Maysaa K. Al-Malkey* , Sinai W. Mohammed
, Sinai W. Mohammed , Noorulhuda F Khalaf
, Noorulhuda F Khalaf  , Mohammed J. Al-Obaidi
, Mohammed J. Al-Obaidi and Fadhaa O. Sameer
and Fadhaa O. Sameer 
Tropical Biological Research Unit, College of Science, University of Baghdad, Baghdad, Iraq
Corresponding Author E-mail:maysakadhim@uobaghdad.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2729
Abstract
The coronavirus disease 2019 (COVID-19) pandemic and the infection escalation around the globe encourage the implementation of the global protocol for standard care patients aiming to cease the infection spread. Evaluating the potency of these therapy courses has drawn particular attention in health practice. This observational study aimed to assess the efficacy of Remdesivir and Favipiravir drugs compared to the standard care patients in COVID-19 confirmed patients. One hundred twenty-seven patients showed the disease at different stages, and one hundred and fifty patients received only standard care as a control group were included in this study. Patients under the Remdesivir therapy protocol were (62.20%); meanwhile, there (30.71%) were under Favipiravir therapy. From the total number of patients under both protocols, 75.6% of the total patients recovered, and 15.7% were deceased. The mortality rate was shown to be 14 out of 64 patients (22%) in critical COVID-19 patients in the Remdesivir group and 3 out of 37 patients (8%) in the Favipiravir group. Remdesivir drug lowered healing mean time to 6 days in mild-to-moderate. COVID-19 clinical manifestations are different among infected patients, and the therapy required to be carefully designed for critical cases in particular. Remdesivir and Favipiravir therapy tend to have a promising efficacy in reducing the mortality rate and time of recovery, especially among mild-to-moderate patients.
Keywords
COVID-19; Favipiravir; Remdesivir; Survival
Download this article as:| Copy the following to cite this article: Al-Malkey M. K, Mohammed S. W, Khalaf N. F, Al-Obaidi M. J, Sameer F. O. The Significance of Remdesivir and Favipiravir Therapies to Survival of COVID-19 Patients. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Al-Malkey M. K, Mohammed S. W, Khalaf N. F, Al-Obaidi M. J, Sameer F. O. The Significance of Remdesivir and Favipiravir Therapies to Survival of COVID-19 Patients. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3rCu37L |
Introduction
The emergence of a novel pandemic caused by COVID-19 poses a huge burden on humanity’s health since the Spanish flu in 1918 exceeding 297 million nearly 7 million casualties by December 2021 worldwide. By first week of February, 2021, confirmed COVID-19 cases reached 106 million, mortality recorded over 2 and half millions worldwide1. Tremendous global efforts by health workers including clinicians and researchers in an attempt to come up with new drug options to seize the infection spread2. Many clinical trials have been conducted, but the clinical efficacy of any therapeutic agent for coronavirus disease has not been proven yet meanwhile, a few of them (Remdesivir, hINFa-2b, Ribavirin, Chloroquine, and Arbidol) are ongoing investigated under clinical trials for their efficacy against the disease3.
Remdesivir which is an adenosine triphosphate (ATP) structural analog inserted during RNA polymerase instead of the natural substrate into nascent viral RNA resultant in delayed replication due to termination of the chain leading to viral replication inhibition4. Remdesivir was suggested to treat SARS-CoV-2 confirmed infection through viral replication during the first week of the symptoms starts5. The purine nucleotide favipiravir ribofuranosyl-5′-triphosphate, often known as favipiravir or T-705, is a prodrug whose active ingredient inhibits RNA polymerase, stopping viral replication6,7. The Japan Pharmaceuticals and Medical Devices Agency approved favipiravir for use in 2014 to treat novel and re-emerging influenza virus infection. In vitro study by Eloy et al., (2020) stated that Favipiravir 50% effective concentration (EC50) against SARS-CoV-2 in Vero E6 cells was 61.88 µM/L8. Favipiravir, which focused on RNA-dependent RNA polymerase (RdRP) to treat influenza A, received approval in Japan with an IC50 range of (0.13 to 0.48 ug/ml)6. Influenza and SARS-CoV-2 are RNA genomes, they showed similar disease manifestations and organ tropism which they rely on (RdRp) for replication, their inhibitor Arbidol which they license for influenza treatment in Asia like Russia and China as an option of standard care for coronavirus disease9,10. Tocilizumab Actemra® by Roche Pharma Company is attached to membrane-bound and soluble receptors of interleukin 6, so it is IgG1 mAb human recombinant. The signaling pathway inhibition leads to the reduction the proinflammator activity of the interleukin 611.
There are currently significant efforts being made by researchers to carry out clinical trials and research studies to better understand the biology and clinical characteristics of COVID-1912. Information about the age and gender of patients13,14, as well as the effects of various treatment modalities on morbidity and death15,16. However, to the best of our knowledge, no studies from Iraq have been published to cover these pivotal aspects. Therefore, this observational study presenting the first study from Baghdad province to scrutinize the nature of the COVID-19 infection and to evaluate the impact of the proposed treatment protocols on the treated sample of COVID-19 patients seen in a single center from January to August 2020. The study includes the currently therapeutic regimen used such as Remdesivir, Actemra®, and Favipiravir and their effect on time of recovery and mortality rate.
Patients and Methods
Patients
In this prospective, open-label, single-center observational study, adult COVID-19 patients were included. The total number of the patients randomized in this study were 277 confirmed patients with COVID-19, 127 patients were enrolled under therapy protocols beside the supportive treatment and 150 patients were set as a control group received only standard care (Figure 1). The subjects were residents at Al-Forat General Hospital, Baghdad through January-August 2020. The single center patients were mostly inpatients and few were outpatients. They were classified according to the disease severity into mild-to-moderate patients in which were outpatients and all inpatients were severe and critical patients. The COVID-19 patients were evaluated clinically, radiological and confirmed by laboratory polymerase chain reaction (PCR) testing. According to World Health Organization (WHO) recommendations, the COVID-19 patient’s condition was classified as mild, moderate, severe, or critical. The patients were considered recovered according to the symptoms vanishing, radiology of chest x-ray or Computerized tomography scan and PCR clearance. This study was authorized by the scientific committee of the college of science at the University of Baghdad.
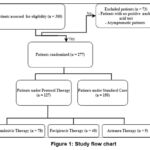 |
Figure 1: Study flow chart |
COVID-19 Patients Therapy Protocols
If we put in mind that hospital stay was 14 days, and recovery rate from COVID-19 symptoms (fever, fatigue, or coughing) in the treatment groups. Senior medical staff at Al-Forat General Hospital in Baghdad implemented the treatment guidelines in accordance with those that the Iraqi Ministry of Health had approved. The therapies groups were as follows:
Remdesivir therapy (D1 group)
Remdesivir 200 mg (Intra venous injection) as a single dose on daily for 3 days, followed by 100 mg once within 7 days of symptom onset17.
Actemra® therapy (D2 group)
Actemra® 80 mg/4 mL (Intra venous infusion bag) as a single dose once. One further Actemra® infusion may be given at least eight hours following the initial infusion if clinical signs or symptoms continue to deteriorate or do not improve after the first dose. Favipiravir therapy (D3 group)
The Favipiravir dosage is the same as the recommended amount for treating influenza, which is 1600 mg given twice daily on day one and 600 mg given twice daily on day two. Favipiravir treatment lasted between 7 and 14 days.18,19.
Control group
According to each patient’s clinical status, the patients in this group only received standard care, which may have comprised all of the following or some of them. The standard of care for SARS-CoV-2 often included supportive therapies such oxygen therapy, antibiotic and anti-inflammatory drugs, and symptomatic medicines, as well as some dietary support. According to the disease severity, patients were provided with COVID-19 standard care adopting the 4th edition of The Protocol for Diagnosis and Treatment of Novel Coronavirus Pneumonia (Table 1).
Table 1: COVID-19 Patients’ standard care
|
Medication |
Dosage |
Recommendations |
|
Acetaminophen Vitamin C Zinc Vitamin D3 Azithromycin Dexamethasone Methylprednisolone |
500 mg 1000 mg 75-125 mg 5000 IU 250 mg/day 6 mg 40 mg |
On need Twice / day Daily Daily Daily for five days course Daily, if needed twice per day, if needed |
|
Oxygen therapy/ C-Pap Mechanical ventilation |
|
If needed If needed |
Assessing study endpoints
The mortality rate and the recovery time were assessed in current study. For each of the three protocol therapies, the patients’ survival, progression, and/or recovery were compared to those receiving only conventional treatment in the control group.
Recovery Time
The time required to heal from the time of taking therapy.
Mortality Rate
The patients with mild-to-moderate, severe, or critical patients in therapy protocol group comparing to controls.
Statistical analysis
The PSS software Version (SAS Institute, Cary, North Carolina) was used to perform the statistical analyses, two-sided, with p < 0.05 being considered statistically significant. Continuous variables were presented with mean ± standard deviation, and compared with independent t-test. Count (percentage) was used to summarize the categorical variables comparing with Chi-square tests.
Results
Patients’ clinical characteristics
About 127 COVID-19 patients were enrolled; their average age was 46.7 years, and they ranged in age from 23 to 87 years. Of these patients, 37% were men and 63% were women. The controls age and gender were matched, their mean age (50.3.2±9.5 year) with 48% male and 52% females (P > 0.05) (Figure 2) (Table 2). The status of COVID-19 patients through January to August 2020. The percentage of patients under the therapies protocol were shown in Figure 3. There were had high recovery rate with 75.6% and 15.7% of patients were deceased (Figure 4).
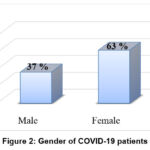 |
Figure 2: Gender of COVID-19 patients |
Table 2: Basic characteristics of the patients
|
Variables |
Remdesivir group (N = 78) |
Actemra® group (N = 9 ) |
Favipiravir group (N = 40) |
Control group (N = 150) |
|
Gender, n (%) |
||||
|
Male |
34 (44%) |
2 (22%) |
11 (27%) |
88 (59%) |
|
Female |
44 (56%) |
7 (78%) |
29 (73%) |
62 (41%) |
|
Age (years), n (%) |
||||
|
< 65 |
54 (69%) |
5 (56%) |
30 (75%) |
98 (65%) |
|
≥ 65 |
24 (31%) |
4 (44%) |
10 (25%) |
52 (35%) |
|
Clinical Classification, n (%) |
||||
|
Mild-to-Moderate |
47 (60%) |
6 (67%) |
33 (82%) |
150 (100%) |
|
Sever |
17 (22%) |
0 |
4 (10%) |
0 |
|
Critical |
14 (18%) |
3 (33%) |
3 (8%) |
0 |
 |
Figure 3: Patients under the therapy protocols |
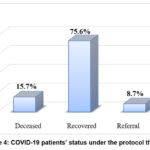 |
Figure 4: COVID-19 patients’ status under the protocol therapy |
Recovery Time
The recovery time was significantly reduced in the Remdesivir therapy (D1) comparing to controls, the mean time in group D1 was 6.23±2.1days, and the recovery mean time in control group, 3.22±2.1days (P<0.05). Hence, Remdesivir drug lowered healing mean time to 6 days in mild-to-moderate (Table 3).
Table 3: Time of recovery of COVID-19 patients(Days) in protocol therapy.
|
Variables |
Patients subgroup |
Remdesivir |
Actemra® |
Favipiravir |
Control |
|
Total |
N = 127 |
N = 78 |
N = 9 |
N = 40 |
N=150 |
|
Time of recovery (day) |
Total |
8.52±5.2 |
5.33±2.2 |
16.9±5.4 |
3.22±2.1 |
|
Mild-to-Moderate |
6.23±2.1 |
0 |
12.44±6.6 |
5.24±3.2 |
|
|
Sever |
17.25±8.2 |
3.24±3.4 |
21.20±8.7 |
0 |
|
|
Critical |
15.75±3.4 |
0 |
0 |
0 |
Mortality rate
The mortality rate was 14/64 patients (22%) in critical COVID-19 patients in D1 group, 3 out of 37 patients (8%) in D2 group, and 3 out of 9 patients (33%) in group D3 (P=0.052). The infected patients taken Remdesivir drug were reduced regarding the death rate especially in COVID-19 critical cases, which in Iraq at the time of performing this observational study was might reach higher than above 50% according to on world data.
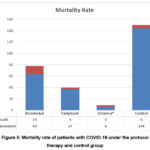 |
Figure 5: Mortality rate of patients with COVID-19 under the protocol therapy and control group |
Discussion
SARS-CoV2 is novel virus which is require emergence of effective treatment methods, the vaccine was not an option during the first wave of the epidemic. There was an urgently need to a new antiviral agent against SARS-CoV-2 to be applied. In this observational study, the aim was to screen the antiviral therapy protocol since January to end of August 2020 in a single health center (Tables 1 and 2). The COVID-19 treatment current recommendations as in Table 3.
Remdesivir has a high potential in many clinical trials to treat moderate to severe case, lowering required time to improve, and reducing mortality20. A placebo-controlled experiment was done by Beigel et al. (2020) on 1062 individuals, 541 of whom were given Remdesivir and 521 received a placebo. The median recovery duration was 10 days (95% confidence interval [CI], 9 to 11 days) for Remdesivir recipients compared to 15 days (95% CI, 13 to 18) for placebo recipients. By day 15, the mortality rate was 6.7% in the Remdesivir group and 11.9% in the placebo group. The study found that Remdesivir reduced the amount of time adults hospitalized with COVID-19 needed to recover more quickly than a placebo21.
Spinner et al. (2020) conducted a randomized, open-label trial of hospitalized patients in which Remdesivir-treated patients were compared with standard care included 596 patients (including 584 patients with moderate COVID-19), and the trial results showed that patients randomized to a 5-day course of Remdesivir had a statistically significant difference in clinical status compared with those receiving standard care.22. The aforementioned clinical trials come in close contact with what this observational study try to highlighted in this single center (Al-Forat General Hospital) which is worked in line with international protocols (COVID-19 Treatment Guidelines Panel, 2021)23.
It is important to note that this study concurs with a local study carried out by Darweesh et al. (2021) in the Kirkuk province, which highlighted the effectiveness of five different therapeutic protocols adopted by the healthcare system in Iraq in reference to WHO guidelines for management of COVID-19 infection and its relationship to mortality rate. This information may be useful for doctors and healthcare providers in choosing the most appropriate therapeutic regimens24.
However, this study disagrees with Goldman et al. (2020) study, which performed an open-label trial among 397 patients underwent randomization compared to standard care to evaluate the efficacy and safety of treatment with Remdesivir for 5 or 10 days in patients with severe COVID-19 patients. This study came to the conclusion that severe COVID-19 patients did not need the mechanical ventilation and that there were no significant differences for the both course duration of Remdesivir25.
A study by Chen and colleagues (2021), in China carried out open-label trail in multi-center to evaluate 2 therapy protocols for treating COVID-19 confirmed patients (Favipiravir Versus Arbidol), included 240 patients. Those under Favipiravir revealed tendency toward improvement in a week period, especially moderate case (71%) versus (56. %) respectively, as well as an earlier lowered in temperature and cough clearance26. Another open-labeled nonrandomized study by (Cai et al., 2020) from China compared the effect of Favipiravir Versus lopinavir/ritonavir among mild-to-moderate COVID-19 cases. The study included 56 patients in which 35 patients were treated by Favipiravir. The authors concluded to safety of Favipiravir administration with no side effects. Favipiravir was used to treat 68% of patients’ fevers, which resolved in 3 days as opposed to 6 days in the control group (standard of care).27.
The systematic and meta-analysis review conducted by Manabe et al. in 2021 concluded that Favipiravir had a great promise for treating COVID-19 patients. Favipiravir enhanced viral clearance in patients with mild-to-moderate COVID-19 after 7 days of treatment, which is consistent with the results of this observational trial28,29. According to Brahmantya et al. (2021), oseltamivir or favipiravir, which is regarded as administering an antibiotic and has become routine therapy in Indonesia, is the antiviral that is advised in that country. Oseltamivir or favipiravir are still prescribed according to the Indonesian COVID-19 management guideline, despite the fact that the WHO does not advise doing so30.
A meta-analysis of observational studies investigating the effectiveness of tocilizumab on COVID-19 patient mortality was carried out by Malgie et al. in 2021. Ten studies including Tocilizumab therapy were included in the analysis, totaling 1358 patients. The findings showed that Tocilizumab group mortality was lower than control group mortality. Patients who took tocilizumab had a mortality rate that was (12%) lower than that of controls31. Ten randomized controlled trials examining Tocilizumab’s effectiveness in COVID-19 patients were included in a review by Arthur et al. (2021). Standard care was provide to the control group. The primary findings were recorded mortality in the (28 to 30 day). A nearly 6500 were enrolled, around 3400 patients (52%) were treated by Tocilizumab. The therapy group was declined mortality (24 % versus 29%)32, meanwhile; Rosas et al (2021) study findings was not conclusive, mortality at day 28 was (20 %) under the Tocilizumab therapy and (19%) under the placebo33. Due to the few numbers of patients included in the current observational study who had been given Tocilizumab, we can roll out the efficacy effect of Tocilizumab.
Study limitations
This study has a number of limitations, including the observational design, which may not account for all risk factors other than age, gender, and clinical classification, such as co-existing chronic conditions, which frequently necessitate the referral of patients to hospitals with a higher level of expertise. Second, it is exceedingly challenging to undertake a thorough study in the midst of such a public health catastrophe. Third, given the length of the patient’s hospital stay, the follow-up period was quite brief. Four, access to such information is closely controlled by higher authorities and was at times prohibited in the initial few months of the pandemic. The data were gathered based on hospital registry data.
Conclusions
Health care providers are facing tremendous efforts in supplying pharmaceutical care. The symptomatology of COVID-19 are varied among patients, the therapy required to be designed towards certain symptoms as well as towards the progression of the infection among patients. Remdesivir and Favipiravir therapy tend to have a promising efficacy in reducing the mortality rate and time of recovery especially among mild-to-moderate patients.
Acknowledgment
The author would like to thank all the medical staff in Al-Forat General Hospital, Baghdad for their cooperation, thanks are extended to all patients who agree to share their affliction through that difficult period.
Conflict of Interests
The authors claim that there are no conflicting interests.
Funding Sources
There is no funding sources
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020; 20(5):533–4.
CrossRef - Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ 2021; 375, no 2697. https://doi.org/10.1136/ bmj.n2697.
CrossRef - Drożdżal S, Rosik J, Lechowicz K, Machaj F, Szostak B, , et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resistance Updates. 2021; 59: 100794. https://doi:10.1016/j.drup.2021.100794
CrossRef - Singh, AK, Singh, A, and Singh R, Misra, A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diab. Metab. Syndr. Clin. Res. Rev. 2020; 14 (4), 641–48. https://doi.org/10.1016/j.dsx.2020.05.018
CrossRef - Angamo MT, Mohammed MA, and Peterson GM. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 2021; 1–15. https://doi.org/10.1007/s15010-021-01671-0.
CrossRef - Goldhill DH, Te Velthuis AJW, Fletcher RA, Langat, et al. The Mechanism of Resistance to Favipiravir in Influenza. Proc. Natl. Acad. Sci. USA. 2018; 115 (45): 11613–18.
CrossRef - Patil SM, Chandana Kumari VB, Shirahatti PS, Sujay S, Tejaswini M, et al. Pharmacotherapy of COVID-19: A Perspective of Pathogenicity and Life Cycle. Biomed and Pharam J. 2020; 13(3): 1579–94. https://dx.doi.org/10.13005/bpj/2033
CrossRef - EloyP, Solas C, Touret F, Mentré F, Malvy D, de Lamballerie X, et al. Dose rationale for Favipiravir use in patients infected with SARS-CoV-2. Clin Pharmacol Ther. 2021; 108(2):188. https://doi.org/10.1002/cpt.1877.
CrossRef - Pshenichnaya NY, Bulgakova VA, Lvov NI, et al. Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR). Ter Arkh. 2019; 91:56–63.
CrossRef - Shi L, Xiong H, He J, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007; 152:1447–55.
CrossRef - Sebba A. (2008). Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health. Syst. Pharm. (2008); 65(15): 1413–18. https://doi.org/10.2146/ajhp070449
CrossRef - TsatsakisA, Calina D, Falzone L, Petrakis D, Mitrut R, et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol. 2020; 146: 111769. https://doi:10.1016/j.fct.2020.111769
CrossRef - Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020; 11(1):1–10. http://doi:10.1038/s41467-020-19741-6
CrossRef - Jin JM, Bai P, He W, Wu F, Liu XF,et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020; 8:152. http://doi:10.3389/fpubh.2020.00152
CrossRef - Al-ZidanRN. Potential drug-drug and drug-disease interactions of selected experimental therapies used in treating COVID-19 patients. J Drug Deliv Ther. 2020; 10(6):219–30.
CrossRef - Gudadappanavar AM and Benni J. An evidence-based systematic review on emerging therapeutic and preventive strategies to treat novel coronavirus (SARS-CoV-2) during an outbreak scenario. J Basic Clin Physiol Pharmacol. 2020; 31(6).
CrossRef - Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020 Jun 11; 382:2327e2336.
CrossRef - DoiY, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. A prospective, randomized, open-label trial of early versus late favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/AAC.01897-20.
CrossRef - Rattanaumpawan P, Jirajariyavej S, Lerdlamyong K, Palavutitotai N, Saiyarin J. Real-world Experience with Favipiravir for Treatment of COVID-19 in Thailand: Results from a Multi-center Observational Study. medRxiv. 2020.06.24.20133249. https://doi.org/10.1101/2020.06.24.20133249.
CrossRef - Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 – preliminary report. N Engl J Med. 2020. https://doi.org/10.1056/nejmc2022236
CrossRef - Beigel, JH, Tomashek, KM, Dodd, LE, et al (2020). Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020; 383 (19), 1813–1826. https://doi.org/10.1056/NEJMoa2007764.
CrossRef - Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324 (11): 1048–57. https://doi.org/10.1001/jama.2020.16349.
CrossRef - COVID-19 Treatment Guidelines Panel, 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www. Covid19treatmentguidelines.Nih.Gov/. Accessed July 8, 2021.
- Darweesh O, Abdulrazzaq GM, Al-Zidan RN, Bebane P, Merkhan M, et al (2021). Evaluation of the Pharmacologic Treatment of COVID-19 Pandemic in Iraq. Curr Pharmacol Rep. 2021; 7: 171–178 https://doi.org/10.1007/s40495-021-00262-9
CrossRef - Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020; 383 (19): 1827–1837. https://doi.org/10.1056/NEJMoa2015301.
CrossRef - ChenC, Zhang Y, Huang J, Yin P, Cheng Z, et al. Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial. Front Pharmacol. 2021; 12:683296. https://doi:10.3389/fphar.2021.683296.
CrossRef - Cai Q, Yang M, Liu D, Chen J, Shu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020; 6(10):1192–98. https://doi:10.1016/j.eng.2020.03.007.
CrossRef - Manabe T, Kambayashi D, Akatsu H, Kudo K (2021). Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021; 21(1):489.
https://doi:10.1186/s12879-021-06164-x
CrossRef - AgrawalU, Raju R, Udwadia ZF. Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020; 76(4):370–76. http://doi:10.1016/j.mjafi.2020.08.004.
CrossRef - Brahmantya IBY, PurnamasidhiCAW and Sumardika IW. COVID-19 Pharmacological Treatment at the Udayana University Hospital in April-May 2020. Biomed and Pharam J. 2020; 14(2): 971–77. https://dx.doi.org/10.13005/bpj/2033
CrossRef - Malgie J, Schoones JW, Pijls BG. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin. Infect. Dis. 2021; 72 (11), e742–e749. https://doi.org/10.1093/cid/ciaa1445
CrossRef - Arthur T, Snow C, Saleem N, Ambler G, Nastouli E and Singer M. Tocilizumab in COVID ‑ 19: a meta‑analysis, trial sequential analysis, and meta‑regression of randomized ‑ controlled trials. Intensive Care Med. 2021. https://doi.org/10.1007/s00134-021-06416-z
CrossRef - Rosas IO, Br¨au N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021; 384 (16), 1503–16. https://doi.org/10.1056/NEJMoa2028700
CrossRef








