Widjiati Widjiati1 , Suryo Kuntjorodjakti1
, Suryo Kuntjorodjakti1 , Aditya Tri Ananda2
, Aditya Tri Ananda2 , Mey Vanda Pusparina Sajida2
, Mey Vanda Pusparina Sajida2 , Alivia Fairuz Ilmi2
, Alivia Fairuz Ilmi2 , Meisa Zalfa Adisti3
, Meisa Zalfa Adisti3 , Dean Chou4
, Dean Chou4 and Epy Muhammad Luqman1*
and Epy Muhammad Luqman1*
1Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya 60115, Jawa Timur, Indonesia,
2Postgraduate Reproductive Biology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya 60115, Jawa Timur, Indonesia
3Graduate Student Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya 60115, Jawa Timur, Indonesia,
4Department of Biomedical Engineering National Cheng Kung University, No. 1, Dasyue Rd, East District, Tainan City, Taiwan
Corresponding Author E-mail: epy-m-l@fkh.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2721
Abstract
Livestock that has stress releases glucocorticoids in response to it, and it causes inhibition of the hypothalamus-pituitary-gonadal axis (HPG) signaling pathway so that it reduces reproductive efficiency. Forest honey can reduce corticosteroid levels as a stress response from physical stress induction which is expected to increase reproductive efficiency including folliculogenesis and the formation of the corpus luteum. This study aims to determine the effect of forest honey on rats (Rattus novergicus) exposed to physical stress on corticosteroid levels, folliculogenesis, and the number of corpus luteum. This study is an experimental laboratory one using 32 rats which were divided into 4 treatment groups; control positive (C) treated with physical stress, treatment 1 (T1) treated with physical stress + honey 2 g/rat/day PO, treatment 2 (T2) treated with physical stress + honey 4 g/rat/day PO and treatment 3 (T3) treated with physical stress + honey 6 g/rat/day PO. All treatments were carried out for 14 days. The results showed that T1 had the lowest corticosteroid level compared to all treatment groups and the corticosteroid level of this group was significantly different (p <0.05) compared to that of C and T3. The folliculogenesis profile showed that the number of primary secondary, tertiary, and Graafian follicles of group T1 was significantly different (p<0.05) compared to that of C, T2, and T3. In terms of the number of corpus luteum, it showed that T1 had the highest number of corpus luteum, and the number of corpus luteum in this group was significantly different (p<0.05) from that of C, T2, and T3. It can be concluded that the administration of forest honey at a dose of 2g/rat/day could reduce corticosteroid levels, improve the folliculogenesis profile, and increase the number of corpus luteum in rats exposed to physical stress. The use of forest honey could reduce corticosteroid levels as a stress response from physical stress induction which was expected to increase reproductive efficiency.
Keywords
Corticosteroid; Folliculogenesis; Health care; Honey; Physical Stress
Download this article as:| Copy the following to cite this article: Widjiati W, Kuntjorodjakti S, Ananda A. T, Sajida M. V, P, Ilmi A. F, Adisti M. Z, Chou D, Luqman E. M. The Effect of Administering Forest Honey to Rats Exposed to Physical Stress on Corticosteroid Levels, Folliculogenesis and the Number of Corpus Luteum. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Widjiati W, Kuntjorodjakti S, Ananda A. T, Sajida M. V, P, Ilmi A. F, Adisti M. Z, Chou D, Luqman E. M. The Effect of Administering Forest Honey to Rats Exposed to Physical Stress on Corticosteroid Levels, Folliculogenesis and the Number of Corpus Luteum. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3YNqFmI |
Introduction
Livestock stress can cause several physical health disorders and various mental and psychosomatic disorders.1 Sources of stress that commonly occur in livestock originate from the environment and also maintenance management such as temperature, weather and climate change, light intensity, feed factors and over-exercise of livestock. Stress resulted from over-exercise leads to disruption of the homeostasis of the endocrine system in livestock and a decrease in livestock productivity.2 Due to the changes in living environment, livestock is more likely to suffer from poor psychophysiological health. Repeated and prolonged stress induces hypothalamic-pituitary-adrenal (HPA) dysregulation, which disrupts homeostasis.3 Activation of the HPA axis results in the secretion of various stress hormones including glucocorticoids, corticotropin-releasing factor (CRF), and cortisol.4 Furthermore, excessive HPA activity is reportedly associated with inadequate activation of the hypothalamus-pituitary-ovarian axis, which controls the growth and development of ovarian follicles and oocytes.5
Livestock that has stress releases glucocorticoids in response to it.2,3 Glucocorticoids during stress are synthesized in the adrenal cortex with stimulation from adrenocorticotropic hormone (ACTH) to stimulate gluconeogenesis during the fight-or-flight response. The body responds to short-term and long-term stress in different ways which refer to a pattern known as the general adaptation syndrome (GAS). Early stage of GAS is called the alarm reaction. This is a short-term stress, the fight-or-flight response, mediated by epinephrine and norepinephrine hormones from the adrenal medulla. They function to prepare the body for extreme physical exertion. Once this stress is relieved, the body quickly returns to normal.6 However, excessive secretion of corticosteroids has a negative effect on the reproductive system because of the allocation of energy to other organ system.7
This suppression mechanism was explicitly described by Mahabadi8 that activation of glucocorticoid receptors on gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus induces apoptosis of neuronal cells, causing hypogonadism due to loss of pulsatility of GnRH. GnRH neuronal cell death also affects the synthesis of dehydroepiandrosterone (DHEA) which occurs in the mitochondria of the reticular zone of the adrenal cortex which functions to synthesize estrogen due to the loss of gonadotropin stimulus.9 The disruption on GnRH release causes inhibition of the hypothalamus-pituitary-gonadal axis (HPG) signaling pathway and reduces reproductive efficiency in livestock.5,10
The use of natural-based ingredients is a trend that starts to emerge globally in the current globalization era, both from the points of view for humans and livestock.11,12 Natural ingredients that positively affect the reproductive system are forest honey.13 Research conducted by Luqman14 showed that the antioxidant content of forest honey also has a high range of phenolic and flavonoid compounds due to its multi-flora nature honey and can reduce cortisol levels in response to physical stress.15
The use of forest honey as a natural ingredient that can reduce corticosteroid level as a stress response from physical stress induction is expected to increase reproductive efficiency including folliculogenesis and the formation of the corpus luteum as parameters in ovulation. Based on this background, the study was conducted to determine the effect of administering forest honey to rats (Rattus novergicus) exposed to physical stress on corticosteroid levels, folliculogenesis and the number of corpus luteum.
Material and Methods
Ethical approval
This research is an experimental animal study. This research has been declared ethically by the Research Ethics Commission of the Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia with the ethical eligibility number: No: 1.KEH.041.04.2022.
This research is an experimental laboratory study using a completely randomized design (CRD) at the Embryology Laboratory and Pathology Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga. This study used 32 rats (Rattus novergicus) which were divided into 4 treatment groups, and each group consisted of 8 rats. Before the treatment, the experimental animals were first acclimatized by resting them and were given enough food and drink for 7 days.
Materials
The tools and materials used in this study were ZD® forest honey, aquadest, oral gavage, Eppendorf tube, centrifuge, Olympus® microscope, Neutraled Buffered Formaldehyde 10% (NBF 10%), tissue pot, Onemed® 5cc syringe, scalpel, surgical scissors, anatomical tweezers, ketamine (Ket-A-100®), and xylazine (Xyla®). The Feed used for mice was Hi-Pro-Vit Medicated 593®.
Methods
There were 4 treatments groups in this study, namely positive control (C) treated with physical stress, treatment 1 group (T1) treated with physical stress + honey 2 g/ rat /day PO, treatment 2 group (T2) treated with physical stress + honey 4 g/ rat /day PO and treatment 3 group (T3) treated with physical stress + honey 6 g/ rat /day PO. All treatments were carried out for 14 days.
Physical Stress Treatments in Rats
Physical stress using rat model was based on a modified by placing the rats in a specific box measuring 50x30x25 cms and two third of the box was filled with water at the temperature of 24-28oC. Next, the rats were forced to swim for 5 minutes once a day and then they were placed in a dark box. It was conducted daily at 09.00 AM. In T1, T2 and T3 groups after swimming treatment, forest honey with doses of 2 g, 4 g, and 6 g was given orally (PO) to each rat daily for 14 days. Forest honey was dissolved in distilled water and was given through the rat’s mouth using a special nasogastric sonde to reach the gastrointestinal organ.
Sample Collection and Histopathology
After 14 days of treatment, all rats were euthanized using anesthetic Ket-A-100® and Xyla®. After the rats were anesthetized, the incision was made using a scalpel in the ventral midline area and then it was also made using scissors on the thorax and abdomen. Blood was drawn using a 5 cc syringe intra-cardiac. The collected blood was allowed to freeze to take off the serum using a centrifuge at a speed of 2500rpm to examine corticosteroid level by ELISA. Ovarian organs were taken and then they were placed in a sample pot that was filled with 10% NBF. The organ samples were then taken to the Pathology Laboratory Faculty of Veterinary Medicine, Universitas Airlangga to make histological preparations.
Corticosteroid Level
Examination of Corticosteroid levels was carried out at the Laboratory of Physiology, Faculty of Medicine, Universitas Brawijaya using the Colorimetric ELISA method with kit. Corticosteroid levels are expressed in ng/ml
Folliculogenesis Profile
Examination of the folliculogenesis profile was carried out on histological preparation of ovarian tissue that was previously prepared. The examination was carried out using an Olympus® microscope with 400x magnification and then the number of primary follicles, secondary follicles, tertiary follicles, de Graff follicles, and corpus luteum were counted. The calculation was carried out in 5 fields of view and then in the average values of the results.
Statistical Analysis
Statistical Analysis was conducted using an application named IBM SPSS 23 using One-Way ANOVA with post hoc Duncan to find out the differences between groups on the variables of corticosteroid levels, number of primary follicles, secondary follicles, tertiary follicles, de Graff follicles and corpus luteum to find out differences between groups of each variable.
Results and Discussion
The results of corticosteroid level examination using a colorimetric ELISA are shown in table 1. The results show that group C that was treated with the physical stress which is swimming has a corticosteroid level of 149.78±32.67 ng/ml. The treatment groups (T1,T2 dan T3) which also obtained physical stress treatment and then were treated with forest honey at doses of 2 g, 4 g, dan 6 g/ rat /day have the following results : T1 shows the lowest corticosteroid level of all treatment group which is at 57.03 ± 3.21 ng/ml and the corticosteroid level of this group is significantly different (p<0.05) compared to that of group C. The corticosteroid level of T3 is significantly different (p<0.05) compared to that of group C, which is lower, however, it is still higher if compared to that of T1 (p<0.05).
Overall, the results of the folliculogenesis profile examination presented in table 2 show that the number of primary follicles, secondary follicles, tertiary follicles and de Graff follicles of T1 is significantly different (p<0.05) when compared to that of the group C and the other treatment groups (T2 dan T3). The lowest folliculogenesis profile is obtained by T3 and in terms of several variables, such as the number of primary follicles, tertiary follicles, and de Graff follicles, it does not have a significant difference with group C (p>0.05), while for secondary follicles it is significantly different from group C (p<0.05).
The calculation of the corpus luteum in Table 3 shows that the T1 has the highest corpus luteum, which is 9.16±1.72 and the number of corpus luteum in this group is significantly different (p<0.05) from that of group C. The number of corpus luteum of group C , T2, and T3 is not significantly different (p>0.05) compared to T3 that has the lowest number of corpus luteum.
Table 1: Corticosteroid levels (ng/ml) of each treatment group using ELISA examination (Mean ± SD).
|
Treatment Groups |
Corticosteroid Levels (ng/ml) |
|
C |
149.78c ± 32.67 |
|
T1 |
57.03a ± 3.21 |
|
T2 |
72.66ab ± 4.87 |
|
T3 |
87.71b ± 14.56 |
Note: Different superscripts show significant difference (p<0.05).
Table 2: Number of Primary follicles, secondary follicles, tertiary follicles, and de Graff follicles of each treatment group (Mean ± SD).
|
Treatment Groups |
Primary Follicles |
Secondary Follicles |
Tertiary Follicle |
De Graff Follicles |
|
C |
1.67 ± 0.81a |
1.67 ± 0.81b |
1.83 ± 0.75a |
0.67 ± 0.13a |
|
T1 |
4.67 ± 1.63b |
3.00 ± 1.09c |
3.83 ± 1.72b |
4.33 ± 1.75b |
|
T2 |
1.00 ± 0.63a |
0.67 ± 0.12ab |
1.00 ± 0.63a |
0 a ± 0 |
|
T3 |
0.50 ± 0.2a |
0.17 ± 0.04a |
0.50 ± 0.08a |
0.17 ± 0.04a |
Note: Different superscripts show significant difference (p<0.05)
Table 3: Number of Corpus Luteum of each treatment group (Mean ± SD).
|
Treatment Groups |
Corpus Luteum |
|
C |
6.50 ± 1.05a |
|
T1 |
9.16 ± 1.72b |
|
T2 |
5.00 ± 1.09a |
|
T3 |
5.83 ± 1.16 a |
Note: Different superscripts show significant difference (p<0.05)
In this study, there was a significant increase in corticosteroid levels in group C that was treated with physical stress in the form of swimming and without supplementing forest honey. Physical stress induced by swimming is Variability in Behavioral Phenotypes after Forced Swimming-Induced Stress is associated with acute stress and it increases the expression of several stress proteins such as glucocorticoid receptor(GR), Nurr1, and IL-1β. The increase in GR expression is caused by an increase in plasma corticosteroid levels in response to physical stress.16
Increased plasma levels of corticosteroids have a negative effect on the reproductive system as reported by Ackerman17 that the increase of cortisol and corticosteroid levels due to physical stress inhibits the hypothalamic-pituitary-gonadal (HPG) signaling pathway so that it becomes a factor that inhibits LH secretion. On the other hand, Mahabadi8 explained that high plasma corticosteroid levels activate GR on GnRH neurons in the hypothalamus and induce apoptosis of the neurons, causing hypogonadism due to loss of pulsatility of GnRH. The results of this study are in line with the study in which in group C, there was a significant decrease in the number of primary, secondary, tertiary, de Graff follicles and corpus luteum when compared to that of T1. Furthermore, GnRH is an important hormone that stimulates FSH and LH as the main factors for folliculogenesis and ovulation. Suppressing these hormones by corticosteroids disrupts these processes.18
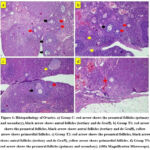 |
Figure 1: Histopathology of Ovaries. a) Group C: red arrow shows the preantral follicles (primary and secondary), black arrow shows antral follicles (tertiary and de Graff). b) Group T1: red arrow shows the preantral follicles, |
Sex hormone-binding globulin (SHBG) and corticosteroid-binding globulin (CBG) mRNAs in corpus luteum have a role to stimulate GnRH in order to release FSH and LH. SHBG binds and transports testosterone, estradiol, and other sex steroids in the plasma, reduces their metabolic clearance rate and affects their bioavailability. SHBG exhibits high affinity for testosterone and a low-capacity binding protein (estradiol), and other low-affinity but higher-capacity binding proteins including human serum albumin (HSA), corticosteroid-binding globulin (CBG) and Orosomucoid or α1 acid glycoprotein. The free active testosterone concentrations in plasma are highly influenced by SHBG concentrations because only 1–2% of testosterone in the circulation is free (unbound) and active; 65% is bound to SHBG and the rest is bound to albumin. So, the levels of SHBG have a correlation with serum steroid hormone levels.19,20
The mechanism of physical stress disorder in the reproductive system is also reported to be caused by low energy availability. This causes a decrease in insulin levels and insulin-like growth factor1 (IGF-1).21 Impaired IGF-1 secretion, which is an essential factor for folliculogenesis process and oocyte maturation, causes apoptosis of granulosa cells and failure of further follicular development into de Graff follicles.22 This is in line with the result of this study, in which in group C de Graff follicles that are produced are lower compared to those of T1.
Increased levels of corticosteroids consistently increase apoptosis of neuron cells and are neurotoxic. Several neurotoxin mechanisms in neuronal cells include reducing the ability of neurons to use Ca2+, increasing Ca2+ intracellular levels uncontrollably, changing mitochondria and endoplasmic reticulum function, and decreasing Bcl-2 expression due to translocations in the mitochondria of neuron cells.9 Several studies show that the potential protective effects of forest honey against organic compounds that induce neurotoxicity in the reproductive system.
Honey is known to be rich in enzymatic and non-enzymatic antioxidants, including catalase, ascorbic acid, flavonoids, alkaloids, glucose oxidase, phenolics acid, carotenoid derivatives, Maillard reaction products, amino acids and proteins. A unique flavonoid, known as pinocembrin, is present in propolis and honey; other types of flavonoids, including quercetin, chrysin, galangin, luteolin and kaempferol, are also found in honey. Previous study found that quercetin and kaempferol contained in honey exerted the free radical scavenging activity. In addition, estrogenic properties of quercetin and kaempferol might compete with EDC to bind to estrogen receptors.23,24
Ruslee24 conducted a research that showed that Tualang honey had protective effects in reducing the ovarian toxicity induced by Cd. The protective effects observed were in the form of a reduction in morphological abnormalities in the ovary, restoration of the gonadotropin hormones, reduction in the lipid peroxidation level and increase in the levels of enzymatic antioxidants. Many studies show that flavonoids and phenolic acids are responsible for the antioxidant activity of honey as it has the ability to scavenge free radical formation.25,26,27 Phenolic compounds work against oxidative stress as they have the properties of reducing agents (they have hydrogen- or electron-donating capacity) with chemical structure of hydroxyl groups.28 The more hydroxyl groups there are in phenolic compounds, the more efficiently they can react as antioxidant agents due to their ability to donate hydrogen atoms to free radicals then free radical formation will be reduced. Also, vitamin E and C contained in honey also have a protective effect against oxidative stress. Vitamin E helps to prevent lipid peroxidation reactions by inhibiting the production of lipid radicals in cellular membranes, whereas vitamin C is a water-soluble antioxidant that can interact directly against free radicals in cytosol and extracellular fluids to reduce oxidative damage.29
The decrease in Bcl-2 levels causes the ability of buffer calcium in neuronal cells to decrease, therefore it increases ROS production, which will cause neuronal cell death.30 On the other hand, GnRH secretion requires Ca2+ and ROS at normal levels, but the overload of Ca2+ and ROS production has an effect on GnRH secretion (hypogonadism) and interferes with normal reproductive function.8
Forest honey has a high antioxidant content and consists of flavonoids, phenolic components, an enzymatic antioxidant such as catalase and glucose oxidase, carotenoids, several amino acids and vitamin C.14 The potential of antioxidants contained in forest honey was measured by Apak31 which used DPPH method to measure scavenging ability and obtained a relatively high IC50 result of 5453.57 ppm. Usman32 also explained in his research that administering honey was able to reduce cortisol levels in plasma and to increase glutathione levels which counteracted the forced swimming stress effects. Increased levels of glutathione can exert a protective effect on neurons against oxidative stress caused by overloaded ROS and maintain the secretory function of GnRH. Azman33 said that forest honey (200 mg/kg/body weight) that was administered for 28 days successfully counteracted the forced swimming stress effects in which the honey treated rats exhibited significant decrease in depressive-like behaviour and levels of ACTH, corticosterone, and oxidative stress markers, with significant increase in antioxidant enzymes activities and total antioxidant status. The honey mediated antidepressant-like effects in stressed rats, possibly acting via restoration of hypothalamic-pituitary-adrenal axis through its antioxidant properties. Similarly, our current study demonstrated that forest honey was able to reverse the increase of corticosterone levels in the stressed rats. These findings suggested that forest honey reduced the adverse effects of stress and may be beneficial for the nervous system and vasculature, and protect the brain and body from stress-induced damage. Azman33 said that honey may have modulated corticosterone and ACTH levels either by suppressing HPA mobilization in response to stress or by facilitating elevated plasma corticosterone and ACTH levels back to baseline following the termination of stress.
Another mechanism of honey to prevent neuronal cell damage due to oxidative stress is by decreasing the expression of pro-inflammatory cytokines, such as TNF-α, NF-kB, and MAPK and increasing anti-inflammatory cytokines such as IL-13.34 Forest honey also provides energy and stimulates the secretion and stimulation of IGF-1, which is important for the folliculogenesis process and oocyte maturation.35 The mechanism was proven in this study in which group T1 with a dose of honey 2 g/ rat /day was the most effective for providing protection to neuron cells as evidenced by the lowest corticosteroid levels, a good folliculogenesis profile and high number of corpus luteum that indicated good ovulation process (enough LH).
Although limited, the clinical evidence described above suggests that honey has great potential in the management of asthma resistant of corticosteroid. The anti-asthmatic effect of raw Gelam honey (Apis mellifera) which is originated from Malaysia showed that oral administration of Gelam honey (40% and 80% (v/v)) exhibited a significant dose-dependent reduction in the airway epithelium thickening and infiltration of inflammatory cells (lymphocytes, neutrophils, and eosinophils) at peribronchiolar region and in the BALF of OVA-induced BALB/c mice.36 Another study investigated the effectiveness of aerosolized Tualang honey (25 and 50% (v/v)) as both rescue and preventative agents in OVA-induced rabbits. Regardless of the dosage and treatment method (pretreatment or co-treatment), aerosolized Tualang honey was able to significantly inhibit goblet cell hyperplasia, mucus overproduction, and infiltration of inflammatory cells (eosinophils, mononuclear, neutrophils, and macrophage) in the peribronchial region and BALF in OVA-induced rabbits.37
In this study, group T1 which received the physical stress treatment of swimming and then was treated with forest honey at dose of 2 g/rat/day showed that the number of primary follicles, secondary follicles, tertiary follicles and de Graff follicles were significantly different (p<0.05) when compared with those of the other treatment groups and the number of the corpus luteum of this group had the highest rate at 9.16±1.72 and it was significantly different (p<0.05) from group C. This showed significantly higher number of corpus luteum and antral follicles (primary follicles, secondary follicles, tertiary follicles and de Graff follicles) compared to those of the control group. The higher number of corpus luteum indicates an increase in ovulation rate, while the antral follicle shows that the normal folliculogenesis process occurs. Forest honey was reported to improve oxidative stress status and normalize the hormonal and oestrus cycle disturbances. These hormonal and oestrus cycle corrections bring back physiological healthy folliculogenesis, which is manifested by improvement in folliculogenesis-related factors and histological findings in this study. Oxidative stress and its enzymatic markers play roles to regulate the folliculogenesis progress, oocyte development, and ovarian steroidogenesis.38,39
In rat ovaries, Cyp17a1 is localized in theca cells of large antral and preovulatory follicles. Kamal40 study recorded a similar finding in which the Cyp17a1 protein distribution was significantly higher in theca cells of the large antral and preovulatory follicles of PCOS rats compared with that of the normal control rats. Similar with that study, Kakuta in his study showed that LH hypersecretion was one of the causes of Cyp17a1 overexpression.41 Tualang honey was reported to regulate the hypothalamic–pituitary–adrenal axis in ovariectomised rats.42 In sexually mature animals, aromatase is reported to be present in the granulosa cell layer of large antral healthy follicles, preovulatory follicles, and corpus luteum.43,44 In this study, group T1 showed that the number of primary follicles, secondary follicles, tertiary follicles and de Graff follicles was higher.
Ruslee24 study found that in BPA-exposed rats, Tualang honey, when consumed orally on a daily basis, could serve as an effective natural supplement to reduce the toxic effects of BPA by restoring the level of FSH and LH hormones. This result reflected the normalization of GnRH in the brain. In BPA-exposed rats treated with Tualang honey, morphological abnormalities, such as the formation of large antral cystic-like follicles (anovulation follicles), the insufficiency of the corpus luteum and preantral follicles and the number of atretic follicles, were slightly reduced. In BPA-exposed rats, honey supplementation significantly improved ovarian and uterine morphological abnormalities, reduced lipid peroxidation and normalized ERα, ERβ and C3 expression levels and distribution. Quercetin and kaempferol are the main naturally occurring flavonols in forest honey that share structural similarities with 17β-oestradiol. Therefore, the potential oestrogenic effects of these compounds are comparable with those of other xeno-oestrogens.45
In this study, group T2 and T3 showed poor results compared to group T1. Researchers suspect that forest honey with a dose 4 g/rat/day and 6 g/rat/day has a very high viscosity. This is in accordance with the results of Bakier’s research46 that honey in liquid form has a high viscosity which is influenced by temperature and water concentration. Administering honey with those doses causes stress in experimental animals because viscosity is quite high.47 Physical stress coupled with stress due to honey administration makes the therapy with honey ineffective.
Another hypothesis to consider is the antioxidant paradox. Antioxidants become effective to prevent cell damage if used with the right dose. Forest honey that has protective effects is observed in the form of reduction in morphological abnormalities in the ovary, restoration of the gonadotropin hormones, reduction in the lipid peroxidation level and increase in the levels of enzymatic antioxidants. Forest honey contains flavonoids and phenolic acids that are responsible for antioxidant activity as it has the ability to scavenge free radical formation.27 Phenolic compounds work against oxidative stress as they have properties of reducing agents (they have hydrogen- or electron-donating capacity) with chemical structure of hydroxyl groups. The more hydroxyl groups there are in phenolic compounds, the more efficiently they can react as antioxidant agents due to their ability to donate hydrogen atoms to free radicals then free radical formation will be reduced. 28
Then, vitamin E and C contained in honey also have protective effects against oxidative stress. Vitamin E helps to prevent lipid peroxidation reactions by inhibiting the production of lipid radicals and vitamin C is a water-soluble antioxidant that reduces oxidative damage in cytosol and extracellular fluids.29,48 Excessive dose can cause reductive stress that increases ROS levels and pro-inflammatory cytokines and activates the inflammatory cascade with increased expression of NF-kB and MAPK. Group T2 and T3 which used forest honey at doses of 4 g/rat/day and 6 g/rat/day caused reductive stress and increased GnRH neuronal damage which was caused by physical stress and interfered with the process of folliculogenesis and ovulation
Conclusion
Administration of forest honey at a dose of 2 g/rat/day to rats treated with physical stress for 14 days decreases corticosteroid levels with reductive stress which increases ROS levels and pro-inflammatory cytokines and activates inflammatory cascade with increased expression of NF-kB and MAPK because forest honey contains flavonoids and phenolic acids: antioxidants that have the ability to scavenge free radical formation, increase folliculogenesis profile, and the number of corpus luteum because Cyp17a1 is localized in theca cells of large antral and preovulatory follicles as well as LH hyper-secretion and increase corpus luteum development.
The use of forest honey could reduce corticosteroid levels as a stress response from physical stress induction which was expected to increase reproductive efficiency.
Conflict of interest
All authors declare that there is no conflict of interest.
Funding
The authors received financial support for the Implementation of Internal Research Universitas Airlangga Number 978/UN3/2022 and publication of this article.
References
- Verma R, Balhara Y. P. S and Gupta C. S. Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J, 2011; 20(1): 4–10.
CrossRef - Gebregeziabhear E and Ameha N. The effect of stress on productivity of animals: a review. J Biol Agric Healthcare. 2015; 3:165-172.
CrossRef - Hotamisligil G. S, and Davis R. J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol.2016;8:a006072.
- Karin O, Raz M, Tendler A, Bar A, Kohanim YK, Milo T, and Alon U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol. Syst. Biol. 2020;16:e9510.
CrossRef - Zhai Q.-Y, Wang J.-J, Tian Y, Liu X, and Song Z. Review of psychological stress on oocyte and early embryonic development in female mice. Reprod. Biol. Endocrinol.2020;18:1–10.
CrossRef - Aljerf L, Williams M, Ajong AB, Onydinma UP, Dehmchi F, Pham VT, Bhatnagar S, and Belboukhari N. Comparative study of the biochemical response behavior of some highly toxic minerals on selenosis in rats. Rev. Chim. 2021; 72(2):9-18.
CrossRef - Whirledge S and Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva endocrinol, 2010; 35: 109-114.
- Mahabadi N, Doucet A, Lun WA and Mahabadi V. Glucocorticoid induced hypothalamic-pituitary axis alterations associated with hypogonadotropic hypogonadism. Osteol Rheumatol–Open J, 2019; 1: 30-34.
- Du J, Wang Y and Hunter R. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A; 2009; 106: 3543-3548.
CrossRef - Saple S, Agrawal M and Kawar S. Precycle estradiol in synchronization and scheduling of antagonist cycles. J Obstet Gynaecol India. 2016; 66(4): 295–299.
CrossRef - Nono F, Yulianti D. L and Krisnaningsih ATN. The effect of using herbal ingredients as a feed additive on the income over feed cost of broiler chickens. J Anim Sci, 2017; 5: 100-105.
CrossRef - Mohamad TAST, Islahudin F, Jasamai M and Jamal J. A. Preference, perception and predictors of herbal medicine use among Malay women in Malaysia. Patient preference and adherence, 2019; 13: 1829-1832.
CrossRef - Lowore J, Meaton J, and Wood A. African forest honey: an overlooked NTFP with potential to support livelihoods and forests. Environ Manage. 2018; 62(1): 15–28.
CrossRef - Luqman EM, Ananda AT, Widjiati W and Hendrawan VF. Protective effect of apis dorsata honey on chronic monosodium glutamate-induced testicular toxicity in Mus musculus mice. Turkish J Pharm Sci, 2022; 19: 246-250.
CrossRef - Moniruzzaman M, Khalil M I, Sulaiman SA and Gan SH. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement Altern. Med, 2013; 13(1): 13-43
CrossRef - Ruiz-Sánchez E, López-Ramírez AM, Ruiz-Chow Á, Calvillo M, Reséndiz-Albor AA, Anguiano B, and Rojas P. Variability in behavioral phenotypes after forced swimming-induced stress in rats is associated with expression of the glucocorticoid receptor, Nurr1, and IL-1β in the Hippocampus. Int J Mol Sci, 2021; 22(23): 12700.
CrossRef - Ackerman KE, Patel KT, Guereca G, Pierce L, Herzog DB, Misra M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin. Endocrinol, 2013; 78(1): 114-119.
CrossRef - Stamatiades GA, Carroll RS, Kaiser UB. GnRH—a key regulator of FSH. Endocrinology, 2019; 160(1): 57-67.
CrossRef - Gyawali P. Sex Hormone-Binding globulin: regulation and role as a marker of chronic disease risk. Dissertation. Adelaide Medical School Faculty of Health and Medical Sciences. 2019.
- Qu X, and Donnely R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int J Mol Sci. 2020; 21: 8191.
CrossRef - Martin B, Pearson M, Kebejian L, Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology, 2007; 148(9): 4318–4333.
CrossRef - Han Y, Wang S, Wang Y, Zeng S. IGF-1 inhibits apoptosis of porcine primary granulosa cell by targeting degradation of BimEL. Int J Mol Sci., 2019; 20(21): 5356.
CrossRef - Zaid SSM, Ruslee SS, and Mokhtar MH. Protective roles of honey in reproductive health: A Review. Molecules. 2021;26(11):3322.
CrossRef - Ruslee SS, Zaid SSM, Bakrin IH, Goh YM, and Mustapha NM. Protective effect of Tualang honey against cadmium-induced morphological abnormalities and oxidative stress in the ovary of rats. BMC Complement Med Ther. 2020; 20:160.
CrossRef - Khalil MI, Alam N, Moniruzzaman M, Sulaiman SA, and Gan SH. Phenolic acid composition and antioxidant properties of Malaysian honeys. J Food Sci. 2011;76(6):C921–8.
- Mohamed ZBH, and Alfarisi HAH. Tualang honey: composition, physiochemical properties and clinical importance. Int Res J Pharm. 2017;8(9):1–5
CrossRef - Ahmed S, Sulaiman SA, Baig AA, Ibrahim M, Liaqat S, Fatima S, Jabeen S, Shamim N, Othman NH. Honey as a potential natural antioxidant medicine: an insight into its molecular mechanisms of action. Oxidative Med Cell Longev. 2018;2018:1–19.
CrossRef - Silva FAM, Borges F, Guimarães C, Lima JLFC, Matos C, and Reis S. Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity, and physicochemical parameters. J Agric Food Chem. 2000;48(6):2122–6
- Ryan MJ, Dudash HJ, Docherty M, Geronilla KB, Baker BA, Haff GG, Cutlip RG, and Alway SE. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp Gerontol. 2010;45(11):882–95.
CrossRef - Anilkumar U, and Prehn JH. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front Cells neurosci, 2014; 8: 281.
CrossRef - Apak R, Güçlü K, Demirata B, zyürek M, Çelik SE, Bektaşoğlu KB, Berker I, Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007; 12(7):1496-1547.
CrossRef - Usman AN, Raya I, Yasmin R, Dirpan A, Arsyad A, Permatasari AE, Sumidarti A, and Umami N. Ginger honey affects cortisol, estrogen and glutathione levels; preliminary study to target preconceptional women. Gac Sanit, 2021; 35: S251-S253.
CrossRef - Azman KF, Zakaria R, Abdul Aziz CB, and Othman Z. Tualang honey exerts antidepressant-like effects and antioxidant properties in stress-exposed rats. Malaysian J App Sci. 2019; 4(1): 15-25.
- Safitri E, Purnobasuki H, Purnama MTE, and Chhetri S. Effectiveness of forest honey (Apis dorsata) as therapy for ovarian failure causing malnutrition. F1000Research. 2022; 11(512): 512.
CrossRef - Ajibola A, Olusakin J, and Oyewale AA, Growth and metabolic response of suckling rats fed with natural honey supplements. Int J Food Sci Nutr, 2016; 3(1): 199-203.
CrossRef - Shamshuddin NSS, and Mohd Zohdi R. Gelam honey attenuates ovalbumin-induced airway inflammation in a mice model of allergic asthma. J. Tradit Complement Med. 2018; 8:39–45.
CrossRef - Kamaruzaman NA, Sulaiman SA, Kaur G, and Yahaya B. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement Altern Med. 2014;14:176.
CrossRef - Agarwal A, Gupta S, and Sharma RK. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005;3:28-34.
CrossRef - Wang S, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Oxid. Med. Cell. Longev.2017; 2017:4371714.
CrossRef - Kamal DAM, Ibrahim SF, Ugusman A, Zaid SSM, and Mokhtar MH. Kelulut honey improves folliculogenesis, steroidogenic, and aromatase enzyme profiles and ovarian histomorphology in letrozole-induced polycystic ovary syndrome rats. Nutrients. 2022;14(20):4364.
CrossRef - Kakuta H, Iguchi T, and Sato T. The involvement of granulosa cells in the regulation by gonadotropins of Cyp17a1 in theca cells. Vivo. 2018;32:1387–1401.
CrossRef - Al-Rahbi B, Zakaria R, Othman Z, Hassan A, and Ahmad AH. Enhancement of BDNF concentration and restoration of the hypothalamic-pituitary-adrenal axis accompany reduced depressive-like behaviour in stressed ovariectomised rats treated with either tualang honey or estrogen. Sci. World J. 2014; 2014:310821.
CrossRef - Stocco C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids. 2008;73:473–487.
CrossRef - Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77:27–35.
CrossRef - Stanner SA, Hughes J, Kelly CNM, Buttriss J. A review of the epidemiological evidence for the antioxidant hypothesis. Public Health Nutrition. 2004;7(3):407-422.
CrossRef - Bakier S, Rheological properties of honey in a liquid and crystallized state. In: Honey analysis, Licensee IntechOpen, 2017: 115-137.
CrossRef - Stuart SA, Robinson ES. Reducing the stress of drug administration: implications for the 3Rs. Sci rep, 2015; 5(1): 1-8.
CrossRef - Dutta S, Sengupta P, Roychoudhury S, Chakravarthi S, Wang CW, Slama P. Antioxidant Paradox in Male Infertility:‘A Blind Eye’on Inflammation. Antioxidants, 2022; 11(1): 167.
CrossRef







