Ade Rifka Junita1,2 , Firdaus Hamid3
, Firdaus Hamid3 , Budu Budu4
, Budu Budu4 , Rosdiana Natzir5
, Rosdiana Natzir5 , Yusmina Hala6
, Yusmina Hala6 , Gemini Alam7
, Gemini Alam7 , Rosana Agus7
, Rosana Agus7 , Burhanuddin Bahar8
, Burhanuddin Bahar8 , Ahmad Syukri1,2
, Ahmad Syukri1,2 , Muhammad Reza Primaguna9
, Muhammad Reza Primaguna9 , Ressy Dwiyanti10,11
, Ressy Dwiyanti10,11 , Andini Febrianti11, Azhar Azhar12
, Andini Febrianti11, Azhar Azhar12 and Mochammad Hatta2*
and Mochammad Hatta2*
1Postgraduate School, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
2Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
3Department of Microbiology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
4Department of Ophthalmology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
5Department of Biochemistry, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
6Department of Biology, Faculty of Sciences, Makassar University, Makassar, Indonesia
7Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia
8Department of Biostatistic, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
9Department of Internal Medicine, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
10Department of Medical Microbiology, Faculty of Medicine, Tadulako University, Palu, Indonesia
11Department of Forensic and Medicolegal, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
12Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Corresponding Author E-mail: hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2710
Abstract
Background: Miana, also known as Coleus scutellariodes, is a supplement agent frequently used to treat infectious disorders. Its antibacterial and anti-inflammatory mechanisms are not well understood. Nuclear factor-kappa B (NF-kB), which acts as a major regulator in these infectious processes, strongly induces proinflammatory cytokines via I-kB through its connection with the NF-kB receptor, which affects cytokine production, and angiogenesis via the role of VEGF and HIF-1. VEGF is an angiogenic factor that can trigger cellular responses on the surface of endothelial cells via the NF-kB pathway. HIF-1 has a critical role in the cellular response to systemic oxygen levels of cells. This article's objective is to provide a thorough analysis of molecular mechanisms of NF-kB in relation to infectious diseases treated by Miana. Methods: To obtain the data for this study, relevant reference lists were manually searched in the PubMed, EMBASE, and Scopus databases using the keywords "Miana", "Coleus scutellariodes", "NF-kB", "antibacterial", "anti-inflammation" and "Infectious diseases." as significant reference lists. This review article included and narratively covered each appropriate article from the database above. Results: It was found in several articles that NF-kB and molecular mechanisms of Miana in infectious diseases are strongly related, and that these mechanisms may be used to cure and prevent infectious diseases. The molecular mechanism of Miana containing the active component of flavonoids is broad and complex, in which the induced NF-kB has two main pathways, namely canonical and non-canonical initially from the upstream and downstream of NF-kB activities and there is intricate crosstalk of NF-kB. Conclusions: Miana treats infectious diseases through NF-kB, which functions mainly through a variety of mechanisms. Miana's treatment of infectious diseases with NF-kB leads to the conclusion that NF-kB is a stimulator of several proinflammatory cytokines. Additionally, Miana can reduce HIF-1 expression, and HIF-1 is also in function of upregulating some angiogenic factors in infectious diseases, therefore Miana may suppress NF-kB activities both in vitro and in vivo. Miana contains an active component of flavonoid, which has broad capabilities in both inflammatory and non-inflammatory processes, thus research is urgently needed that links from upstream to downstream of its molecular mechanisms. Besides that, a more detailed study is needed on the intricate crosstalk in the inflammatory process due to microorganism infection through NF-kB activity in Miana interventions containing flavonoid active substances.
Keywords
Antibacterial; Anti-inflammation; Miana (Coleus scutellariodes); Infectious Diseases; Nuclear Factor-kappa B (NF-kB)
Download this article as:| Copy the following to cite this article: Junita A. R, Hamid F, Budu B, Natzir R, Hala Y, Alam G, Bahar B, Syukri A, Primaguna M. R, Dwiyanti R, Febrianti A, Azhar A, Hatta M. Miana (Coleus scutellariodes) Inhibits Nuclear Factor-kappa B (NF-kB) Activity and its Antibacterial and Anti-inflammatory Benefits in Infectious Diseases: Review Article. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Junita A. R, Hamid F, Budu B, Natzir R, Hala Y, Alam G, Bahar B, Syukri A, Primaguna M. R, Dwiyanti R, Febrianti A, Azhar A, Hatta M. Miana (Coleus scutellariodes) Inhibits Nuclear Factor-kappa B (NF-kB) Activity and its Antibacterial and Anti-inflammatory Benefits in Infectious Diseases: Review Article. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3Pc12J6 |
Introduction
Herbal and its products including Miana (Coleus scutellariodes) widely used as supplements and treatment in traditional medicine in several tropical countries such as Indonesia for both infectious and non-infectious diseases. Several studies have demonstrated the efficacy of herbal medicine, such as virgin coconut oil for Alzheimer’s disease1, Valerian extract for antidepressant2, Moringa Oleifera Lam for neuroprotective and hepatoprotective3 and Maina for antibacterial against Aggregatibacter actinomycetemcomitans4 and Porphyromonas gingivalis.5
Previous studies revealed that in rats with traumatic brain injury, caffeic acid phenethyl ester (CAPE), one of the bioactive components of propolis extract, is shown to reduce cerebral vasospasm6 and neuroprotective.7 Another study on propolis showed antibacterial effects against Klebsiella pneumoniae10 and Salmonella typhi.8,9 Another study revealed that banana components have an anti-inflammatory effects.10 and is strongly associated with the treatment of Alzheimer’s disease.11 Furthermore, curcumin exhibits an antimicrobial effect against Salmonella typhi12 and Toxoplasma gondii.13 Other studies showed that MLC901 is a traditional Chinese medicine for protects the ischemia of the brain14
The study by Korbecki, et al, showed that the hypoxic cycle in infection causes an increase in systemic Reactive Oxygen Species (ROS) which has the effect of inducing HIF-I and activating NF-kB.15 Miana and Quercetin’s anti-inflammatory properties may be mediated by the suppression of NF-kB activation and cytokine release.16,17 Besides that, a study conducted by Wahyuni, et al, stated that using Miana orally showed a significant increase in levels of Natural Resistance Associated Macrophage Protein1 (NRAMP-1) as a macrophage activator in Klebsiella pneumonia infection.18
A study to more fully comprehend the effects of Miana (Coleus scutellariodes) as an anti-inflammation and antibacterial on alterations in Nuclear factor-kappa B (NF-kB), which may be a mechanism for infectious diseases, would be urgently required based on the background and previous findings.
Methods
Using keyword combinations of the medical subject headings (MeSH) of “Miana,” “Coleus scutellariodes”, “NF-kB”, “antibacterial, “anti-inflammation”, and “Infectious disease” an in-depth review of the literature was carried out in the PubMed (NIH), Scopus, EMBASE, and Google Scholar databases. Relevant reference lists were also manually searched. This review article included and narratively discussed any relevant publications from the database above.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA) guidelines were also followed.19 All relevant publications of any research design published in the previous data set in either the English or Indonesian language were included and narratively evaluated.
Results
Miana (Coleus scutellariodes)
Miana (Coleus scutellariodes) is a plant for many years, the plant height is between 15-30 cm with a very wide variety of plant colors or leaf colors. People in Indonesia commonly use Miana as traditional medicine.20 The composition of Miana useful chemical compounds, namely essential oils, tannins, flavonoids, saponins, thymol, carvacrol, and eugenol, and the content of active substances such as essential oils, alkaloids, flavonoids, and phenolic derivatives (polyphenols) can be antibacterial.21,22
Miana is a plant that grows in the tropics and is a shrub with a height of up to 1.5 m. The leaves are efficacious as a remedy for hemorrhoids, acne vulgaris, puerperal fever, ear inflammation, and irregular menstruation.
Miana’s taxonomy is Kingdom : Plantae; Division : Spermatophyta; Sub Division : Angiosperms; Class : Dicotyledonae; Order: Solanales; Family: Lamiaceae; Genus: Coleus
Species : Coleus scutellariodes (L) Benth.23
This plant has other names, namely Bulunangko (Toraja), Jawek Kotok (Sunda), Serewung (Minahasa), Ati-ati (Bugis), Sigresing (Batak), Iler (Central Java), and Adong-adong (Palembang). Miana (Coleus scutellarioides (L) Benth) is originally from Thailand and India.
Miana also has a different Latin name, such as Solenostemon scutellariodes Codd , Plectranthus scutellariodes, (Linn), C. ingrates. Benth, Coleus atropurpureus. Bent, , Coleus laciniatus. Benth, Coleus hybridus Hort, Coleus blunei.24,25
The morphology of the Miana root is in the form of a taproot, which is indicated by the presence of 1 enlarged root stem. Miana plants include herb plants, where the stems are soft and easily broken. The structure of the stem is upright or lying at the base. Grows up to 1.5 m tall.
Miana leaves include single leaves, heart-shaped, rounded, or curved bases to resemble the shape of a heart. Each edge of the leaf has continuous thin grooves and long stalks measuring 3-4 cm with various colors. The tip of the leaf is tapered and the veins are pinnate. Miana flowers are shaped like a strand of flowers in layers and are red and purple in color. It has a distinctive aroma and a slightly bitter taste. (Figure 1)
 |
Figure 1: Miana Tree (Coleus scutellariodes). |
Based on the literature and publications that have been done before, it can be concluded that until now the level of resistance tends to increase and it is likely that many patients infected with microorganisms have not been successful with antibiotics.26 According to these conditions, they began to be directed to look for additional therapies that have almost the same effect as antibiotic therapy, one of which is by administering herbal medicines that have useful phytochemicals to suppress the inflammatory process that occurs. There are 32 plants that have been tested and have a similar antimicrobial effect as antibiotics, but in Indonesia, the mechanism of action of these herbal plants has not been studied in depth both molecularly and immunologically.9
One of the plants that are commonly found in Indonesia is Miana leaves (Coleus scutellariodes), which based on a study assessing the ethnopharmacology of Miana in West Halmahera assessed that in this area the use of Miana leaves varies widely from cultivation to being mixed to be used as medicine for several diseases including low back pain, coughs, ulcers, and hemorrhoids, but no one has reported direct consumption in typhoid sufferers. Miana’s effectiveness in treating the disease is thought to be due to the phytochemical content in Miana, including flavonoids, tannins, saponins, phytol, rosmanic acid, stroptozocin, steroids, eugenol, essential oils, quercetin.25 The phytochemical content in Miana leaves such as flavonoids as anti-inflammatories was also assessed to be able to have an effect on the expression of the HMGB-1 gene as a pro-inflammatory cytokine, especially in S. typhi infection. Among these phytochemicals, which have antibacterial activity are flavonoids, steroids, tannins, saponins, and alkaloids. Apart from being antibacterial, the flavonoid content in Miana plants is considered to have an effect as an anti-inflammatory.21,22,26 Miana leaves from the purple plant are known to have antioxidant properties. This antioxidant property is due to the presence of secondary metabolites of the phenolic group. Flavonoids are the largest group of phenolic compounds found in nature. The purple color of purple leaves shows that antioxidant properties come from compounds belonging to the flavonoid group.27
The chemical structure of the flavonoid derivatives contained in Miana (Figure 2) where Flavonoids can be divided into several classes include anthocyanins, aurones, biflavones, chalcones, dihydrochalcones, dihydroflavonols, flavans and proanthocyanidins, flavanones, flavones, flavonols, isoflavonoids.28 The basic structure of Anthocyanins is a flavilium salt (Fig. 2; no.1). Flavones have substitutions at rings A and B but lack oxygenation at position 3 of ring C (Fig. 2; no. 2). Although flavones are generally present in cell vacuoles as O- and/or C glycosides (C-glycosyl flavone), some compounds, especially simple and polymethoxylated flavone, are present in heartwood and as farinose exudate, bud wax, and so on. Flavonols are flavone attached to the hydroxyl group at position 3 (Figure 2; no.3). The chalcone is a double bond between the and positions, but not the dihydrochalcone. So that the color of many chalcones becomes yellow (Fig. 2; no. 4).
Auron glycosides act as a water-soluble yellow pigment in Mina flowers (Fig. 2; no. 5)
Dihydroflavonol, namely 3-hydroxy-flavanone is an intermediate required in the pathway to flavonols via one pathway and to anthocyanins via flavan 3,4-diol via another pathway (Fig. 2; no. 6). Isoflavonoids differ from other classes of flavonoids in that they have the basic structural feature of binding to C-3 but not C-2, and are divided into several classes, e.g. isoflavones, coumestans, coumaronochromones, pterocarpans, and rotenoids (Fig. 2; no. 7)
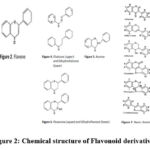 |
Figure 2: Chemical structure of Flavonoid derivatives28 |
A previous study has been carried out by assessing the potential of Miana leaves content flavonoid as an immunomodulator in cases of Klebsiella pneumonia infection and the results demonstrate that NRAMP-1 (Natural Resistance Associated Macrophage Protein-1) expression can be elevated by Miana leaf extract (Coleus scutellariodes).18
Effects of flavonoid in Miana
A study by Vezza, et al, showed that flavonoid derivatives have very varied effects (Table 1). By inhibiting enzymatic activity and suppressing inflammatory processes both in vivo and in vitro, the biological action of flavonoids including their antioxidant properties can reduce the severity of inflammatory diseases related to the digestive system.29
Table 1: Effects of flavonoid derivate as anti-inflammation in infectious diseases.29
|
Plant |
Chemical compound |
Mechanisms |
|
|
Anthocyanins |
|
|
Hibiscus sabdariffa |
Cyanidin-3-glucoside |
Decrease in the generation of inflammatory mediators Blocking the STAT pathway Reducing PGE-2 production by controlling COX-2 activity |
|
|
Chalcnes |
|
|
Alfinia katsumadai Alfinia conchigera |
Cardamomin |
Suppression of NF-kB activity Leukocyte migration restriction Preventing the production of reactive nitrogen species Pro-inflammatory mediators reduced |
|
|
Flavanones |
|
|
Grapefruit (Citnes paradise)
|
Naringenin |
Suppression of NF-kB activity Reduction of the production of pro-inflammatory mediators Enhanced epithelial barrier performance Suppression of leukocyte migration Modulation of the gut microbiota and the antimicrobial action Suppressing COX-2 activity Suppression of the generation of reactive nitrogen species
|
|
|
Flavones |
|
|
Picea crassifolia |
Chrysin |
Reduction of NF-KB activity Preventing the production of reactive nitrogen species Pro-inflammatory mediators are reduced Leukocyte migration restriction |
|
Scutellaria baicalensis |
Baicalin |
Suppression of NF-KB activity Modulation T cell activities |
|
|
Flavonols |
|
|
Disosma reitchii |
Quercetin |
Suppression of NF-kB activity Decrease in the production of inflammatory mediators Preventing the generation of reactive nitrogen species |
|
Ruta graviolens |
Rutin |
Reduction of NF-kB activity Enhanced epithelial barrier performance Leukocyte migration restriction Reduction of COX-2 activity Reduction of pro-inflammatory mediators |
|
Tartaryy buckwheat (Fagopyrum tataricumm) Oaks species (Quercus sp.)
|
Quercitrin |
Reduction of NF-kB activity Decrease in the generation of inflammatory mediators Enhanced epithelial barrier performance Leukocyte migration restriction Preventing the production of reactive nitrogen species |
The Toll-like receptors (TLRs) pathway, a protein that is crucial in triggering the body’s immune response, especially for infections carried on by the S. typhi bacteria, maybe the mechanism through which Miana leaves have an antibacterial impact.30
Previous studies using the UV-V spectrophotometer qualitative method revealed that the total flavonoid component of the 96% ethanol extract of Miana leaves (Coleus atropurpereus) was 8.59 mg RE/gram of extract. This compound can be used as an immunostimulator in the prevention and treatment of various diseases.21,31
By decreasing the NF-kB signal transduction pathway, the flavonoid concentration in Miana may reduce NF-kB activity (Figure 3). After the NF-kB receptor is induced by the components of microorganisms, the NF-kB protein complex (p65-p50) will bind to each other and be inhibited by the IK-kB protein. The IKK complex (IKK, IKK, and IKK) is activated by pro-inflammatory cytokines such LPS from S. typhi, which phosphorylates the IK-B protein. IB is phosphorylated, which results in proteasomal breakdown and the release of NF-kB. The active NF-kB protein is then activated by post-translational modifications (phosphorylation, acetylation, glycosylation), and it is translocated to the nucleus where it induces the expression of its target genes and regulates a number of biological processes, including innate and adaptive immunity, inflammation, stress response, cell development, and lymphoid organogenesis. Thus, the inhibition of the NF-kB signal transduction pathway may be responsible for some flavonoid derivatives’ ability to reduce inflammation.29
The results above support the hypothesis that administering Miana leaf extract can inhibit the expression of proinflammatory cytokines including MCP-1-1, IL-6, TNF-α , and IL-1 through the NF-kB pathway.
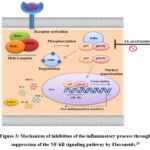 |
Figure 3: Mechanism of inhibition of the inflammatory process through suppression of the NF-kB signaling pathway by Flavonoids.29 |
Previous research on Mycobacterium tuberculosis-infected Balb/c mice has demonstrated that Miana has anti-inflammatory properties by suppressing the mRNA expression of vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1 (HIF-1), and intercellular adhesion molecule-1 (ICAM-1).17,32 A previous study has demonstrated that Miana can stimulate the expression of IL-37 mRNA in Balb/c mice that have been injected with Candida albicans.33,34
By inducing the transcription of proinflammatory genes, NF-kB transcriptional activation, which results from infection with the translocation core complex in the cytoplasm, is a key factor in the inflammatory process. When cells are appropriately stimulated, most frequently by signals from pathogenic or hypoxic microorganisms, these pathways become activated.35,36
Through the innate immune response, NF-kB is crucial to the host’s defense against microbial infection. NF-kB is activated by multiple signaling pathways originating from many different cellular receptors and sensors. Most pathogenic microorganisms are the most capable of regulating NF-kB activation and will then induce signals for various types of proinflammatory cytokines. 37Despite differences in signaling processes, NF-kB activation involves two major signaling mechanisms: canonical and noncanonical (or alternative) pathways. Both of these pathways are crucial to regulate immunological and inflammatory responses. After being activated by microbial or product, NF-kB will be stimulated from upstream, for example IKK upstream signaling factors to downstream, for example transforming growth factor-β-activated kinase 1 (TAK1).36
Transcriptional regulator immune system function, apoptosis, differentiation, and stress response are all crucially regulated through NF-kB. Multiple stimuli, such as microbial infection or its products, interact to activate NF-kB, which in turn can mediate alternative transcriptional programs. As a result, both positive and negative regulatory mechanisms tightly regulate and closely coordinate NF-kB-dependent transcription with other signaling pathways. In order to optimize the numerous biological roles of NF-kB for certain responses, this complex crosstalk is required.38
According to Martin et al. (2016) and Lin et al. (2016), activation of NF-kB signaling results in the production of a variety of inflammatory cytokines, chemokines, and transcription factors that start and modulate inflammatory reactions as well as control the host response to tissue damage. In addition, NF-kB is crucial for controlling the survival, activation, and differentiation of innate immune cells and inflammatory T cells.39,40 Based in large part on the activation of NF-kB by proinflammatory cytokines like interleukin-1 (IL-1) and tumor necrosis factor (TNF), nuclear factor-kappa (NF-kB) has long been thought of as an archetypal proinflammatory signaling pathway.40 One of the most significant molecules connecting chronic inflammation to infection is nuclear factor-k (NF-kB), a transcription factor required for the inflammatory response. The activity of NF-kB is closely controlled by a number of mechanisms.42
Bacterial endotoxins such lipopolysaccharides and pro-inflammatory cytokines like IL-1 and TNF-α are the major mediators of NF-kB activation. Most solid tumors and hematological malignancies exhibit NF-kB activation in cancer cells as well as in the tumor microenvironment.43 Additionally, systemic lidocaine administration affects TNF-α and NF-kB gene expression on musculoskeletal injury.44 as well as VEGF, HIF-I, and HMGB1 as a crucial regulator in DOX-induced cardiomyocyte damage through NF-kB pathway.45
The molecular mechanisms of Miana through Nuclear factor-kappa B (NF-kB) activities on infectious diseases
By preventing the synthesis of nucleic acids and affecting the permeability of bacterial cell walls, microsomes, and lysosomes, the flavonoids and quercetin found in Miana are known to directly inhibit the growth of microorganisms (both in vitro and in vivo). According to Taufik et al. and Yanto et al, the mechanism involves interactions between flavonoids and bacterial DNA, the formation of complex compounds with external proteins, the destruction of the latter through dissolution in bacterial cell membranes, and mixing with intracellular chemicals.8,34
In a state of infection, apart from causing PAMP and DAMP events caused by microorganisms, it will also increase ROS and NF-kB activity, where proinflammatory cytokines including TNF-α, MCP-1, IL-8, and IL-6, and IL-1 are produced more frequently as a result of inflammation stimulation.
This increase in proinflammatory cytokines will have an impact in the form of biological effects such as symptoms of inflammation, bacterial growth, endothelial activation, cell migration, tissue damage, and sepsis. Besides that, the increase in ROS activity due to infection will induce NF-kB activity and also end up with an increase in the production of proinflammatory cytokines. ROS will also induce HIF-I and can affect NF-kB activity. In addition, hypoxia and increased HIF-I activity are closely related to inflammation, bacterial growth, endothelial activation, cell migration, tissue damage, and sepsis.
Microorganism antigens can bind to TLR-4 ligands and then TLR-4 will induce NF-kB which will produce inflammatory stimulation and end with increased production of proinflammatory cytokines.
Miana’s active components such as flavonoids and quercetin as strong antioxidants can inhibit the inflammatory process through the ROS pathway thereby preventing hypoxia. Also, Miana can inhibit inflammatory processes through the NF-kB, TLR-4, and HIF-1 alpha pathways and the production of proinflammatory cytokines. Thus, the effect of Miana (Coleus scutellariodes) to inhibit NF-kB via several pathways while NF-kB has a pivotal regulator of proinflammatory cytokines in infectious diseases. In addition, the increase of various angiogenic factors in infectious disease, both in vitro and in vivo, may be caused by Miana’s inhibition of HIF-1 expression and TLR-4 function.
Downstream of the body’s defense system against infection, Miana’s effect can inhibit proinflammatory cytokines such as TNF-α , MCP-1-1, IL-8, IL-6, and IL-1. Thus, it can be concluded that the effect of Miana (Coleus scutellariodes) to inhibit NF-kB via several pathways such as PAMP and DAMP. The summary of molecular mechanisms of Miana through Nuclear factor-kappa B (NF-kB) activities on infectious diseases could be shown in Figure 4.
 |
Figure 4: Pathomechanisms of Miana (Coleus scutellariodes) via Nuclear factor-kappa B (NF-kB) activities on Infectious Diseases |
In cases of bacterial infection, including S. typhi, extracellular HMGB1, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs) are known to interact with a variety of receptors and immunological sensors.46,47 In order to produce pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-alpha), monocyte chemoattractant protein-1 (MCP-1-1), IL-6, and IL1, HMGB1 must bind to the TLR-4 receptor, which activates the nuclear factor kappa- (NF-kB) signaling pathway through the IKK kinase complex (I-kB) binding.48 Similar to quercetin, a flavonoid derivative, NF-kB is suppressed in its transcriptional activity by quercetin, which inhibits the NF-kB pathway.49.50,51
Inflammatory stressors triggered by infection by S.typhi can cause an exaggerated immune response and sepsis which will cause damage to organs.36 The mechanism of anti-inflammatory action of Miana and Quercetin is related to their ability to inhibit signals from several Toll-like receptors (TLRs) which mediate the inflammatory response. Miana which contains flavonaoids and quercetin as anti-inflammatories, works to stabilize cell membranes by reducing the release of proteases from neutrophils or macrophages. Miana and quercetin are able to inhibit the release of inflammatory cytokines such as interleukin-37, IL-1, and VEGF. Miana and parenteral quercetin also show the ability to inhibit neutrophil adhesion, migration and accumulation, macrophage activity and enzyme release.34,52,53,33
According to a study by Canton et al. from 2021, NF-kB is engaged in hypoxic circumstances, and there is increasing evidence that HIF-1 and NF-kB interact to cause HIF-1 to be upregulated in situations when NADPH oxidase-mediated ROS are present.54 By inhibiting NF-kB activation and altering the expression of HIF-1 and ICAM-1 and cytokine release, Miana contain flavonoid and Quercetin may be able to reduce inflammation.55,16,17 Additionally, a study by Wahyuni et al. in 2021 found that administering Miana orally caused induced Balb/c mice with Klebsiella pneumonia to have significantly higher plasma levels of NRAMP-1, which is responsible for activating macrophage cells.18
Table 2 provides an overview of Miana’s prior publications in a number of infectious disorders, including vulvoginal candidiasis, TB, typhoid fever, A. actinomycetemcomitans, and Klebsiella pneumoniae infections.
Table 2: Effects of Miana extract as anti-inflammation and antimicrobial in infectious diseases.
|
Disease/ Microrganisms
|
Mechanisms |
Reference |
|
Typhoid fever/ Salmonella typhi |
Inhibition TLR-4 activity Antimicrobial effect |
[30] |
|
Typhoid fever/ S.typhi |
Inhibition NF-kB activity Antimicrobial effect |
This study |
|
Pneumonia/ Klebsiella pneumoniae |
Increase Natural Resistance Associated Macrophage Protein 1 (NRAMP-1) Antimicrobial effect |
[18] |
|
Vulvovaginal candidiasis/ Candida albicans |
Induce mRNA IL-37 expression Antioxidant Antimicrobial effect Decrease IgM |
[33] [20]
|
|
Tuberculosis/ Mycobacterium tuberculosis |
Inhibition TLR-4 activity Alterations in HIF-1 and ICAM-1 Expression Inhibition VEGF Antimicrobial effect |
[56] [17] [32] |
|
Periodontitis/ A. actinomycetemcomitans |
Increase IL-10 Antimicrobial effect |
[4] |
|
Pseudomonas aeruginosa Escherichia coli/ Streptococcus sp./ Staphylococcus sp./ |
Inhibition HIF-I Antimicrobial effect |
[34] |
The interaction between LPS S. typhi and TLR-4 that activates MyD88 is crucial in regulating bacterial exponential development. Inducible NO synthase (iNOs), NF-KB, and TNF-cytokines will all turn into nuclear translocated as a result of LPS stimulation of TLR-4 and the effect of Miana significantly reduced the TLR-4 mRNA expression.30 Meanwhile, Miana’s molecular pathomechanisms can suppress NF-kB activity in infectious conditions.
According to Wahyuni’s study, the administration of Miana will result in an increase in NRAMP-1 protein levels, and clinical impact has shown a comparable effect on inhibiting Klebsiella pneumoniae. A metal ion transporter called NRAMP-1 can export iron and manganese from the macrophage phagosome in order to reduce the number of metals that an intracellular pathogen can access. The expression of NRAMP-1 (cytosolic iron transport) and iron-carrying protein is induced by microbial infection in macrophages. These alterations in iron homeostasis improve iron bioavailability, which makes it easier to obtain iron and boosts intracellular microbe survival. The regulation of NRAMP-1 during microbial infection shows that NRAMP-1 helps phagocytes fight off infections and, in mammalian hosts, isolates iron, zinc, and manganese ions to restrict microbial development through a mechanism known as nutritional immunity.18
Miana may act as an anti-inflammatory through its role as an antioxidant, so it could potentially be used as an alternative treatment in humans, particularly patients with vulvovaginal candidiasis. According to another study, Miana has fungistatic effects on the expression of mRNA IL-37 in vulvovaginal candidiasis. The body needs antioxidants to stop and get eliminated pathogen invasion and produce an appropriate and effective immune response. Flavonoids, one type of antioxidant found in Miana, have been shown to elevate levels of IFN-γ and CD4+ T-cells with decreasing microbe numbers.
Miana are herbal medicines that at the molecular level exhibit anti-inflammatory properties in patients with vulvovaginal candidiasis caused by Candida albicans, as well as the potential to inhibit the production of prostaglandin, pro-inflammatory signaling molecules. It has been identified over the years the significance of traditional remedies for treating vulvovaginal candidiasis and how the cytokine IL-37, an IL-1 derivative that has been shown to naturally inhibit the non-specific immune system, has a similar effect as an immune mediator with anti-inflammatory properties. Although the mechanisms of cytokine IL-37 action are still unknown, lipopolysaccharide induction has been shown to be the basis of its pro-inflammatory features.33
Currently, azoles, polyenes, echinocandins, allylamines, and fluoropyrimidines are utilized as antifungals to treat fungal infections. However, the restricted use of these antifungals for C. albicans infection has increased patient toxicity and drug resistance. It has been demonstrated that the Miana is safe and effective in pharmacological activities, such as antifungal activity, and that it has the potential to create new antifungals to treat Candida infection. The metabolites of allicin, diterpene, coumarin, terpenoids, curcumin, xanthorrhizol, thymol, essential oils, eugenol, and [6]-shogaol may show the mechanism of action of Miana against C. albicans. T The antifungal mechanisms of medicinal plant metabolites include disruption of hyphal production by allicin, filament and biofilm formation by [6]-shogaol, curcumin, and xanthorhizol, envelope by eugenol, membrane permeabilization by essential oils, thymol, and diterpene, and cell wall by terpenoids, thymol, and coumarin. Metabolite primarily exerts its antifungal effects through the cell wall, membrane, and growth inhibition active mechanisms.20
According to Amsyah et al., Miana treatment against A. actinomycetemcomitans had a substantial impact on the expression of IL-10 mRNA, and Miana administration will increase the expression of IL-10 mRNA in periodontitis caused by A. actinomycetemcomitans. Lipopolysaccharides (LPS), cytolethal distending toxin (cdtABC), leukotoxins, and A.actinomycetemcomitans all have virulence factors that can affect the host immune system and contribute to periodontal disease. CdtB entrance into the cell is facilitated by interactions between CdtA and CdtC and the host membrane. When CdtB enters the cell, an active mechanism that requires amino acid residues in its N terminus transports it into the nucleus. Through its DNAse activity, CdtB induces apoptosis and damages DNA in the nucleus. Aa Cdt has the ability to increase the production of receptor activator of nuclear factor-KB ligand in human gingival fibroblast, which is involved in pathological bone resorption that is a hallmark of localized aggressive periodontitis. Lipopolysaccharides (LPS) activate macrophages to create interleukin-1, tumor necrosis factor, mRNA, and protein that are implicated in potent inhibitors of fibroblast proliferation, bone resorption, and tissue inflammation, among other immunological and endotoxic actions.4
By altering the ratio of pro- and anti-inflammatory cytokines as well as the strength and quantity of the immune system’s reaction to T cells, B cells, and cytokines, Miana can improve immunity. Miana administration influenced the expression of IL 10 mRNA, which thus impacted host immunity. The specific genotypes with low IL-10 expression may exacerbate the inflammatory response and lead to the expansion of the gingival. IL-10 is a significant anti-inflammatory cytokine that was involved in the development of periodontal disease. By producing reactive oxygen species (ROS) and nitrogen intermediates, IL-10 suppresses the activity of proinflammatory cytokines and prevents phagocytosis and microbial death. Because proinflammatory cytokines have not been regulated by anti-inflammatory cytokines when IL-10 levels are low, their activity can increase.4
Based on a previous study, quercetin, and flavonoid extracted from herbals and their derivatives, including Miana, have been proven to strongly inhibit the activities of NF-kB and HIF-1. In addition, it has been revealed that the transcriptional regulator NF-kB has a critical role in controlling the immune system, apoptosis, differentiation, and stress response. Flavonoids such as rutin and quercetin activated Sirtuin1. Sirtuin1 is essential in the NF-kB regulation and the immune system, apoptosis, and oxidative stress transcriptional regulation of a variety of transcription factors. Sirtuin1’s role in infectious diseases makes it possible to explore the pathomechanisms of Miana.57-61
Furthermore, NF-kB has a key role in activating the pro-inflammatory genes that produce cyclooxygenase-2, IL12, IL6, and TNF-α . TLR-4 assists NF-kB in mediating the differentiation of macrophages towards the M1 phenotype. M1 stimulates the release of cytokines that cause inflammation and the proliferation of T cells that cause inflammation. Several studies have reported that herbals and their derivatives including Miana contain flavonoid and quercetin which is effective in strongly inhibiting NF-kB and HIF-1 activities.62,34,63,64.
Conclusions
Miana (Coleus scutellariodes) is a commonly used supplement agent for infectious diseases and it is unclear exactly how the mechanisms of Miana are against bacteria and inflammation. Nuclear factor-kappa B (NF-kB) strongly induces proinflammatory cytokines through I-kB by interacting with the NF-kB receptor, which affects cytokine release and angiogenesis. Because VEGF is an angiogenic factor and activates the NF-kB pathway, it can drive cellular responses on the surface of endothelial cells, where HIF-1 plays a significant role in the cellular response to systemic oxygen levels of cells.
Under conditions of Miana used to treat infectious diseases, NF-kB plays a crucial role as a regulator and mainly functions through multiple pathways. Miana’s treatment of infectious diseases could inhibit NF-kB activity leading to the conclusion that NF-kB is a stimulator of several proinflammatory cytokines. The production of HIF-1, which is also responsible for the elevation of various angiogenic factors in infectious disease both in vitro and in vivo, can be decreased by Miana treatment through reduced NF-kB.
Because Miana contains active components of flavonoid, which have broad and complex abilities, both in inflammatory and non-inflammatory processes that involve NF-kB, research is urgently needed to link upstream, for example, IKK upstream signaling factors to downstream, for example transforming growth factor-β -activated kinase 1 (TAK1) of the mechanisms of canonical and non-canonical of NF-kB pathway.
Besides that, studies are needed to be related to the intricate crosstalk in the inflammatory process due to microorganism infection through NF-kB activity in Miana interventions containing flavonoid active substances.
Acknowledgement
Authors would like to thank Romi Usman, Mus Jubaru, Wani, and Markus of Molecular Biology and Immunology Laboratory for Infection Diseases, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia who helped in the search of journals.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Funding Sources
No specific funding are available.
References
- Chatterjee P, Fernando M, Fernando B, Dias CB, Shah T, Silva R, Williams S, Pedrini S, Hillebrandt H, Goozee K, Barin E, Sohrabi HR, Garg M, Cunnane S, Martins RN. Potential of coconut oil and medium chain triglycerides in the prevention and treatment of Alzheimer’s disease. Mech Ageing Dev. 2020 Mar;186:111209. doi: 10.1016/j.mad.2020.111209
- Das G, Shin HS, Tundis R, Gonçalves S, Tantengco OAG, Campos MG, Acquaviva R, Malfa GA, Romano A, Robles JAH, Clores MQ, Patra JK. Plant Species of Sub-Family Valerianaceae-A Review on Its Effect on the Central Nervous System. Plants (Basel). 2021 Apr 22;10(5):846. doi: 10.3390/plants10050846.
- Kou X, Li B, Olayanju JB, Drake JM, Chen N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients. 2018; 10(3):343. https://doi.org/10.3390/nu10030343
- Amsyah UK, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in A. actinomycetemcomitans Induced Rat Treated by Purple Miana Leaves. Biomed Pharm J. 2019;12(4); 2099-2104. doi : http://dx.doi.org/10.13005/bpj/1845
- Faisal Madhloom A, Bashir Hashim Al-Taweel F, Sha AM, Raad Abdulbaqi H. Antimicrobial Effect of Moringa Oleifera L. and Red Pomegranate against Clinically Isolated Porphyromonas gingivalis: in vitro Study. Arch Razi Inst. 2022 Aug 31;77(4):1405-1419. doi: 10.22092/ARI.2022.357513.2051.
- Palaz MN, Akcay E. The Impact of Propolis Factor Caffeic Acid Phenethyl-Ester on the Cerebral Vasospasm and Early Brain Damage in the Experimentally Induced Subarachnoid Hemorrhage on Rats. World Neurosurg. 2020 Jun;138:e736-e742. doi: 10.1016/j.wneu.2020.03.058.
- Balaha M, De Filippis B, Cataldi A, di Giacomo V. CAPE and Neuroprotection: A Review. Biomolecules. 2021; 11(2):176. https://doi.org/10.3390/biom11020176
- Taufik FF, Natzir R, Patellongi I, Santoso A, Bukhari, Hatta M, Junita AR, Syukri A, Primaguna MR, Dwiyanti R, Febrianti A. In Vivo and In Vitro Inhibition Effect of Propolis on Klebsiella pneumoniae: A Review. Annals of Medicine and Surgery, 2022; 81,2022,104388.DOI: https://doi.org/10.1016/j.amsu.2022.104388
- Syarif LI, Ade Rifka Junita, Mochammad Hatta, Ressy Dwiyanti, Cahyono Kaelan, Muhammad Sabir, Muhammad Reza Primaguna, Nur Indah Purnamasari. A Mini Review: Medical Plants for Typhoid Fever in Indonesia. Syst Rev Pharm. 2020;11(6): 1171-1180. DOI: https://doi.org/10.31838/srp.2020.6.170
- Hong Y-H, Kao C, Chang C-C, Chang F-K, Song T-Y, Houng J-Y, Wu C-H. Anti-Inflammatory and T-Cell Immunomodulatory Effects of Banana Peel Extracts and Selected Bioactive Components in LPS-Challenged In Vitro and In Vivo Models. Agriculture. 2023; 13(2):451. https://doi.org/10.3390/agriculture13020451
- Oyeyinka BO, Afolayan AJ. Suitability of Banana and Plantain Fruits in Modulating Neurodegenerative Diseases: Implicating the In Vitro and In Vivo Evidence from Neuroactive Narratives of Constituent Biomolecules. Foods. 2022 Jul 29;11(15):2263. doi: 10.3390/foods11152263.
- Rahayu SI, Nurdiana N, Santoso S. The effect of curcumin and cotrimoxazole in salmonella typhimurium infection in vivo. ISRN Microbiol. 2013 Aug 29;2013:601076. doi: 10.1155/2013/601076.
- Simanjuntak TP, Hatta M, Tahir AM, Sirait RH, Karo MB, Tambaib T, Dwiyanti R, Noviyanthi RA, Junita AR. Analysis of Anti-toxoplasma Immunoglobulin G and Immunoglobulin M Antibody Levels after Intervention with Curcuma Longa Extract on Early Pregnant Mice with Acute Toxoplasmosis. J Global Infect Dis. 2019;11:25-9. DOI: https://doi.org/10.4103/jgid.jgid_28_18
- Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C. MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology. 2011 Sep;61(4):622-31. doi: https://doi.org/10.1016/j.neuropharm.2011.05.003.
- Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-kB Activation: A Review of the Molecular Mechanisms. Int J Mol Sci. 2021;22(19):10701. https://doi.org/10.3390/ijms22191070
- Cheng S-C, Huang W-C, S. Pang J-H, Wu Y-H, Cheng C-Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kB Signaling Pathways. Int J Mol Sci. 2019; 20(12):2957. https://doi.org/10.3390/ijms20122957
- Rosamarlina R, Hatta M, Djaharuddin I, Patellongi I, Susanto A. D, Islam A. A, Massi M. N, Bukhari A, Santoso A, Tabri N. A, Murtiani F, Junita A. R, Saleh A. S, Dwiyanti R, Pakadang S. R. The Changes of HIF-I and ICAM-1 Expression after Miana (Coleus Scutellariodes [L]) Treatment in Balb/c Mice with Mycobacterium Tuberculosis Infection. Biomed Pharm J. 2022;15(1), 73-81.DOI: https://dx.doi.org/10.13005/bpj/2344
- Wahyuni TD, Hatta M, Bukhari A, Santoso A, Massi MN, Increasing Natural Resistance Associated Macrophage Protein 1 serum level after Miana treatment in BALB/c induced Klebsiella pneumoniae experimental research, Annals of Medicine and Surgery. 2021; 65:102262. DOI: https://doi.org/10.1016/j.amsu. 2021.102262
- Moher A, Liberati J, Tetzlaff DG, Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Int. J. Surg. 8 (2010) 336–341, https://doi.org/10.1016/j.ijsu.2010.02.007
- Karo MB, Tambaip T, Hatta M, Simanjuntak T, Irmawaty L, Rina T, Kamelia E, Rahmawati F, Bintang M. A mini Review of Indonesian Medical Plants for vulvovaginal candidiasis. Rasayan J Chem. 2017;10(4):1280-1288. DOI: https://dx.doi.org/10.7324/RJC.2017.1041887
- Anita, Arisanti D, Fatmawati A. Isolasi dan identifikasi senyawa flavonoid ekstrak etanol danun Miana (Coleus atropurpereus). Prosiding Seminar Hasil Penelitian (SNP2M) 2018:199-203. doi: 978-602-60766-4-9.
- Rahmawati F. Isolasi dan Karakterisasi Senyawa Antibakteri Ekstrak Daun Miana (Coleus stecullariodes (L) Benth). Tesis. Sekolah PascaSarjana IPB 2008
- Tabalubun. Efek Analgesik Daun Iler (Coleus atropurpureus L. Benth) Dengan Metode Rangsang Kimia Pada Mencit Betina. Skripsi. 2013. Yogyakarta: Universitas Sanata Dharma.
- Hariana A. 262 Tumbuhan Obat dan Khasiatnya. 2013. Jakarta : Penebar Swadaya.
- Wakhidah AZ, Silalahi M. Etnofarmakologi Tumbuhan Miana (Coleus scutellaroides (L) Benth) Pada Masyarakat Halmahera Barat, Maluku Utara. Jurnal Pro-Life. 2018; 5 (2):23-28.
- Tushar K, Uttpal A, Abhijit D, Yehuda YG. Zhe-Sheng G, Zhijun L, Vinay K. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Frontiers in Pharmacology. 20121;12. DOI: 10.3389/fphar.2021.720726
- Abdul Rahim R, Jayusman PA, Lim V, Ahmad NH, Abdul Hamid ZA, Mohamed S, Muhammad N, Ahmad F, Mokhtar N, Mohamed N, Shuid AN, Naina Mohamed I. Phytochemical Analysis, Antioxidant and Bone Anabolic Effects of Blainvillea acmella (L.) Philipson. Front Pharmacol. 2022 Jan 17;12:796509. doi: 10.3389/fphar.2021.796509. PMID: 35111063; PMCID: PMC8802550.1.
- Iwashina T, Agrawal PK. Editorial. Natural Product Communications. 2020;15(12). doi:10.1177/1934578X20982118
- Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients. 2016;8(4):211. https://doi.org/10.3390/nu8040211
- Syamsuri F, Hatta M, Natzir R, Alam G, Massi MN, Bahar B, Rahardjo SP. Expression of TLR-4 in Salmonella typhi-Induced Balb/c Mice Treated by Miana Leaves (Coleus scutellaroides (L) Benth). Indian J Pub Health Res Dev. 2018; 9(12):1449-1454
- Reny SR, Fauza D, Selonni, Taslim T. Kadar fenolat flavonoid siungu Mentawai (Graptophyllum pictum (L)Griff). J Katalisator. 2021;6 (1):34-54. http://ejournal.kopertis10.or.id/ index.php/katalisator
- Rosamarlina, Hatta M, Sridiana E, Djaharuddin I, Patellongi I, Murtian F. The effect of Miana (Coleus scutellariodes [L]) on Vascular Endothelial Growth Factor expression in Balb/c mice infected with Mycobacterium tuberculosis. Biomed Pharm J. 2021;14 (2): 525-532. DOI: https://dx.doi.org/10.13005/bpj/2154
- Karo M, Hatta M, Salma W, Patellongi I, Natzir R. Effects of Miana (Coleus Scutellariodes [L] Benth) to Expression of mRNA IL-37 in Balb/c Mice Infected Candida albicans. Pharmacog J. 2018;10(1):16-19. DOI : https://doi.org/10.5530/pj.2018.1.3
- Yanto TA, Hatta M, Bukhari A, Natzir R. Molecular and Immunological Mechanisms of Miana Leaf (Coleus Scutellariodes [L] Benth) in Infectious Diseases. Biomed Pharm J. 2020;13(4): 1607-1618. https://dx.doi.org/10.13005/bpj/2036
- Liu SF, Malik AB.NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622-L645. doi: 10.1152/ajplung.00477.2005.
- Liu T, Zhang L, Joo D, Sun SC. NF-kB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023–. doi: 10.1038/sigtrans.2017.23. Epub 2017 Jul 14. PMID: 29158945; PMCID: PMC5661633.
- Su CM, Wang L, Yoo D. Activation of NF-kB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep. 2021 Jun 29;11(1):13464. doi: 10.1038/s41598-021-92941-2. PMID: 34188167; PMCID: PMC8242070.
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011 Jul 19;12(8):695-708. doi: 10.1038/ni.2065. PMID: 21772278.
- Martins GR, Gelaleti GB, Moschetta MG, Maschio-Signorini LB, Zuccari DA. (2016). Proinflammatory and Anti-Inflammatory Cytokines Mediated by NF-kB Factor as Prognostic Markers in Mammary Tumors. Mediators Inflamm. 2016:9512743. doi: 10.1155/ 2016/9512743.
- Lin TH, Pajarinen J, Lu L, Nabeshima A, Cordova LA, Yao Z, Goodman SB. NF-kB as a Therapeutic Target in Inflammatory-Associated Bone Diseases. Adv Protein Chem Struct Biol. 2017;107:117-154. doi: 10.1016/bs.apcsb.2016.11.002.
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harbor Perspectives in Biology. 2014; 1(6): a001651–a001651. doi: 10.1101/cshperspect. a001651
- Chandan, K., Gupta, M., & Sarwat, M.Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Frontiers in Immunology. 2020;10. https://doi.org/10.3389/fimmu.2019.03081
- Taniguchi, K., Karin, M. NF-kB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 2018; 18, 309–324. https://doi.org/10.1038/nri.2017.142
- Karnina R, Arif SK, Hatta M, Bukhari A, Natzir R, Hisbullah , Patellongi I, Kaelan C, Systemic lidocaine administration influences NF-kβ gene expression, NF-kβ and TNF- α protein levels on BALB/c mice with musculoskeletal injury, Annals of Medicine and Surgery. 2921; 69:102660. DOI: https://doi.org/10.1016/j.amsu.2021.102660
- Syukri A, Budu, Hatta M, Amir M, Rohman MS, Mappangara I, Kaelan C, Wahyuni S, Bukhari A, Junita AR, Primaguna MR, Dwiyanti R, Febrianti A. Doxorubicin induced immune abnormalities and inflammatory responses via HMGB1, HIF-I-α and VEGF pathway in progressive of cardiovascular damage. Ann Med Surg (Lond). 2022 Mar 21;76:103501. DOI: https://doi.org/10.1016/j.amsu.2022.103501
- Adjemian, S., Branco, L. M., Zanetti, L. C., Weinlich, R., & Bortoluci, K. R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Frontiers in Immunology. 3028; 9. https://doi.org/10.3389/fimmu.2018.02379
- Taban, Q., Mumtaz, P.T., Masoodi, K.Z. et al. Scavenger receptors in host defense: from functional aspects to mode of action. Cell Commun Signal. 2022; 20:2. https://doi.org/10.1186/s12964-021-00812-0
- Wang Y, Weng H, Song JF, Deng YH, Li S, Liu HB. Activation of the HMGB1‑TLR-4‑NF‑κB pathway may occur in patients with atopic eczema. Mol Med Rep. 2017 Sep;16(3):2714-2720. doi: 10.3892/mmr.2017.6942.
- Granado-Serrano AB, Martín MÁ, Bravo L, Goya L, Ramos S. Quercetin attenuates TNF-induced inflammation in hepatic cells by inhibiting the NF-kB pathway. Nutrition and Cancer. 2012 ;64(4):588-598. DOI: 10.1080/01635581.2012.661513
- Chekalina N, Burmak Y, Petrov Y, Borisova Z, Manusha Y, Kazakov Y, Kaidashev I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018;70(5):593-597. doi: 10.1016/j.ihj.2018.04.006.
- Ruiz PA, Braune A, Hölzlwimmer G, Quintanilla-Fend L, Haller D. (2007). Quercetin inhibits TNF-induced NF-kappa B transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J Nutr. 137(5):1208-15. doi: 10.1093/jn/137.5.1208.
- Cui S, Wu Q, Wang J, Li M, Qian J, Li S. Quercetin inhibits LPS-induced macrophage migration by suppressing the iNOS/FAK/paxillin pathway and modulating the cytoskeleton. Cell Adh Migr. 2019 Dec;13(1):1-12. doi: 10.1080/19336918.2018.1486142.
- Pereira GS, Percebom I, Mendes S, Souza PSS, Diniz LFA, Costa MF. Quercetin inhibits neutrophil extracellular traps release and their cytotoxic effects on A549 cells, as well the release and enzymatic activity of elastase and myeloperoxidase. Braz J Biol [Internet]. 2024;84(Braz. J. Biol., 2024 84):e252936. https://doi.org/10.1590/1519-6984.252936
- Canton M, Sánchez-Rodríguez R, Spera I, Venegas FC, Favia M, Viola A, Castegna A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front Immunol. 2021 Sep 30;12:734229. doi: 10.3389/fimmu.2021.734229.
- Choy KW, Murugan D, Leong XF, Abas R, Alias A, Mustafa MR. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front Pharmacol. 2019;31(10):1295. doi: 10.3389/fphar.2019.01295.
- Pakadang SR, Ratnah S, Salasa AM, Hatta M. Toll Like Receptor 4 Expression Profile in Mice Infected Mycobacterium Tuberculosis Given with Miana Leaves Extract (Coleus scutellarioides (L.) Benth) (Tuberculosis Preventive and Curative Mechanisms). Pharmacog J. 2022;14(3):497-505. DOI: https://doi.org/10.5530/pj.2022.14.63
- Martins IJ. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Advances in Aging Research. 2016; 5:9-26. http://dx.doi.org/10.4236/aar.2016.51002
- Martins IJ. Nutrition Therapy Regulates Caffeine Metabolism with Relevance to NAFLD and Induction of Type 3 Diabetes. J Diabetes Metab Disord. 2017;4:019. DOI: 10.24966/DMD-201X/100019
- Martins IJ. Single Gene Inactivation with Implications to Diabetes and Multiple Organ Dysfunction Syndrome. J Clin Epigenet. 2017;3:24. doi: 10.21767/2472-1158.100058
- Martins IJ. Appetite Control and Biotherapy in the Management of Autoimmune Induced Global Chronic Diseases. Clin Immunol Res. 2018; 2(1): 1-4.
- Martins IJ. Bacterial Lipopolysaccharides and Neuron Toxicity in Neurodegenerative Diseases. Neurol Res Surg. 2018; 1(1): 1-3.
- Samec M, Liskova A, Koklesova L, Mersakova S, Strnadel J, Kajo K, Pec M, Zhai K, Smejkal K, Mirzaei S, Hushmandi K, Ashrafizadeh M, Saso L, Brockmueller A, Shakibaei M, Büsselberg D, Kubatka P. Flavonoids Targeting HIF-1: Implications on Cancer Metabolism. Cancers (Basel). 2021 Jan 3;13(1):130. doi: 10.3390/cancers13010130.
- Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules. 2022; 27(9):2901. https://doi.org/10.3390/molecules27092901
- Sethi G, Rath P, Chauhan A, Ranjan A, Choudhary R, Ramniwas S, Sak K, Aggarwal D, Rani I, Tuli HS. Apoptotic Mechanisms of Quercetin in Liver Cancer: Recent Trends and Advancements. Pharmaceutics. 2023; 15(2):712.https://doi.org/10.3390/pharmaceutics15020712
Abbreviation
DAMP = Damage-associated molecular pattern
HIF-I = Hypoxia-inducible factor-1
HMGB1 = High mobility group box protein 1
IL = Interleukin
TNF-α = Tumor Necrosis Factor alpha
MCP-1 = Monocyte Chemoattractant Protein-1
MMP = Matrix metalloproteinase
NF-kB = Nuclear factor kappa beta
NRAMP-1 = Natural resistance associated macrophage protein-1
PAMPs = Pathogen-associated molecular patterns
RAGE = Receptor for advanced glycation end-product
ROS = Reactive oxygen species
STAT = Signal transducer and activator of transcription
TLR-4 = Toll like receptor-4
TNF-α = Tumor Necrosis Factor Alpha
VEGF = Vascular endothelial growth factor








