Kamrun Nahar1 , Fatema-Tuz-Zohora2*
, Fatema-Tuz-Zohora2* , Rayhana Begum1
, Rayhana Begum1 , Maruf Hasan1
, Maruf Hasan1 , Abdul Aziz1
, Abdul Aziz1 , Yasmin Jui1, Muhammad Abdullah Al-Mansur 3
, Yasmin Jui1, Muhammad Abdullah Al-Mansur 3 and Md. Rafi Anwar4
and Md. Rafi Anwar4
1Department of Pharmacy, Primeasia University, Bangladesh. Star Tower, 12 Kemal Ataturk Ave, Dhaka.
2Department of Pharmacy, University of Asia Pacific, Bangladesh. 74/A Green Rd, Dhaka.
3Institute of National Analytical Research and Service (INARS), BCSIR; P9RP+375, Dr. Qudrat-E-Khuda Road Dhaka.
4University of Louisiana at Monroe, College of Pharmacy, Louisiana, USA.
Corresponding Author E-mail:fatema.zohora41@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2746
Abstract
The current study was undertaken to provide scientific validation for the traditional medicinal applications of Ceriops decandra leaves in treating gastrointestinal disorders and inflammation. Additionally, the study aimed to isolate a pure component from the extracted leaves for further analysis. Lupeol was extracted from the crude methanolic extract of Ceriops decandra leaves by column chromatography as part of a phytochemical inquiry. Its structure was determined using 1H and 13C NMR spectroscopy. In order to assess the cytotoxicity, the unrefined methanolic extract was divided into two fractions: a petroleum fraction and an aqueous fraction, employing the modified Kupchan method. The brine shrimp lethality test revealed that both the aqueous and petroleum ether fractions had significant cytotoxic activity, with LC50 values of 1.93 µg/l and 2.04 µg/l, respectively. These values were compared to the LC50 value of the standard Vincristine Sulphate, which was found to be 0.02 µg/l. The results of the anti-inflammatory trial demonstrated that the administration of the extract at doses of 250mg/kg and 500mg/kg resulted in protection rates of 62.5% and 87.5%, respectively, as compared to the carrageenan control group after 3 hours post-injection. It is worth noting that Ibuprofen exhibited a higher level of protection, with a rate of 91.7%. In the context of ethanol-induced stomach ulcer, the administration of extracts at doses of 250 mg/kg and 500 mg/kg resulted in 45.5% and 59.1% protection against gastric ulcer, respectively. These findings were compared to the protective effect of Omeprazole, which demonstrated 63.6% protection and served as the standard reference. The findings suggest that the methanolic leaf extract of Ceriops decandra possesses robust cytotoxic and potent anti-inflammatory and anti-ulcer properties. These results provide support for the traditional application of this extract in the management of gastrointestinal diseases, inflammation, and cancer.
Keywords
Anti-Inflammatory; Anti-Ulcer; Cytotoxicity; Isolation; LC50; NMR
Download this article as:| Copy the following to cite this article: Nahar K, Zohora F. T, Begum R, Hasan M, Aziz A, Jui Y, Al-Mansur M. A, Anwar M. R. Isolation and Evaluation of Cytotoxic, Anti-Inflammatory, Anti-Ulcer Activity of Methanolic Extract of Ceriops decandra leaves. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Nahar K, Zohora F. T, Begum R, Hasan M, Aziz A, Jui Y, Al-Mansur M. A, Anwar M. R. Isolation and Evaluation of Cytotoxic, Anti-Inflammatory, Anti-Ulcer Activity of Methanolic Extract of Ceriops decandra leaves. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3PxWxYd |
Introduction
Only 124 nations and regions have at least one natural mangrove species, with Asia having the most mangrove acreage. Mangrove is essential to the ecosystem by protecting the seashore and providing a habitat for marine and terrestrial organisms. Mangroves grow in the intertidal zone of extreme environmental conditions like inundation, anaerobic soil, strong wind, and high salinity1. Because of their extreme morphological and physiological adaptions, mangroves have a higher chance of producing enhanced bioactive compounds. Traditionally, it is used to treat gastrointestinal disorders, inflammation, cancer and infection2.
Ceriops decandra is a mangrove plant of the family Rhizophoraceae. It is found throughout tropical Asian countries like Bangladesh and Eastern Africa, Queensland and Madagascar. It is rich in phytochemicals such as protein, cumarin, phenols (catechin, procyanidins), triterpenoids (lupeol, α-amyrin, oleanolic acid, ursolic acid), flavonoids, saponins, glycosides, alkaloids diterpenoids (ceriopsin A-G), and tannins3.
Despite severe cardiovascular and gastrointestinal side effects, NSAIDs are widely used as analgesic and anti-inflammatory agents. COX 2 selective analgesics such as Celecoxib are safe for Gastro intestinal tract than a nonselective agent. The search for newer and safer analgesic and anti-inflammatory agents continues for this4. The lack of knowledge regarding Ceriops decandra methanolic extracts cytotoxic, anti-inflammatory, and anti-ulcer properties in relation to Bangladeshi species inspired this work. These findings were accompanied by histological analysis of the anti-inflammatory and anti-ulcer actions as well as chemical component separation.
Materials and method
Plant materials
The fresh leaves of Ceriops Decandra were collected from the Sundarbans mangrove forest in June-July. The plant’s identity was confirmed by the Bangladesh National Herbarium, where a voucher specimen was kept (Voucher no- DACB-85929).
Chemicals and drugs
Eskayef Bangladesh Limited provided the omeprazole and ibuprofen that were collected. Carrageenan was acquired from Sigma Aldrich compounds in Germany, and analytical-grade Petroleum ether, ethyl acetate, and other compounds were purchased from Merck in Germany.
Extraction of plant materials
2.5 kg of fresh C. decandra leaves were separated, shade-dried, and ground into a coarse powder. The coarse powder was then extracted with methanol using a Soxhlet apparatus at Jahangirnagar University and vacuum-dried.
Extraction and isolation from Ceriops decandra
For approximately six months, 40 g of crude methanolic extract were stored in a refrigerator at 3-5 °C. The substance underwent a natural separation process, resulting in the formation of two distinct layers: a lower layer characterized by its sticky and resinous properties, and an upper layer consisting of a solid component. In this study, the solid component was utilized to extract and separate Lupeol. The solid component was separated into a fraction that is soluble in petroleum ether using a separating funnel. The organic fraction, which is soluble in petroleum ether, was subsequently dried at room temperature, resulting in a yield of 9 grams.
The petroleum ether fraction was subjected to fractionation using silica column chromatography (CC) utilizing a gradient elution system consisting of petroleum ether, ethyl acetate, and methanol in varying proportions (ranging from 99:1 to 20:80, v/v). Therefore, the entirety of the dissolved fraction of petroleum ether (9 g) was partitioned into 75 fractions, each containing a solvent system of 20 ml.
The impure, colorless crystalline substance was formed through the process of evaporating the solvents (85:15-60:40, v/v) of fractions 22 to 31, which consisted of Petroleum ether and ethyl acetate. The production of compound 1 (30 mg, Rf=0.55) involved the repeated washing of crystal bodies with n-hexane (100:0, v/v) and subsequent recrystallization using a mixture of n-hexane and ethyl acetate (50:50, v/v). The resulting crystals were pure.
Modified Kupchan Partitioning Method
Crude methanolic extract (5gm) was fractionated using petroleum ether (100ml, 3 times). Again, the aqueous fraction from this extraction was fractionated using ethyl acetate. The ethyl acetate soluble fraction (4.8 gm) and aqueous fraction (1.75 gm) were then used for the brine shrimp cytotoxicity assay.
Brine shrimp lethality bioassay
The lethality test on brine shrimp was used to determine the plant extract’s cytotoxic activity. The concentrations of the dried extract preparations were serially diluted to range from 5 g/ml to 320 g/ml. Positive control was provided by the conventional medication vincristine sulphate (VS), whose concentrations ranged from 0.312 g/ml to 5 g/ml. The brine shrimp’s (Artemia salina) eggs were obtained from a neighborhood aquarium store in Dhaka, Bangladesh, and they were incubated in brine for 24 hours with a continual supply of oxygen. After 24 hours, the number of alive nauplii out of 10 were counted. These experiments were conducted on triplets. The lethal concentration (LC50) required to eliminate 50% of the nauplii was calculated using the application “Microsoft Excels 2007”9.
Anti-inflammatory Activity
Experimental Animal
This experiment was designed following the previously described method discussed by Begum et al. 5,6. Male Albino wistar rats weighing 120 and 150 g were collected from Jahangirnagar University’s pharmacy department. They were kept in groups of six rats in polypropylene cages, with a room temperature of 25 2 °C and a 12-hour light/dark cycle. The rats were given a week of acclimation before the studies, during which they had unrestricted access to free standard laboratory animal food and water. They went without eating the night before the test. All procedures used the anesthetic isoflurane (5% in 100% oxygen) to reduce preventable harm. The principles and guidelines outlined in the Guide for the Care and Use of Laboratory Animals served as the cornerstone for the treatment of animals and research procedures (NIH document no. 85-23; updated in 1985).
Induction of Inflammation in experimental animals
The reference standard utilized was ibuprofen (20 mg/kg), and the anti-inflammatory action was carried out as advised by Winter et al. by injecting carrageenan as an edematogenic agent on adult albino rats.
The following five groups, each with six rats, were formed:
|
Group |
Description |
|
Group I |
oral administration of 0.5ml distilled water |
|
Group II |
oral administration of 0.5ml distilled water and 0.1ml of Carageenan injection (Carageenan control, CC) |
|
Group III |
Oral administration of Ibuprofen (20 mg/kg) and 0.1ml of Carageenan injection (IP 20) |
|
Group IV |
250 mg/kg methanolic extract of C.decendra and 0.1ml of Carageenan injection (MECD-250) |
|
Group V |
500 mg/kg methanolic extract of C.decendra and 0.1ml of Carageenan injection (MECD-500) |
The extracts were given orally to the treatment group as a suspension containing 2 to 3 drops of tween 80 at 250 and 500 mg/kg doses, respectively. Thirty minutes before injecting carrageenan (0.1 ml of 1% w/v solution of distilled water) (0.5 ml), all test samples were administered orally. Carrageenan was injected into the sub-planter area of the left paw of each rat. However, the normal control group received no carrageenan. Slide callipers were used to gauge and record the paw swelling every hour. The percentage inhibition of paw edema was calculated after three hours.
The formula for the percentage of inhibition in control and the increase in paw thickness is as follows:
Increase of paw thickness in control or treatment group = PC or PT= Pt – Po
Percentage of inhibition in paw thickness in the treatment group

Where, Pt = paw thickness at time t,
Po = initial paw thickness,
PC = Increase in thickness of paw of the control group and
PT = Increase in thickness of paw of the treatment group.
Anti-ulcer activity
Induction of Ulcer
This experiment was performed following Begum et al. 6,7. Five groups of animals were not allowed to access food for a day, other than water, for two hours before the test. The rats were kept apart in their cages to avoid coprophagy throughout the fasting phase. Gastric ulcers were induced in the test sample (MECD at doses of 250 and 500 mg/kg p.o.) and standard (omeprazole at doses of 20 mg/kg, p.o.) using ethanol-acid after 30 minutes of pretreatment. Under isoflurane, 5% in 100% oxygen anesthesia, these rats were euthanized 90 minutes later, and their stomachs were immediately removed. The stomachs were sliced open along the larger curvature, removed, cleaned with distilled water, and stored in 10% buffered formalin. The gastrointestinal mucosa was examined under a microscope for ulcers, and the ulcers were graded as follows:
|
Tissue architecture |
Ulcer score |
|
Normal stomach |
0 |
|
Red coloration |
0.5 |
|
Spot ulcer |
1 |
|
Hemorrhagic streak |
1.5 |
|
Ulcers |
2.0 |
|
Perforation |
3.0 |
The average ulcer score for each animal is represented by the ulcer index. The percentage of ulcer prevention was calculated using the formula below:

The animals were grouped into five groups, each with six rats:
|
Group |
Description |
|
Group I |
Oral administration of 5 ml/kg/day distilled water (Normal Control) |
|
Group II |
Oral administration of 5 ml/kg/day distilled water and 25 ml per kg of 0.3M HCl in 60 % ethanol (Negative control) |
|
Group III |
Oral administration of 20 mg/kg/day omeprazole and 25 ml per kg of 0.3M HCl in 60 % ethanol |
|
Group IV |
Oral administration of 250 mg/kg methanolic extract of C.decendra 25 ml per kg of 0.3M HCL in 60 % ethanol |
|
Group V |
Oral administration of 500 mg/kg methanolic extract of C.decendra 25 ml per kg of 0.3M HCL in 60 % ethanol |
Histological investigation
Carrageenan-induced paw tissues were collected for this study 3 hours after the induction and kept in freshly made 10% neutral buffered formalin for at least 24 hours. The specimens for the anti-ulcer study were kept in a 10% natural buffer. Following that, tissue slices were paraffin-embedded. Using a microtome, the samples were cut into cross-sections stained with hematoxylin and eosin (H and E) and mounted on Canada balsam. Each segment was inspected under a light microscope, and any histopathological alterations, such as edema, erosion, infiltration, and inflammation, were photographed using an Olympus photomicroscope.
Statistical Analysis
The values are tabulated as mean ± S.E.M, and statistical significance between treated and control groups was analyzed using One-way ANOVA, where P<0.05 was considered statistically significant.
Results
Characterization of isolated compounds
Compound 1 is a pentacyclic triterpene. It was a white powder. Seven tertiary methyl singlets and one secondary hydroxyl group were seen in the 1H NMR spectra. Additionally, it displayed olefinic protons at 4.71 and 4.59. The compound’s 1H NMR spectra showed signals at δ 4.71 and δ 4.59, both of which were focused on the furan exomethylene group, as one proton doublets with 2.4 Hz coupling constants and one proton double quartets with 1.2 Hz and 2.4 Hz coupling constants, respectively. A secondary carbinol group may be given to the signals at δ 3.21 that appeared as a doublet of doublets with coupling constants of 11.2 Hz and 5.2 Hz. A wide doublet at 1.66 with a coupling constant of 1.2 Hz was also visible in the spectrum and was attributed to a methine group. Six tertiary methyl singlets could be seen in the resonance signals at 0.79, 0.81, 0.85, 0.97, 0.99, and 1.05. These results closely matched those that had previously been published for a common pentacyclic triterpenoid called lupeol7.
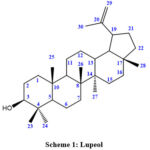 |
Scheme 1: Lupeol |
13C NMR value: (δ 178.2, C-28), (δ 151.6, C-20), (δ 126.0, C-29), (δ 178.2, C-28), (δ 79.1, C-3), (δ 55.8, C-17), (δ 56.1, C-5), (δ 51.1, C-9), (δ 50.0, C-19), (δ 46.8, C-18), (δ 41.8, C-14), (δ 41.0, C-24), (δ 40.0, C-4), (δ 40.1, C-1), (δ 37.9, C-13), (δ 38.0, C-10), (δ 37.5, C-22), (δ 178.2, C-28), (δ 34.8, C-7), (δ 32.5, C-16), (δ 31.0, C-15), (δ 29.9, C-21), (δ 28.1, C-23), (δ 28.4, C-2), (δ 26.0, C-12), (δ 21.0, C-11), (δ 19.0, C-30), (δ 18.7, C-6), (δ 16.0, C-25), (δ 15.2, C-27)8.
Brine Shrimp Cytotoxicity
The effect of methanolic extract of Ceriops decandra as well as Vincristine sulphate on brine shrimp nauplii has been tabulated and graphically represented.
Table 1: Effect of aqueous and petroleum ether fraction of Ceriops decandra as well as Vincristine sulphate on brine shrimp nauplii
|
Ceriops decandra leaf extract |
Vincristine Sulphate |
|||||
|
Concentration, C (µg/ml) |
Log C |
% mortality (aqueous fraction) |
% mortality (Petroleum ether fraction) |
Concentration, CV (µg/ml) |
Log CV |
% mortality |
|
5 |
0.693 |
25 |
5 |
0.312 |
-0.505 |
10 |
|
10 |
1 |
30 |
15 |
0.625 |
-0.204 |
30 |
|
20 |
1.301 |
35 |
20 |
1.25 |
0.096 |
60 |
|
40 |
1.602 |
40 |
30 |
2.50 |
0.397 |
80 |
|
80 |
1.903 |
50 |
45 |
5.0 |
0.698 |
100 |
|
160 |
2.204 |
55 |
55 |
|
|
|
|
320 |
2.505 |
65 |
70 |
|
|
|
Table 2: LD50 Value of extracts Ceriops decandra aqueous and petroleum ether part as well as vincristine sulphate
|
Sample |
LC50 (μg/ml) |
Regression line equation |
R2 |
|
Ceripos decendra (Aqueous fraction) |
1.93 |
y = 21.899x + 7.7936 |
0.9831 |
|
Ceripos decendra (petroleum ether fraction) |
2.04 |
y = 35.513x – 22.575 |
0.9822 |
|
Vincristine sulphate |
0.02 |
y = 76.485x + 48.627 |
0.9934 |
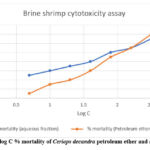 |
Figure 1: Plot of log C % mortality of Ceriops decandra petroleum ether and aqueous fraction. |
Anti-inflammatory activity of Ceriops decandra
In this study, animals given methanolic extract (250 and 500 mg/kg, p.o.) and ibuprofen (20 mg/kg, p.o.) for 1 to 3 hours showed considerably reduced paw thickness and redness than the carrageenan control group. After the investigation, it was found that when compared to the carrageenan control group at 3 hours after carrageenan injection, oral administration of methanolic extract of 250mg/kg and 500mg/kg reduced paw edema by 62.5% and 87.5%, respectively.
Table 3: Anti-inflammatory effect of Ceriops decandra methanolic extract on the carrageenan induced changes in paw thickness in experimental rats
|
Groups Dose mg/kg |
NC
|
CC
|
IP-20 (20mg/kg Ibuprofen) |
MECD-250 (250mg/kg extract) |
MECD-500 (500mg/kg extract) |
|
Initial paw thickness (cm) |
0.48±0.004 |
0.49±0.005c |
0.46±0.003b** |
0.49±0.003b |
0.48±0.004c |
|
Paw thickness (cm) after 1h |
0.48±0.004 |
0.67±0.004a** |
0.57±0.006b** |
0.64±0.004b |
0.53±0.007b** |
|
Paw thickness (cm) after 2h |
0.48±0.002 |
0.70±0.007a** |
0.54±0.003b** |
0.61±0.007b* |
0.52±0.004 b** |
|
Paw thickness (cm) after 3h |
0.48±0.003 |
0.73±0.004a** |
0.48±0.003b** |
0.58±0.003b** |
0.51±0.004 b** |
Each value is presented as Mean SEM (n=6). NC stands for “Healthy Control,” CC stands for “Carrageenan Control,” IP-20 stands for “Ibuprofen 20 mg/kg/day,” MECD-250 stands for “Methanolic Extract of C. Decendra 250 mg/kg,” and MECD-500 stands for “Methanolic Extract of C. Decendra 500 mg/kg.” Dunnett’s multiple comparison test (*p 0.05 and **p0.01) is used after one way analysis of variance to show statistically significant differences from the respective group. uses one-way analysis of variance to demonstrate that there is no statistically significant difference from the corresponding group, followed by Dunnett’s multiple comparison test (p > 0.05). when contrasted with the control (carrageenan) and the control (normal).
Effect of methanolic extract of Ceriops decendra on the carrageenan induced paw edema
In the healthy control (2a) there was no inflammation or erythema observe. However, in Carrageenan control (2b) significant erythema and edema were observed. In case of Ibuprofen 20 mg/kg (2c), minor erythema and edema is seen. The paw of C. decendra 250 mg/kg (2d) methanolic extract treated rat shows mild erythema and edema and 500 mg/kg methanolic extract of C. decendra (2e) treated rat shows comparatively less edema and erythema.
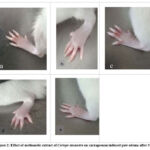 |
Figure 2: Effect of methanolic extract of Ceriops decandra on carrageenan induced paw edema after 3 h. |
Histopathological Evaluations of Paw Tissue
When paw tissue was examined histopathologically, it can be deduced that tissues from normal controls (3a) have few leukocytes. In contrast, tissues from carrageenan-induced rat paws (3b) have a large number of inflammatory cells. On the other hand, tissues from rat paws treated with the standard drug Ibuprofen (3c) and C. decandra methanolic extract at dosages of 250 (3d) and 500 mg/kg (3e) exhibit only a moderate to low level of inflammatory cell infiltration.
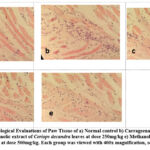 |
Figure 3: Histopathological Evaluations of Paw Tissue of a) Normal control b) Carrageenan control c) 20mg/kg Ibuprofen d) Methanolic extract of Ceriops decandra leaves at dose 250mg/kg |
Ethanol-Induced Gastric Ulcer
After dissecting the stomachs in the greater curvature, the ethanol-treated rat tissues (4b) have thick, dark red and black lesions in the glandular portion. As compared to normal control (4a), Omeprazole (20mg/kg) (4c), Methanolic extract at 250mg/kg (4d) and 500 mg/kg (4e) treated rat tissues have much better ulcer protection with a significant protection index of 63.6 %, 45.5% and 59.1% respectively.
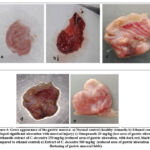 |
Figure 4: Gross appearance of the gastric mucosa: a) Normal control (healthy stomach) b) Ethanol control (developed significant ulceration with mucosal injury) c) Omeprazole 20 mg/kg (less area of gastric ulceration) |
Histological Evaluation of Gastric Lesions
Figure 5a shows a section of normal control which only received distilled water. On the contrary, the ethanol control group 5b shows ulceration with broken mucosal barrier and migration of numerous inflammatory cells. However, omeprazole (20 mg/kg) (5c) administration changed histopathology significantly, with minimal hemorrhage presence and intact mucosal lining. Methanolic extract of C. decendra at 250 mg/kg (5d) showed moderate regeneration of gastric mucosa, while 500 mg/kg (5e) showed significant regeneration.
 |
Figure 5: Histological Evaluation of Gastric Lesions a) Normal control, b) Ethanol control, c) Omeprazole 20 mg/kg, d) Methanolic extract of C. decendra (250 mg/kg), e) Extract of C. decendra (500 mg/kg). |
Discussion
Based on the 1H and 13C NMR values for each proton and carbon that were determined and provided in the results and discussions lupeol skeleton for compound 1, the lack of a hydroxyl group, and the appearance of a carbonyl group in the 13C NMR spectrum data of 1. As a result, structure 1 was determined to be that of the well-known chemical lupeol, which was supported by the physical and spectral information in the literature9.
In this work, a methanolic extract of Ceriops decandra showed a dose-dependent anti-inflammatory activity in paw edema caused by carrageenan. The outcome is nearly as significant as the standard drug Ibuprofen. The suppression of mediators such as prostaglandins, leukotrienes, histamine, serotonin, and bradykinins, which cause pain and fever, may cause anti-inflammatory action10.
The histological analysis of the hind paw tissue in our study showed that the methanolic extract of Ceriops decandra reduced the massive influx and buildup of inflammatory cells in the paw tissue after carrageenan induction in a dose-dependent manner. TNF-a is a crucial element of inflammation. Among other things, it can trigger the release of pro-inflammatory cytokines and the activation and chemotaxis of leukocytes. Leukocyte influx-induced inflammation can be avoided by reducing the release of mediators such MDA, CRP, NO, MPO, and pro-inflammatory cytokines (TNF-a and IL-1b)11.
Since ethanol may permeate the stomach mucosa, the mucosal barrier will break down, increasing the permeability of sodium and water and causing calcium ions to accumulate. Additionally, it increases the production of superoxide ions and hydroperoxyl free radicals, which results in stomach mucosal ulcers10. The stomach cryoprotection through the anti-oxidation mechanism and the decreased release of gastric acid and prostaglandins may all contribute to the anti-ulcer effects of the methanolic extract of Ceriops decandra11. The anti-ulcer effect was dose-dependent, which is almost identical to the standard drug omeprazole. The considerable decrease in the intracellular antioxidant Glutathione (NP-SH/GSH) may also be to blame for this result. Lupeol, a compound derived from the leaves of Ceriops decandra, has demonstrated free radical scavenging abilities that are on par with misoprostol12.
As a functional, living food supply for marine ecology, artemia is regarded as one of the most critical marine ecotoxicity testing organisms13. Cytotoxicity and cell death are also brought on by certain polyphenols’ nonspecific attachment to the membrane14,15,16,17,18,19.
Conclusion
The results of our study revealed that different fractions of the methanolic extract from Ceriops decandra possess cytotoxic properties. These findings suggest the need for further investigation utilizing human cell lines, both in vivo and in vitro. The utilization of this plant for the production of novel anti-cancer medications involves the identification and isolation of its primary cytotoxic compound.
Hence, more investigation is warranted to ascertain the specific phytoconstituents and underlying processes that account for the observed activities as indicated by the findings.
Conflicts of Interest
There is no conflict of interest.
Funding sources
This study was partly funded by Primeasia University, Banani, Dhaka and the Institute of National Analytical Research and Services (INARS), BCSIR.
Acknowledgement
Authors are grateful to the Department of Pharmacy, Primeasia University, Dhaka and the Institute of National Analytical Research and Services (INARS), BCSIR.
References
- Ruang-areerate, Panthita, Thippawan Yoocha, Wasitthee Kongkachana, Phakamas Phetchawang, Chatree Maknual, Wijarn Meepol, Darunee Jiumjamrassil, Wirulda Pootakham, and Sithichoke Tangphatsornruang. 2022. “Comparative Analysis and Phylogenetic Relationships of Ceriops Species (Rhizophoraceae) and Avicennia lanata (Acanthaceae): Insight into the Chloroplast Genome Evolution between Middle and Seaward Zones of Mangrove Forests” Biology 11, no. 3: 383.
CrossRef - Nabeel, Mannalamkunnath Alikunhi, Kandasamy Kathiresan, and Subramanian Manivannan. “Antidiabetic activity of the mangrove species Ceriops decandra in alloxan‐induced diabetic rats.” Journal of Diabetes 2, no. 2 (2010): 97-103.
CrossRef - Mahmud, Imran, Naznin Shahria, Sabina Yeasmin, Asif Iqbal, Emdadul Hasan Mukul, Sudipta Gain, Jamil Ahmad Shilpi, and Md. Khirul Islam. “Ethnomedicinal, Phytochemical and Pharmacological Profile of a Mangrove Plant Ceriops Decandra Griffdin Hou.” Journal of Complementary and Integrative Medicine 16, no. 1 (2018). https://doi.org/10.1515/jcim-2017-0129.
CrossRef - John A Crairns. “The coxibs and traditional nonsteroidal anti-inflammatory drugs: A current perspective on cardiovascular risks.” Canadian Journal of Cardiology 13, no. 2 (2007). https://www.onlinecjc.ca/article/S0828-282X(07)70732-8/pdf
- Mostofa, R., Ahmed, S., Begum, M.M. et al. “Evaluation of anti-inflammatory and gastric anti-ulcer activity of Phyllanthus niruri L. (Euphorbiaceae) leaves in experimental rats.” BMC Complement Altern Med 17, 267 (2017). https://doi.org/10.1186/s12906-017-1771-7
CrossRef - Akter, Saleha, Taslima Begum, Rayhana Begum, Tamanna Sharmin Tonny, Mousumi Yasmin, S. Shifa, and F. Afroze. “Phytochemical analysis and investigation of anti-inflammatory and anti-ulcer activity of Terminalia bellirica leaves extract.” Int J Pharmacogn 6 (2019): 54-65
- Gandagule, Upendra B., B. Duraiswamy, Mayur R. Bhurat, and Sanjay A. Nagdev. “Isolation and Characterization of Lupeol a Triterpenoid from Stem Bark of Ziziphus xylopyrus (Retz) Willd.” Inventi rapid: Pharm Analysis & Quality Assurance 4 (2018): 1Ǧ7. https://doi.org/10.1021/acsomega.1c05647
CrossRef - Fatema Tuz-Zohora, Md. Abdul Muhit, Hasan CM, Ahsan M. Quinolone Alkaloids Along with Other Constituents from Zanthoxylum rhetsa and their Chemotaxonomic Significance. Records of Natural Products. 2018;12(6):634-637. doi:https://doi.org/10.25135/rnp.57.17.11.182
CrossRef - Rayhana Begum, Manjur Ali Sheliya, Showkat R. Mir, Ekta Singh, Manju Sharma, “Inhibition of proinflammatory mediators by coumaroyl lupendioic acid, a new lupane-type triterpene from Careya arborea, on inflammation-induced animal model”, Journal of Ethnopharmacology, 206, 376-392 (2017) https://doi.org/10.1016/j. jep.2017.05.014.
CrossRef - Sumsunnahar Shifa, Taslima Begum, Faruquzzaman, Farzana Afroze and Mst Kaisarunnahar Shraboni. “Antibacterial activity and cytotoxic activity of leaves extract of Carissa carandas Linn.” Journal of Pharmacognosy and Phytochemistry 8, 4 (2019). https://www. researchgate.net/publication/361475887_Preliminary_phytochemical_screening_antibacterial_activity_and_cytotoxic_activity_of_leaves_extract_of_Carissa_carandas_Linn
- Ayertey, Frederick, Ebenezer Ofori-Attah, Stephen Antwi, Michael Amoa-Bosompem, Georgina Djameh, Nathaniel Lartey Lartey, Mistuko Ohashi et al. “Anti-inflammatory activity and mechanism of action of ethanolic leaf extract of Morinda lucida Benth.” Journal of traditional and complementary medicine 11, no. 3 (2021): 249-258. https://doi.org/10.1016/j.jtcme.2020.07.001
CrossRef - Begum, Rayhana, Manju Sharma, K. K. Pillai, Vidhu Aeri, and Manjur Ali Sheliya. “Inhibitory effect of Careya arborea on inflammatory biomarkers in carrageenan-induced inflammation.” Pharmaceutical biology 53, no. 3 (2015): 437-445.
CrossRef - Sahoo, Saroj Kumar, Himanshu Bhusan Sahoo, D. Priyadarshini, G. Soundarya, Ch Kishore Kumar, and K. Usha Rani. “Antiulcer activity of ethanolic extract of Salvadora indica (W.) leaves on albino rats.” Journal of clinical and diagnostic research: JCDR 10, no. 9 (2016): FF07. doi: 10.7860/JCDR/2016/20384.8470
CrossRef - de S Lira, Silvéria Regina, Vietla Satyanarayana Rao, Ana Carla S. Carvalho, Marjorie M. Guedes, Talita C. de Morais, Antonia L. de Souza, Maria Teresa S. Trevisan, Alana F. Lima, Mariana H. Chaves, and Flávia A. Santos. “Gastroprotective effect of lupeol on ethanol-induced gastric damage and the underlying mechanism.” Inflammopharmacology 17, no. 4 (2009): 221-228.
CrossRef - Arulvasu, Chinnasamy, Samou Michael Jennifer, Durai Prabhu, and Devakumar Chandhirasekar. “Toxicity effect of silver nanoparticles in brine shrimp Artemia.” The Scientific World Journal 2014 (2014)x.
CrossRef - Lim DY, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–G75. doi: 10.1152/ajpgi.00248.2006
CrossRef - Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z.
CrossRef - Dey P, Kundu A, Chakraborty HJ, Kar B, Choi WS, Lee BM, et al. Therapeutic value of steroidal alkaloids in cancer: current trends and future perspectives. Int J Cancer. 2019;145:1731–1744. doi: 10.1002/ijc.31965.
CrossRef - Hossain SJ, Tsujiyama I, Takasugi M, Islam MA, Biswas RS, Aoshima H. Total phenolic content, antioxidative, anti-amylase, anti-glucosidase, and antihistamine release activities of Bangladeshi fruits. Food Sci Technol Res. 2008;14:261–268. doi: 10.3136/fstr.14.261.
CrossRef








