
One of the most prominent roles of insulin in human body is to convert the excess glucose to glycogen, which is stocked in liver tissues for future energy usage. Therefore, in diabetic conditions, the diminished insulin level has a direct impact on the glycogen store as well28. The diabetic control group displayed reduced glycogen level due to insulin deficiency as expected in diabetes. On the other hand, the glycogen levels progressed steadily in all three treatment groups. Both CNPT and GBT produced comparable replenishment of glycogen store as observed in Figure 5 (A). Effect of CNPT can be attributed to the size-guided enhanced delivery of the extract to the hepatic tissues. Glycation of hemoglobin is a result of sustained hyperglycemia that eventually led to the HbA1c development. The HbA1c value is directly proportional to the blood glucose level which is evident from its elevated values in diabetes group. In case of treatment groups the HbA1c values were found to normalize with passage of time as shown in Figure 5 (B). Similar to influence on glycogen stores marketed antidiabetic agent and CNPT produced maximum normalization of HbA1c signifying better glycaemic control over extract treated group.
 |
Figure 5: Effect of GBT, AEVPT and CNPT on (A) Liver glycogen (B) HbA1C. Results expressed as mean ± SD (n=6). |
*P-value < 0.0001 highly significant difference with the diabetic group.
Ω P-value < 0.001 significant difference with the diabetic group
Serum fructosamine estimation
Fructosamine is the representative product which includes all ketoamine linkages formed by glycation of serum proteins in diabetes conditions. Chemistry of fructosamine formation reveals that at first condensation occurs amid the free aldehyde group of the carbohydrate and the N-terminal amino acid of the protein that leads in an intermediate reversible Schiff base end product. This product endures Amadori rearrangement to form fructosamine derivatives through reversible ketoamine bonds51 52. The degree of fructosamine in the serum is exponentially high in case of diabetic group as shown in Figure 6. The diabetic rats receiving treatment displayed a declined trend in the fructosamine levels. CNPT treated group exhibited better restoration of the elevated levels of fructosamine as compared to both extract as well as glibenclamide treated group.
 |
Figure 6: Serum fructosamine levels in different study groups. Results expressed as mean ± SD (n=6). |
*P-value < 0.0001 highly significant difference with the diabetic group.
Ω P-value < 0.001 significant difference with the diabetic group
Serum MDA estimation
The alloxan model operates through two discrete pathological avenues: primarily by prevention of glucose-stimulated insulin secretion and secondly by reactive oxygen species (ROS) generation which judiciously causes death of pancreatic beta cells 42,43. ROS mainly targets the lipids and also associate with transition metals to form stable aldehydes, like malondialdehyde which disrupts cell membranes53. So the measurement of MDA levels produced by lipid peroxidation of thiobarbituric acid reactive species (TBARS) is vital to understand the oxidative stress level in animals. The diabetic group displayed a significantly higher MDA level as compared to the control group due to enhanced oxidative stress levels. On the other hand, the treatment groups displayed depreciation in MDA levels. Precisely the influence CNBT group to mitigate oxidative stress was even improved than that induced by clinically approved agent GBT as represented in Figure 7.
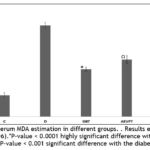 |
Figure 7: Serum MDA estimation in different groups. . Results expressed as mean ± SD (n=6). |
Assessment of superoxide dismutase (SOD), Catalase and GSH in liver tissue
Alloxan nullify the action of glucokinase enzyme by reaction with its -SH group resulting in disulfide bond formation. A redox cycle originates between alloxan and dialuric acid which culminates in reactive oxygen species (ROS) and superoxide radical formation. These radicals converts ferritin to ferrous and ferric ions also generate hydrogen peroxide in the company of superoxide dismutase. Ferrous and hydrogen peroxide interact by Fenton reaction to form highly reactive hydroxyl radicals 54. As indicated in the Table 2, the SOD and catalase levels were found to be inversely proportional in the diabetic condition compared to the control. These might have occurred as a result of glycosylation or ROS-mediated inhibition of the mentioned enzymes55. From the Table 2 it is evident that the treatment with GBT, AEVPT and CNPT demonstrated an improved antioxidant status however profound improvement was observed in CNPT and GBT treated groups. The GSH level on the other hand is found to comparatively decrease in diabetic condition, mainly due to the enhanced action of aldose reductase and glutathione reductase or due to oxidative stress induced degradation as observed in the diabetic group 56. CNPT treated group favored better in replenishment of GSH compared to all other treatment groups which may be linked to enhanced availability of the extract in hepatic environment.
Table 2: SOD, Catalase and GSH levels in different treatment groups.
|
Animal groups |
SOD (U/gm of tissue) |
Catalase (mU/mg of tissue) |
GSH (µM/mg of tissue) |
|
Control (C) |
7.73±0.69 |
150.5±7.70 |
4.41±0.21 |
|
Diabetic (D) |
2.12±0.55 |
85.9±4.26 |
1.94±0.31 |
|
GBT |
6.33±0.33* |
139.76±8.76* |
3.73±0.27* |
|
AEVPT |
5.24±0.58* |
105.53±10.34 Ω |
2.23±0.12# |
|
CNPT |
6.47±0.25* |
137.39±7.64* |
3.93+±0.18* |
Results expressed as mean ± SD (n=6)
*P-value < 0.0001 highly significant difference with the diabetic group.
ΩP-value < 0.05 significant difference with the diabetic group.
#P-value < 1 no significant difference with the diabetic group.
Histopathological studies
The histopathological modulations of the pancreatic tissues related to each study group are represented in Figure 8. Histopathological analysis of control animal reveals compact pancreatic architecture with nearly round shaped islet cell, well defined nuclei and grainy cytoplasm indicates the healthy nature of organ. Alloxan induced damage was evidenced in group D animals from reduced number of islets cell and shrinkage of existing islet cells. Lymphocyte infiltration in peripheral islets was also identified. AEVP treated animal showed an enhanced histological modifications with respect to group B animal. The said observation was confirmed by the presence of mild necrotic lesions and partial recovery of islet cells in comparison to diabetic group. CNPT group however showed significant regeneration of cells of islets of Langerhans with marked improvement in structural integrity in line with other findings27. Additionally, in comparison to group B animals, peripheral lymphocyte infiltration of islets appeared to be significantly improved in the CNPT group. The recovery in histoarchitecture brought about by nanoparticle treatment was comparable with the effect of standard diabetic drug in GBT group and as reported by other researchers 28.
 |
Figure 8: Liver histopathological study after 21 days of treatment – A: Control (C) B: Diabetic (D), C: Glibenclamide treated (GBT) |
Conclusion
Diabetes can be induced by diverse agents like dithizone, monosodium glutamate, gold thioglucose, anti-insulin serum etc. but among them alloxan and streptozotocin mostly gets scientific preference due to easy availability, low-cost and high degree of reproducibility. Our earlier report already established the antidiabetic potential of Vitis pedata extract. Present study is focused to evaluate the influence on pharmacological activity by nanoparticulate drug delivery through oral route which enjoys patient compliance. Nanoparticle treatment showed a profound influence in reducing the blood glucose level in the alloxan induced diabetic rats when compared with the plant extract. The nano-scale formulation also provided better restoration of diabetes related serum biomarkers, liver glycogen store and HbA1C levels. Thus we can conclude that the formulation provided better control of diabetic management compared to the extract and the effect was at par with standard antidiabetic agent glibenclamide. This efficacy enhancement may be at-tributed to the increased availability of the extract at cellular level due to nanoparticulation. Additionally the extract and its nanoparticle also replenished the diabetic induced oxidative stress and barred lipid peroxidation which prevented cellular damage caused by the disease. Similar results were obtained with Momordica charantia extract associated silver nanoparticles which produced antidiabetic activity via genetic modulation in the pancreas of diabetic animals with a remarkable restorative influence on Takeda-G-protein-receptor-5, glucagon-like peptide-1, insulin and NFE2-related factor 2 genes 57. Ramulus mori extract functionalized polyacrylic gold nanoparticles was reported to be successful for the management of gestational diabetes mellitus58.
These findings provide scientific rationale and warrants further clinical investigations to consider the nanoformulation of Vitis pedata as a newer antidiabetic agent. Further, the work could be extended to analyze the therapeutic efficacy of the presented formulation against hyperglycemia at its cellular level and investigate the mechanism of action for the same.
Acknowledgement
Prof. Partha Roy would like to thank Dean, GITAM School of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India for his continuous support and encouragement.
Conflict of Interest
There is no conflict of interest exist among the authors. The authors have no relevant financial or non-financial interests to disclose
Funding Source
Dr. Suvadra Das would like to acknowledge the financial assistance from University of Engineering and Management, Kolkata through Grant-in-aid project. Grant number is UEMK/13/4/2022/1
References
- Ravichandiran V, Masilamani K, Senthilnathan B, Maheshwaran A, Wong TW, Roy P (2017) Quercetin-Decorated Cur-cumin Liposome Design for Cancer Therapy: In-Vitro and In-Vivo Studies. Curr Drug Deliv 14(8).
- Gelperina S, Kisich K, Iseman M D, &Heifets L (2005) The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Cirt. Care Med. 172(12): 1487–1490.
- Pinto Reis C, Neufeld R J, Ribeiro A J, &Veiga F (2006)Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: NBM 2(1): 8–21.
- Ventola CL (2017) Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T, 42(12):742-755.
- Mohammed M, Syeda J, Wasan K, &Wasan E. (2017) An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics, 9(4): 53.
- Adel Abdel-Moneim, Ahmed El-Shahawy, Ahmed Ismail Yousef, Sanaa Mahmoud Abd El-Twab, ZienabEssam Elden, Mohamed Taha (2020) Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches.Int. J. Biol. Macromol. 154:1496-1504.
- Mukhopadhyay P, Sarkar K, ChakrabortyM, BhattacharyaS, MishraR, KunduP.P (2013) Oral insulin delivery by self-assembled chitosan nanoparticles: In vitro and in vivo studies in diabetic animal model.Mater. Sci. Eng. C., 33 (1): 376-382.
- https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html
- Diabetes. Who.int. Retrieved 4 January 2022, from https://www.who.int/news-room/fact-sheets/detail/diabetes,2022
- Zheng Y, Ley SH, Hu FB(2013) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14:89.
- Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti, S, Chada A, Ravilla R, Mirza W (2017) Clinical Review of Antidia-betic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 8.
- Wilcox G (2005) Insulin and insulin resistance. ClinBiochem Rev. 26(2): 19-39.
- Ekor M (2005) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 4: 177.
- Sharmila S, Kalaichelvi K, Dhivya SM, Premamalini P, Abirami P, Jayanthi G (2017) PharmacognosticAssessment of the Endemic and Vulnerable Medicinal Climber-Cayratia pedata (Lam.) Gagnep. var. glabra Gamble and Its Antibacterial Activity. Pharmacognosy Res. 9.
- Ahmad R, AlLehaibi LH, AlSuwaidan HN, Alghiryafi AF, Almubarak LS, AlKhalifah KN, AlMubarak HJ, Alkhathami MA (2021) Evaluation of clinical trials for natural products used in diabetes: An evidence-based systemic literature re-view. Medicine (Baltimore), 23;100(16):e25641.
- Anna TB, Parnell JAN, Watson MF (2017) Nomenclatural notes on species of Asian Vitaceae. Taxon 66.6, 1500-1501.
- Aswathy TR, Gayathri E, Praveen J, Achuthsankar S Nair and Sugunan VS (2019) Phytoprofiling of medicinal plant Cayratia pedata by qualitative and quantitative method. J.pharmacogn. Phytochem., 8(2), 1637-1642.
- Sharmila S, Kalaichelvi K, Dhivya SM (2018) Pharmacognostical and phytochemical analysis of Cayratia pedata var. glabra—A vitaceae member. Int. J. Pharm. Sci. Res, 9: 218-226.
- Nayak BK, Lazar J (2014) Antimicrobial efficacy of leaf extract of Cayratia pedata Lam., Vitaceae. International Journal of ChemTech Research, 6(14): 5721-5725.
- Rajendran V, Rathinambal V, Gopal V (2011) A preliminary study on anti-inflammatory activity of Cayratia pedata leaves on Wister albino rats. Der Pharmacia Lettre 3(2):433-437.
- Selvarani K, Bai GVS (2014) Anti-arthritic activity of Cayratia pedata leaf extract in Freund’s adjuvant induced arthritic rats. Int. J Res. Plant Sci 4(2), 55-59.
- Bajaj S, Khan A (2012) Antioxidants and diabetes. Indian J EndocrinolMetab, 16(2):S267-S271,
- Mahmoudi R, TajaliArdakani M, HajipourVerdom, Bagheri A, Mohammad-Beigi H, Aliakbari F, BardaniaH (2019) Chi-tosan nanoparticles containing Physalis alkekengi-L extract: preparation, optimization and their antioxidant activity, Bull. Mater. Sci 42(3).
- Das S, Roy P, Pal R, Auddy RG, Chakraborti AS, Mukherjee A (2014) Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS One. 3;9(7): e101818.
- Dewanjee S, Chakraborty P, Mukherjee B, De Feo V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int J Mol Sci. 2020 Mar 23;21(6):2217.]
- Bhattacharya J, Ata S, ChakrabartyS, JhaS K, RoyP (2022) Evaluation of antidiabetic activity of Vitis pedata in alloxan induced diabetic rats, Indian J. Pharm. Sci. 84(3): 631-641.
- Perumal V, Manickam T, Bang K.-S, Velmurugan P, & Oh B.-T (2016) Antidiabetic potential of bioactive molecules coat-ed chitosan nanoparticles in experimental rats. Int. J. Biol. Macromol. 92: 63–69.
- Bindu RH, Lakshmi SM, Himaja N, Nirosha K, Pooja M (2014) Formulation characterization and antidiabetic evaluation of Talinum portulacifolium (Forssk.) loaded solid lipid nanoparticles in Streptozotocin and high fat diet induced dia-betic rats. J. Glob. Trends. Pharm. Sci., 5(4): 2108-14.
- Kim MJ, Ha BJ (2013) Antihyperglycemic and Antihyperlipidemic Effects of Fermented Rhynchosia volubilis in Allox-an-induced Diabetic Rats. Toxicol Res, 29(1):15-9.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1959) Protein measurement with the Folin phenol reagent. J BiolChem 193, 265–275.
- Murat JC, Serfaty A. (1974) Simple enzymatic determination of polysaccharide (glycogen) content of animal tissues. ClinChem 20, 1576–1577.
- Liu SH, Chang YH, Chiang MT. (2010) Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal mus-cle in streptozotocin-induced diabetic rats. J Agric Food Chem 58: 5795–5800.
- Johnson RN, Metcalf PA, Baker JR (1983) Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. ClinChimActa 127, 87–95.
- Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S (2013) Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med 13, 171.
- Yagi K (1987) Lipid peroxides and human diseases. ChemPhys Lipids 45, 337– 351.
- Koyu A, Gokcimen A, Ozguner F, Bayram DS, Kocak A (2006) Evaluation of the effects of cadmium on rat liver. Molec-ular and cellular biochemistry 284(1): 81-5.
- Ellman GL, (1959) Tissue sulfhydryl groups. Arch BiochemBiophys 82, 70–77,
- Leonida M, Ispas-Szabo P, Mateescu MA (2018) Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery, Bioact. Mater. 3(3): 334-340.
- George M, Abraham TE (2006) Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chi-tosan-a review. J. Control. Release 114(1): 1-14.
- Pedroso‐Santana S, Fleitas‐Salazar N (2020) Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 69(5): 443-7.
- Sreekumar S, Goycoolea FM, Moerschbacher BM et al (2018) Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci Rep 8: 4695.
- Macdonald IO, Adeosun MA, Akinloye AO (2017) Alloxan-induced diabetes, a common model for evaluating the gly-cemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina, 53: 365-374.
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50(6):537-46.
- Swanston-Flat SK, Day C, Bailey CJ, Flatt PR (1990) Traditional plant treatment for diabetes: Studies in normal and streptozotocin diabetic mice. Diabetologia33: 462–4.
- Dinger K, Mohr J, Vohlen C, Hirani D, Hucklenbruch-Rother E, Ensenauer R, Dötsch J, Alejandre Alcazar MA (2018) In-traperitoneal Glucose Tolerance Test, Measurement of Lung Function, and Fixation of the Lung to Study the Impact of Obesity and Impaired Metabolism on Pulmonary Outcomes. J Vis Exp 15(133): 56685.
- Matsuda M, Defronzo RA (1999) Insulin Sensitivity Indices Obtained from Oral Glucose Tolerance Testing. Diabetes Care22: 1462-14.
- BarathManiKanth S, Kalishwaralal K, Sriram M, Pandian S, Youn H, Eom S,Gurunathan S (2010) Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotech. 8(1): 16.
- Pupim LB, Heimbürger O, Qureshi AR, Ikizler TA, Stenvinkel P (2005) Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus, Kidney Int.68(5): 2368-74.
- Shen G (2007) Lipid Disorders in Diabetes Mellitus and Current Management. Curr. Pharm. Anal.3(1): 17-24.
- Salehi B, Ata A, V Anil Kumar N. Sharopov F et al (2019) Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomol. 9(10): 551.
- Vermes I, Zeyen LJ, van Roon E, Brandts H (1989) The role of serum fructosamine as a screening test for gestational di-abetes mellitus. HormMetab Res. 21(2): 73-6.
- Danese E, Montagnana M, Nouvenne A, Lippi G (2015) Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes SciTechnol. 9(2): 169-76.
- Tiwari BK, Pandey KB, Abidi AB, Rizvi SI (2013)Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 1–8.
- Rohilla A, Ali S(2012) Alloxan Induced Diabetes: Mechanisms and Effects. Int. J. Res. Pharm. Biomed. Sci. 3 (2).
- Sozmen EY, Sozmen B, Delen Y, Onat T (2001) Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ra-tios may implicate poor glycemiccontrol. Arch. Med. Res. 32: 283–287.
- HakkiKalkan I, Suher M (2013) The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak. J. Med. Sci. 29(4): 938-42.
- Elekofehinti, O.O. Momordica charantia nanoparticles potentiate insulin release and modulate antioxidant gene expression in pancreas of diabetic rats. Egypt J Med Hum Genet 23, 63 (2022). https://doi.org/10.1186/s43042-022-00282-0
- Xianghong Cheng, Yahui Xu, Qian Jia, Ning Guo, Zhenzhen Wang & Yu Wang (2020) Novel greener approached synthesis of polyacrylic nanoparticles for therapy and care of gestational diabetes, Drug Delivery, 27:1, 1263-1270, DOI: 10.1080/10717544.2020.1809555







