Ananya Kuanar1 , Bibhudutta Pattnaik1
, Bibhudutta Pattnaik1 , Guru Charan Nayak2
, Guru Charan Nayak2 , Anindiya Bose3
, Anindiya Bose3 , Somadatta Das4
, Somadatta Das4 , Pratap Keshari Pattnaik5
, Pratap Keshari Pattnaik5 , Dattatreya Kar6*
, Dattatreya Kar6*
1Center For Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India.
2Department of Botany, Samanta Chandrasekhar Autonomous College, Puri, Odisha, India.
3School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha.
4Central Research Laboratory, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India.
5Department of Microbiology, College of Basic Science and Humanities, Odisha University of Agriculture and Technology, Baramunda, Bhubaneswar, Odisha, India.
6Department of Medical Research, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha-751003, India.
Corresponding Author E-mail: drdkar2@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2717
Abstract
The oxidative compounds at a certain level positively affect the body's immune functions; however, inappropriate lifestyles and dietary habits can trigger an imbalance in the body's antioxidant defense mechanisms and the production of free radicles, can cause molecular damages which can be observed through different biomarkers. These free radicles can cause undesirable health, leading to different degenerative diseases and pathogenesis. Antioxidants are highly effective in reducing the frequent occurrence of such chronic diseases. FAV (fruits and vegetables) and food plants have been well known for its antioxidant contain. This study interprets to determine the total phenolic content which ranges from 8.6 μg (Tomato of Cuttack) to 27.8 μg (Amla of Jajpur) of gallic acid equivalents per 100 gm of extract. Flavonoid content ranges from 3.6-34.2 μg of quercetin equivalents per 100 gm of the extract, with Karela of Jajpur having the maximal (34.2 μg) content, with banana (3.6 μg) being the least of Cuttack. Antioxidant content ranges from 5.1-10.8 μg/ml. Banana of Jajpur has maximal IC50 value through DPPH based scavenging assay method, with Amla of Angul, Cuttack and Dhenkanal as the lowest capacity. The significant output of the research will convey the habitant of these localities and the selection as well as the inclusion of the finest fruits and vegetables in their day to day regular diet. The researchers may utilize the data for geographical based epidemiological studies where the intake of reported foods can be used to measure their antioxidant values, which may further be utilized to verify the impact of antioxidants and their synergistic effect on the cell. Animal based experimental studies or human based clinical trials may interpret the role of dietary phytochemical based antioxidants in preventing different chronic and degenerative diseases.
Keywords
Antioxidant; Fruits; Flavonoids; Health; Phenolic; Vegetables
Download this article as:| Copy the following to cite this article: Kuanar A, Pattnaik B, Nayak G. C, Bose A, Das S, Pattnaik P. K, Kar D. Multi-Locational Based Comparative Antioxidant Study of Some Commonly Consumed Fruits And Vegetables in a Part of Eastern India. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Kuanar A, Pattnaik B, Nayak G. C, Bose A, Das S, Pattnaik P. K, Kar D. Multi-Locational Based Comparative Antioxidant Study of Some Commonly Consumed Fruits And Vegetables in a Part of Eastern India. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/45ZF3L9 |
Introduction
The metabolic activity of cells in biological systems results in the production of highly reactive compounds known as free radicles.1,2 These oxidative compounds, at a certain level, positively affect the body’s immune functions; however, inappropriate lifestyles and dietary habits can trigger an imbalance in the body’s antioxidant defense mechanisms and the production of free radicles, which causes molecular damages observed through different biomarkers.3-6 These free radicles can make the event to cause undesirable health which leads to the pathogenesis of different degenerative diseases like atherosclerosis, neurodegenerative diseases, carcinogenesis, including Alzheimer as well as Parkinson’s diseases, aging etc. 7,8,9 Therefore adhering, the habit of healthy consumption is now a growing social concern, which can be overcome with high intake of bioactive antioxidant compounds.10-13
Consumption of fruits and vegetables is included as worldwide dietary recommendation, for disease prevention strategy because along with fiber content and their micro as well as macronutrient. Fruits and vegetables also contain compounds in the form of photochemical that stand out for their antioxidant properties.14,15,16 It has also been reported that health-improving benefits are found in various FAV (fruits and vegetables) and food plants have been well known to contain antioxidants, such as apples, bananas, carrots, cabbage, citrus fruits (lemon, lime, orange, grapes), dates, dark leafy greens, vegetables yellow and green (peppers) pomegranates and strawberries.17-23 In order to reduce the frequent occurrence of such chronic diseases, antioxidants are highly effective as they exert both synergistic and additive effects.24,25,26 Vegetables and fruits play a vital protective role against chronic diseases such as hypertension, diabetes, cancer, strokes, ocular, cerebrovascular, neurological diseases and blood-related diseases.27-31 FAV are potent to combat for primary health conditions as they contain natural compounds that have presented compelling evidence with several epidemiological studies.17,20
World’s 11% of stroke and 31% of ischemic heart disease were estimated to be responsible for low intake of FAV. The regular intake of FAV (400–500 g/day) as recommended by the joint report of FAO/WHO, may be the prevention of chronic diseases like cardiovascular diseases, stroke, high blood pressure, and other micronutrient related deficiencies.20,24 A significant risk factor is observed with intake of inadequate FAV and may cause several nutritionally based NCDs (non-communicable diseases).3,8,29-32 So the role and requirement of the antioxidants are not only to work as a nutritional supplement, but the health professionals should recommend the level of intake and their impact on health.33,34,35
The objective of the current study is to interpret and determine the total phenolic and flavonoid content along with antioxidant models of various vegetable fruit extracts that are done by amassing samples from various locations in eastern part of India. The significant output of the research will convey the habitant of this locality and the selection for inclusion of the finest fruits and vegetables in their day to day regular diet.
Material and Methods
Vegetable and fruit samples of Amla (Phyllanthus emblica), Apple (Malus domestica), Banana (Musa acuminate), Capsicum (Capsicum annum), Carrot (Daucus carota), Grapes (Vitis vinifera), Green chilli (Capsicum frutescens), Karela (Momordica charantia), Pomegranate (Punica granatum), Orange (Citrus Sinensis), Lemon (Citrus limon) and Tomato (Solanum lycopersicum) were randomly collected from local market of Angul, Cuttack, Dhenkanal and Jajpur during November 2022 were clean dried and kept in a different sterile bowl marked with the location on it. Further, the samples were refrigerated for storage purposes until they were used for analysis.
Processing of Extract
Using distilled water the vegetables and fruits were washed and cleaned. Then at room temperature, the samples were blended and dried. The dried and blended samples were mixed with methanol (1:1) with the help of a magnetic stirrer at room temperature for 30 minutes with low rpm. The mixture obtained was filtered with the dry, sterilized, clean cotton cloth and refiltered with Whatman filter paper. The final concentration was prepared at 0.5 gm/ml by dilution with the solvent of each extract.36
TFC (Total Flavonoid Content) Determination
From the stock concentration, each sample was prepared for a working concentration of 50 μg/ml separately according to sample location. In each working concentration, 2% ammonium chloride was added to 1ml of working solution. By sonication, each solution was appropriately mixed and at 434 nm the absorbance was recorded using a UV-Visible spectrophotometer.
A 20-50 μg/ml concentration of quercetin was processed to get a standard curve for flavonoid content estimation. The total flavonoids were expressed with the quercetin equivalents (μg) per 100 gram of extract.37
TPC (Total Phenolic Content) Determination
Each sample was prepared for a working concentration of 50 μg/ml from the stock concentration, and Folin Ciocalteu reagent was added to it of 1.0ml. Then, a sodium carbonate solution of 20% w/v was added to 2.0 ml. At 641 nm, absorbance was recorded by using a UV-Visible spectrophotometer. In the 50-250 μg/ml range, the concentration of gallic acid was processed to get a standard curve for the estimation of TPC. Gallic Acid equivalents (μg) per 100 grams of extract were used to express.37
Determination of Free Radicle Scavenging Activity by DPPH Assay Method
DPPH radicle scavenging activity method of in-vitro antioxidant study was carried out by preparing 50 μg/ml concentration of a working solution from a stock solution of 1mg/ml. 1ml of working concentration of different samples, a solution of 2,2-diphenyl-1-picryl-hydrazine-hydrate solution (DPPH) 500μl with 0.004% w/v solution was mixed and was sequence with 4ml methanol.
At 516 nm, the sample’s absorbance was recorded using a UV-Visible spectrophotometer. The percentage of DPPH radicle scavenging (IC50) for the samples was recorded using a calibration curve of quercetin using the following formula:
% DPPH radicle scavenging activity = (Abs control –Abs sample)/ Abs control × 100, where Abs control and Abs samples are the absorbance readings of the solvent (control) and sample respectively.38,2
Method Validation
Validation of all the antioxidant methods was done through precision, linearity, wavelength selection and percent of recovery by the method of standard spectrophotometric.39-41
Statistical analysis
This study has been repeated in triplicate and the report was presented in the mean ± standard deviation format. ANOVA was done using SPSS (version 25), where the p value was less than 0.005 and was reasonably significant.
Results
The quantitative estimations of TPC and TFC of the methanolic extracts of various fruits and vegetables are briefed in Figures 1, 2 and 3 respectively. The current study identified a reasonable amount of phenolic and flavonoid compounds in the tested samples of vegetables and fruits.
TFC Estimation
The TFC values of vegetables and fruits ranged from about 3.6-34.2 μg of quercetin equivalents per 100 gm of the extract, with Karela of Jajpur having the maximal content, with banana (3.6 μg) being the least of Cuttack (Figure 1). Comparing flavonoid content in the fruits and vegetables concerning four different locations, in Amla, it was observed that Dhenkanal (32 μg) has the highest and lowest in Jajpur (28 μg), the apple was Jajpur (32 μg) has the highest and lowest in Angul (28 μg), in banana was Jajpur (7 μg) has highest and lowest in Angul (3.6 μg), in capsicum highest is Jajpur (33.6 μg) and lowest is Cuttack (30.6 μg), in Carrot highest, is Jajpur (34.6 μg) and lowest is Cuttack (30.1 μg), in grapes highest is Dhenkanal (23.4 μg) and lowest is Angul (20.33μg), in green chilly highest, is Jajpur (25.3 μg) and lowest is Cuttack (21.12 μg), in Karela highest is Jajpur (34.2 μg) and lowest is Cuttack (33.14 μg), in lemon highest, is Jajpur (23.6 μg) and lowest is Cuttack (19.4 μg), in orange highest, is Jajpur (15.4 μg) and lowest is Angul (10.42 μg), in pomagranat highest, is Jajpur (25.2μg) and lowest is Angul (20.12 μg), in tomato highest, is Jajpur (18 μg) and lowest is Cuttack (13.26 μg). On average when it is observed that Jajpur has the highest flavonoid content, whereas the lowest was observed in Cuttack, Dhenkanal, and Angul, being in the intermediate position. (Figure 1)
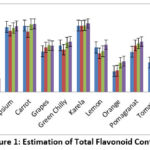 |
Figure 2: Factors Influencing the Knowledge, Attitude and Use of CAM Modalities. |
TPC Estimation
While estimating the samples, TPC values varied from 8.6 μg (Tomato of Cuttack) to 27.8 μg (Amla of Jajpur) of gallic acid equivalents per 100 gm of extract (Figure 2). Comparing phenolic content in the fruits and vegetables concerning four different locations, in Amla, it was observed that Jajpur (27.8 μg) has the highest and lowest in Angul (25.2 μg), in the apple Jajpur (19.22 μg) has the highest and lowest in Angul (16.3 μg), in banana was Jajpur (19.12μg) has highest and lowest in Cuttack (13.6 μg), in capsicum highest is Jajpur (19 μg) and lowest is Cuttack (17.6 μg), in Carrot highest, is Jajpur (17.33 μg) and lowest is Cuttack (14.32μg), in grapes highest is Jajpur (17.24μg) and lowest is Angul (14.8μg), in green chilly highest is Jajpur (19.34μg) and lowest is Cuttack (17.22 μg), in Karela highest, is Jajpur (18.4μg). The lowest is Cuttack (13.3 μg) in lemon highest is Jajpur (14 μg) and the lowest is Cuttack (12.2 μg), in orange highest is Jajpur (13.12μg) and the lowest is Angul (11.2 μg). In pomegranates highest, is Jajpur (22.6μg) and lowest is Cuttack (20.5 μg), in tomato highest, is Angul (11.3μg) and the lowest is Cuttack (8.6 μg). On average, Jajpur has the highest Phenol content, whereas the lowest was observed in Cuttack, Dhenkanal and Angul, being in the intermediate position.(Figure 2)
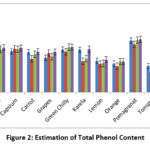 |
Figure 2: Estimation of Total Phenol Content. |
In vitro antioxidant activity
In DPPH assay, good antioxidant activity was found in the samples (Figure 3). In our study, the DPPH radicle scavenging assay ranges from 5.1-10.8 μg/ml. Banana of Jajpur location exerted the maximal IC50 value through DPPH based scavenging assay method, with Amla of Angul, Cuttack and Dhenkanal having the lowest capacity of DPPH radicle scavenging activity (Figure 3). Comparing the DPPH assay in the fruits and vegetables with reference to four different location, in Amla it was observed Jajpur (5.2 μg/ml) has highest and lowest in Angul, Dhenkanal and Cuttack (5.1 μg/ml), in apple was Dhenkanal (19.22 μg/ml) has highest and lowest in Cuttack(6 μg/ml), in banana Jajpur (10.8 μg/ml) has highest and lowest in Angul and Cuttack (10.4 μg/ml), in capsicum highest is Jajpur (6.4 μg/ml) and lowest is Angul and Dhenkanal (6 μg/ml), in Carrot highest is Jajpur (6.7 μg/ml) and lowest is Cuttack (6.2 μg/ml), in grapes highest is Cuttack (6.7 μg/ml) and lowest is Angul (6 μg/ml), in green chilly highest is Angul (6.5 μg/ml) and lowest is Cuttack (6.43 μg/ml), in Karela highest is Jajpur and Dhenkanal (6.8μg) and lowest is Cuttack (6.5 μg), in lemon highest is Jajpur (7.7μg) and lowest is Cuttack (7 μg/ml), in orange highest is Cuttack (6.2 μg/ml) and lowest is Jajpur and Angul (6 μg/ml), in pomagranat highest is Dhenkanal (5.9 μg/ml) and lowest is Angul (5.6 μg/ml, in tomato highest is Jajpur and Angul (8.8 μg/ml) and lowest is Dhenkanal and Cuttack (8.9 μg/ml). Comparing the antioxidant property of all the fruits and vegetables consumed locally in eastern India, it was the same for all locations with a very minimal difference.
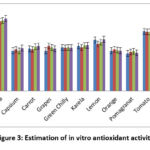 |
Figure 3: Estimation of in vitro antioxidant activity |
Discussion
In the current research, TFC, TPC and free radicle scavenging assay was done with 12 fruits and vegetables commonly consumed in Eastern India. Vegetables and fruits have earned much attention for their potential benefits for human health as they are rich in phenols and flavonoids that provide free radicle scavenging activity. Thus, the phenolic content of vegetables and fruits was evaluated and reported.42
Flavonoids are the plant-based phenols content in vegetables, fruits, and grains, which are classified into flavonols, flavanones, flavones, flavanols (catechins) and anthocyanins. The flavonoid content in this study was higher in Karela of Jajpur, which was more than that of Sahoo et al. of the Bhubaneswar location.43 The violet, blue and red or orange colouration in the plant, flowers, fruits, vegetables and storage tissues of plants are due to anthocyanins, which are water-soluble natural pigments. Due to loss of oxygen, they reduce to yellow or colorless.44 Thus, the anthocyanins detected in pomegranate, orange, carrot, lemon, tomato and apple may be responsible for the high phenolic content next to Karela in the current study. Also, many fruits and vegetables have been depicted to be enriched with flavonoids, polyphenols, and tannins such as catechins, kaempferol, quercetin, luteolin, syringic acid, coumaric and ferulic, which showed significant antioxidant activities.45,46 However, comparing the current result with those of the bibliography remains onerous as the extraction method used varies in each study.
In most food plants, the phenolic content may get influenced due to several factors such as the place of cultivation (geographic region), altitude, environmental factors such as the harvest season, light quality, temperature range, irrigation, soil (type,pH,etc), drying method, industrial processing, quantification, extraction and storage.46,47,39 Many studies have reported that vegetables and fruits grown in arid zones have shown high antioxidant activity and polyphenol content. This is due to the abiotic stress adaptive property the vegetables and fruits enhance their phytochemicals.47,48
The extracts of vegetables and fruits exhibit free radicle scavenging activity that can be evaluated through DPPH method of assay, as it is a regularly applied method due to its appropriate, effectiveness and rapidity.49 In this assay, the minimum IC50 value is determined as the higher potent antioxidant activity of the extract in terms of hydrogen atom or electron donating capacity. The outcome revealed that the extracts of vegetables and fruits exhibited a better scavenging effect in DPPH assay, which may be linked to their higher polyphenols content. Hence, fruits and vegetables can provide high antioxidant values as they are rich in polyphenols. The recent findings are identical to the literature earlier reported and stated, So foods are more dietary antioxidant rich and functionally potent due to the ingredients like flavonoid or phenolic content.40,43
In the search for functional foods the consumers need to be aware of good health and protection against the onset of various illness, so that the potent antioxidant activity of the bioactive compounds present in the functional food can been highlighted. So these functional foods must be taken frequently to avoid diseases related to oxidative stress like cardiovascular diseases, neurodegeneration, cancer, aging, etc.50,51,52 Although different foods can provide antioxidant properties, vegetables and fruits stand out for their richness.4,53,54 Consumers are highly demanding for the food with minimum synthetic additives content and processed with low chemicals to be available in the market. It is always preferable to consume foods that are rich with higher antioxidant properties that are essentially from the natural origins or extracted from nature-based resources.
Conclusion
This study reflects on the most commonly consumed vegetables and fruits in the parts of eastern India that contain polyphenols and flavonoids that are mainly affected due to the geographical region and by comparing the outcome, a positive correlation was observed between TPC and TFC, whereas in DPPH assay with IC50 value had a negative correlation. This study would benefit the nutritionists, consumers and farmers of the current locality for choosing appropriate vegetables and fruits to be grown and consumed for their diet purpose. Further, the researchers may utilize the data for geographically based epidemiological studies where the intake of reported food may be used to measure their antioxidant values, which also can be utilized to examine the antioxidant impact and the synergy in cells. Animal based experimental studies or human based clinical trials can be done to interpret the role of phytoconstituent based antioxidant dietary for preventing diabetes, cardiovascular-related diseases, cancer and oxidative stress related other chronic diseases in the near future.
Acknowledgment
The Authors are highly grateful to the Dean IMS & SUM Hospital (SOA University), Dean SPS (SOA University) and Director CBSH (OUAT) for providing all the support and encouragement during the study.
Conflict of Interest
All authors disclose that there is no any conflict of interest among them.
Funding Source
No source of financial support was provided for this study.
References
- Ikonne, E.U., Ikpeazu, V.O. and Ugbogu, E.A., 2020. The potential health benefits of dietary natural plant products in age related eye diseases. Heliyon, 6(7), p.e04408.
- Kar, D. and Panda, M.K., 2017. In vitro Antioxidant Potential of Methanolic Extract of Symplocos racemosa Roxb. Asian Journal of Chemistry, 29(10).
- Nimse, S.B. and Pal, D., 2015. Free radicals, natural antioxidants, and their reaction mechanisms. RSC advances, 5(35), pp.27986-28006.
- Dhalaria, R., Verma, R., Kumar, D., Puri, S., Tapwal, A., Kumar, V., Nepovimova, E. and Kuca, K., 2020. Bioactive compounds of edible fruits with their anti-aging properties: A comprehensive review to prolong human life. Antioxidants, 9(11), p.1123.
- Sir Elkhatim, K.A., Elagib, R.A. and Hassan, A.B., 2018. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food science & nutrition, 6(5), pp.1214-1219.
- Mekhilef, S., Saidur, R. and Kamalisarvestani, M., 2012. Effect of dust, humidity and air velocity on efficiency of photovoltaic cells. Renewable and sustainable energy reviews, 16(5), pp.2920-2925.
- Uttara, B., Singh, A.V., Zamboni, P. and Mahajan, R., 2009. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current neuropharmacology, 7(1), pp.65-74.
- Pramod, J., Singh, S. and Singh, J., 2013. Role of free radicals and antioxidants in human health and disease. International Journal of Current Research and Review, 5(19), p.14.
- Swain, S.K. and Kar, D., 2021. Vocal fold leukoplakia–An underestimated premalignant lesion of the larynx: A narrative review. Cancer Research, Statistics, and Treatment, 4(2), pp.321-327.
- Forni, C., Rossi, M., Borromeo, I., Feriotto, G., Platamone, G., Tabolacci, C., Mischiati, C. and Beninati, S., 2021. Flavonoids: A myth or a reality for cancer therapy?. Molecules, 26(12), p.3583.
- Khan, J., Deb, P.K., Priya, S., Medina, K.D., Devi, R., Walode, S.G. and Rudrapal, M., 2021. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules, 26(13), p.4021.
- Popa, D.S. and Rusu, M.E., 2017. Isoflavones: Vegetable sources, biological activity, and analytical methods for their assessment. Superfood and Functional Food-The development of superfoods and their roles as medicine, pp.133-153.
- Tresserra-Rimbau, A., Lamuela-Raventos, R.M. and Moreno, J.J., 2018. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochemical Pharmacology, 156, pp.186-195.
- Ali, M.Y., Sina, A.A.I., Khandker, S.S., Neesa, L., Tanvir, E.M., Kabir, A., Khalil, M.I. and Gan, S.H., 2020. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods, 10(1), p.45.
- Kaur, G., Sandal, A. and Dhillon, N.S., 2017. Lycopene and human health-A review. Agricultural Reviews, 38(4), pp.282-289.
- Di Lorenzo, C., Colombo, F., Biella, S., Stockley, C. and Restani, P., 2021. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Eastwood, M.A., 1999. Interaction of dietary antioxidants in vivo: how fruit and vegetables prevent disease?. Qjm, 92(9), pp.527-530.
- Elmi, M., 2004. Food safety: current situation, unaddressed issues and the emerging priorities. EMHJ-Eastern Mediterranean Health Journal, 10 (6), 794-800, 2004.
- Kuanar, А., Pati, А., Pattnaik, B., Bhuyan, R. and Kar, D., 2021. Biotechnological Approaches for Enhancing the Production of Vegetables–An Updated Overview. Universal Journal of Agricultural Research, 9(6), pp.221-234.
- Serna-Saldivar, S. O., Cereal Grains: Properties, Processing and Nutritional Attributes; Taylor and Francis Group: Boca Raton, FL: 2010; 606–609.
- Rice‐Evans, C. and Miller, N.J., 1995. Antioxidants–the case for fruit and vegetables in the diet. British food journal, 97(9), pp.35-40.
- FSA. 2010. Eatwell: 8 tips for making healthier choices. http://www.food.gov.uk/multimedia/pdfs/publication/eat well0708.pdf. Accessed on: [December 26, 2022].
- Radovich, T. J. K., Biology and Classification of Vegetables. In Handbook of Vegetables and Vegetable Processing; Sinha N., Hui YH, Evranuz E.O, Siddiq M Ahmed J. Eds.; Blackwell Publishing: Iowa; 2011: 43–47
- Jaganath, I.B. and Crozier, A., 2008. Overview of health-promoting compounds in fruit and vegetables. Improving the health-promoting properties of fruit and vegetable products, pp.3-37.
- Hounsome, N. and Hounsome, B., 2011. Biochemistry of vegetables: major classes of primary (carbohydrates, amino acids, fatty acids, vitamins, and organic acids) and secondary metabolites (terpenoids, phenolics, alkaloids, and sulfur-containing compounds) in vegetables. Handbook of vegetables and vegetable processing, pp.23-58.
- Pisoschi, A.M. and Negulescu, G.P., 2011. Methods for total antioxidant activity determination: a review. Biochem Anal Biochem, 1(1), p.106.
- Block, G., Patterson, B. and Subar, A., 1992. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutrition and cancer, 18(1), pp.1-29.
- Ka, S., 1996. Vegetables, fruit, and cancer prevention: a review. J am diet assoc, 96, pp.1027-1039.
- Kaur, C. and Kapoor, H.C., 2001. Antioxidants in fruits and vegetables–the millennium’s health. International journal of food science & technology, 36(7), pp.703-725.
- Hung, H.C., Joshipura, K.J., Jiang, R., Hu, F.B., Hunter, D., Smith-Warner, S.A., Colditz, G.A., Rosner, B., Spiegelman, D. and Willett, W.C., 2004. Fruit and vegetable intake and risk of major chronic disease. Journal of the National Cancer Institute, 96(21), pp.1577-1584.
- Barrett, D.M., Somogyi, L. and Ramaswamy, H.S. eds., 2004. Processing fruits: science and technology. CRC press.
- Liu, R.H., 2003. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. The American journal of clinical nutrition, 78(3), pp.517S-520S.
- Belwal, T., Pandey, A., Bhatt, I.D. and Rawal, R.S., 2020. Optimized microwave assisted extraction (MAE) of alkaloids and polyphenols from Berberis roots using multiple-component analysis. Scientific Reports, 10(1), p.917.
- Shahbazi, S., Kuanar, A., Gade, D.R., Kar, D., Shrivastava, A., Kunala, P. and Mahto, M.K., 2016. Semiemperical investigation of the postmenopausal breast cancer treatment potential of xanthone derivatives. Nat Prod Chem Res, 4(206), p.2.
- Sosa-Hernández, J.E., Escobedo-Avellaneda, Z., Iqbal, H.M. and Welti-Chanes, J., 2018. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules, 23(11), p.2953.
- Shan, S., Huang, X., Shah, M.H. and Abbasi, A.M., 2019. Evaluation of polyphenolics content and antioxidant activity in edible wild fruits. BioMed research international, 2019.
- Luzia, D.M.M. and Jorge, N., 2014. Study of antioxidant activity of non-conventional Brazilian fruits. Journal of Food Science and Technology, 51, pp.1167-1172.
- Saha, M.R., Hasan, S.M.R., Akter, R., Hossain, M.M., Alam, M.S., Alam, M.A. and Mazumder, M.E.H., 2008. In vitro free radical scavenging activity of methanol extract of the leaves of Mimusops elengi Linn. Bangladesh Journal of Veterinary Medicine, 6(2), pp.197-202.
- Almeida, M.G., Chiari, B.G., Correa, M.A., Chung, M.C. and Isaac, V.L., 2013. Validation of an alternative analytical method for the quantification of antioxidant activity in plant extracts. Lat. Am. J. Pharm, 32(1), pp.90-5.
- Hussain, A.I., Anwar, F., Sherazi, S.T.H. and Przybylski, R., 2008. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food chemistry, 108(3), pp.986-995.
- Amponsah, I.K., Orman, E., Mensah, A.Y., Sarpong, F.M., Armah, F.A. and Sarpong, L.M., 2016. Development and validation of a radical scavenging antioxidant assay using potassium permanganate. Journal of Scientific and Innovative Research, 5(2), pp.36-42.
- Gunathilake, K.P.P. and Ranaweera, K.K.D.S., 2016. Antioxidative properties of 34 green leafy vegetables. Journal of Functional Foods, 26, pp.176-186.
- Sahoo, S. K., Gangopadhyay, A., Kar, D., Bhuyan, R., Bose, A., 2021. Comparative Antioxidant Study of Different Fruits and Vegetables Commonly Consumed in Odisha, India. IJCRR. 13(11).pp.142-145.
- Merken, H.M. and Beecher, G.R., 2000. Measurement of food flavonoids by high-performance liquid chromatography: a review. Journal of agricultural and food chemistry, 48(3), pp.577-599.
- Manach, C., Scalbert, A., Morand, C., Rémésy, C. and Jiménez, L., 2004. Polyphenols: food sources and bioavailability. The American journal of clinical nutrition, 79(5), pp.727-747.
- Stafussa, A.P., Maciel, G.M., Rampazzo, V., Bona, E., Makara, C.N., Junior, B.D. and Haminiuk, C.W.I., 2018. Bioactive compounds of 44 traditional and exotic Brazilian fruit pulps: phenolic compounds and antioxidant activity. International Journal of Food Properties, 21(1), pp.106-118.
- Kumari, D., Madhujith, T. and Chandrasekara, A., 2017. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food science & nutrition, 5(3), pp.474-485.
- Taghizadeh, S.F., Davarynejad, G., Asili, J., Nemati, S.H. and Karimi, G., 2018. Assessment of phenolic profile and antioxidant power of five pistachio (Pistacia vera) cultivars collected from four geographical regions of Iran. Avicenna Journal of Phytomedicine, 8(1), p.33.
- Amarowicz, R., Pegg, R.B., Rahimi-Moghaddam, P., Barl, B. and Weil, J.A., 2004. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food chemistry, 84(4), pp.551-562.
- Abreu-Naranjo, R., Paredes-Moreta, J.G., Granda-Albuja, G., Iturralde, G., González-Paramás, A.M. and Alvarez-Suarez, J.M., 2020. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon, 6(10), p.e05211.
- da Silva, L.C., Viganó, J., de Souza Mesquita, L.M., Dias, A.L.B., de Souza, M.C., Sanches, V.L., Chaves, J.O., Pizani, R.S., Contieri, L.S. and Rostagno, M.A., 2021. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chemistry: X, 12, p.100133.
- Kiokias, S. and Oreopoulou, V., 2021. A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules, 26(17), p.5405.
- Saini, A., Panesar, P.S. and Bera, M.B., 2019. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresources and Bioprocessing, 6(1), pp.1-12.
- Samtiya, M., Aluko, R.E., Dhewa, T. and Moreno-Rojas, J.M., 2021. Potential health benefits of plant food-derived bioactive components: An overview. Foods, 10(4), p.839.








