Fitim Alidema1 , Arieta Hasani Alidema1
, Arieta Hasani Alidema1 , Albina Fejza1
, Albina Fejza1 and Albulena Jashari Selmani2
and Albulena Jashari Selmani2
1UBT – Highger Institution – Fakulteti Farmacisë, Prishtinë, Kosovë.
2Regional Hospital in Ferizaj- Prishtinë, Kosovo
Corresponding Author E-mail:arieta.hasani@ubt-uni.net
DOI : https://dx.doi.org/10.13005/bpj/2763
Abstract
Introduction: Micronucleus test (MN) in buccal cells of the mouth presents a new road on the way of studying the degenerative changes directly in the targeted organs which were impacted by the tumoral diseases. Aim of the study: This paper aims to analyze the incidence of the test micronucleus in the epithelial cells of the buccal mucosa of the oral cavity in male patients with breast carcinoma after a cycle of chemotherapy. Material and Methods: In the study, 40 male patients with breast cancer with an average age of 45.8±15.5 years were taken for analysis. From the measured parameters, we analyzed the incidence of degenerative changes and the effect of chemotherapy, after one cycle of chemotherapy. Results: The average number of deteriorative modifications in the exfoliative cells deriving from the buccal mucosa of breast cancer patients, increased significantly after a single cycle of chemotherapy with 15.5±22.4 being the number of degenerated cells before treatments and 27.5±30.1 after the first cycle of chemotherapy, p<0.0002. A significant increase was noticed in all types of nuclear degenerations. The incidence of acute nuclear changes as well as the total number of these modifications was high in males diagnosed with breast carcinoma Conclusion: The results show that cytostatic drugs induced cellular cytotoxicity. However, it did not significantly change chromosomal changes or the micronuclei formation.
Keywords
Acute Nucleus Changes; Breast Carcinoma; Chemotherapy; Incidence
Download this article as:| Copy the following to cite this article: Alidema F, Alidema A. H, Fejza A, Selmani A. J. Degenerative Changes of Buccal Cells After One Cycle of Chemotherapy on Male Breast Cancer Patients. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Alidema F, Alidema A. H, Fejza A, Selmani A. J. Degenerative Changes of Buccal Cells After One Cycle of Chemotherapy on Male Breast Cancer Patients. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3LJLY3o |
Introduction
Micronuclei originate from chromosomes’ fragments and/or entire chromosomes that failed to be incorporated into the nucleus of the cell in the nuclear division process. Analysis of the micronucleus (MN) in the exfoliative cells taken from human, could potentially serve as a great tool to study the modifications of the genome directly in the tumor-affected target organs 1.
In some research, a significant correlation has been shown between the chromosomal aberration’s levels in lymphocytes and MN in exfoliative cells of the buccal mucosa in patients exposed to environmental mutagens 2.
There are different ways by which genome damage can be caused, such as by environmental genotoxins (e.g., radiation and chemical substances), by micronutrient deficiencies (e.g., folates), by life habits (e.g., alcohol, tobacco, drugs, stress, etc.) and lastly by genetic factors which include inherited defects in metabolism and DNA repair 3. Considering these facts, it is assumed that the epithelial cells of the oral mucosa represent one preferred site for the early appearance of genotoxic modifications induced by carcinogenic agents that are introduced in the body either through the inhaling or by ingestion 3.
Thus, in this study we have analyzed the degree of change of the nuclei of exfoliative epithelial in the buccal mucosa of the oral cavity in patients diagnosed with breast cancer and who received chemotherapy treatment.
Material and Methods
Sample collection was done in the Clinical Center of Kosova and a total of 40 male patients were enrolled in the study. Samples were collected before and after the first cycle of chemotherapy, thus the inclusion criteria consisted on patients from the time of diagnosis and after the first chemotherapy. Male breast cancer patients who underwent more than one cycle of chemotherapy at the time of the study were excluded. All the study procedures were approved by the Institutional Ethic Committee of the Oncology Department in the Clinical Center of Kosova.
All the samples were taken the same way with a cytobrush. After the collection of the epithelial cells from the buccal cavity of the mouth, we added 5 ml of physiological solution NaCl 9% to the test tubes. The next step was centrifuging the samples, for 10 minutes at the 1200 rpm in a multiple purpose benchtop centrifuge by Eppendorf. After that the supernatant was carefully removed, leaving 1 ml together with the precipitate. Next, we carefully mixed the precipitate with the solution left on the tube. Then, we took a small quantity of the mixture and spread on the surface of three glasses. After that, the samples were dried for 2-3 hours at room temperature. The next step included the adding of ethanol 96% and after the complete drying of the sample we placed the glasses in Giemsa staining solution from CellaVision (Sweden) diluted 1:5 for a period of 1 hour. Next, after the coloring step, we rinsed with distilled water the glasses and dried. The samples were examined under the optical microscope.
Results are presented in the tabular and graphic form. All statistical analyses were conducted using SPSS 22 software platform and the significant changes were shown. Changes values being P<0.05 were considered significant.
Results
Demographic presentation of patients
As a starting point, demographic data from all the male patients diagnosed with breast carcinomas, involved in the study were analyzed (Table 1). The average age of our patients was 45.8±15.5 years. Interestingly, looking up at the history of the patients, we have observed that a vast majority of the patients were tobacco users while a small percentage declared alcohol consumption, 54,5% and 5,5% respectively (Figure 1A, B).
Table 1: Demographic data of patients with breast cancer treated with chemotherapy
|
Sex |
Number |
% |
|
Male |
40 patients |
100% |
|
Age |
Number |
% |
|
45-61 years old |
5 patients |
13.% |
|
61-80 years old |
35 patients |
87 % |
|
Age, mean (SD). |
45.8 patients |
15.5 |
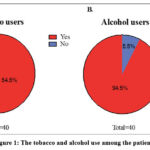 |
Figure 1: The tobacco and alcohol use among the patients. |
Figure 1A shows that a percentage of 54.5% corresponds to regular tobacco users while 1B only 5.5% of the patients declared of using alcohol.
Changes on epithelial cells of the oral mucosa of the patients
The average number of degenerative modifications in the exfoliative epithelial cells taken from breast cancer patients’ buccal mucosa, was shown to be significantly increased after a single chemotherapy. We have examined these types of changes; Micronuclei (MN), Pycnotic Nuclei (Pyk), Karyorrhectic cells (KR), Karyolysis (KL), Binuclear cells (BN) and the total changes before and after the first cycle of chemotherapy treatment. With the MN test we have in fact not found striking differences in Micronuclei cells specifically, but we have noticed differences in other degenerative modifications such as pycnotic nuclei (Pyk) cells with mean of 5.5±7.7 before the treatment and 11.5±13.5 after the treatment and the differences were p<0.00001. The differences in karyorrhectic cells, karyolysis and binuclear cells were also found significant between the samples taken before and after the first chemotherapy cycle with p<0.0001, p<0.0003, p<0.00002, respectively, The mean of total changes before the treatment were 15.5±22.4, while the changes after the first cycle of chemotherapy was 27.9±30.1 with a significant change of p<0.0002. Table 2 summarizes the significant increase for all types of the nuclear change. The numbers represent the manually counting of the degenerated cells per sample.
Moreover, we were curious to see if age if a factor in having a higher number of degenerative cells, thus we have checked the differences in the degenerative modifications in the exfoliative epithelial cells in patients, comparing two groups of patients with differences in age. At this point, we have observed that a higher, despite not significant, total degenerative changes occurred in older patients (group age from 61 to 80 years old) rather than younger patients (group age from 45 to 61 years old), with differences being 13.24 and 8.20 respectively. Confirming that age is not a factor in changes of the cells independently. Data for all type of nuclear changes represented accordingly to the age are shown in Table 3.
Table 2: Mean degenerative modifications in the exfoliative epithelial cells of the male breast cancer patients’ buccal mucosa (n=40).
|
Type of degenerative changes |
Base line |
After the treatment |
Difference |
P |
||
|
|
Mean |
Sd |
Mean |
Sd |
Mean |
|
|
Micronuclei (MN) |
0.2 |
0.0 |
0.0 |
0.0 |
-0.1 |
ns |
|
Pycnotic Nuclei (Pyk) |
5.5 |
7.7 |
11.5 |
13.5 |
9.0 |
0.00001* |
|
Karyorrhectic cells (KR) |
6.9 |
8.5 |
17.7 |
16.5 |
15.6 |
0.0001* |
|
Karyolysis (KL) |
51.9 |
31.6 |
65.8 |
46.9 |
18.9 |
0.0003* |
|
Binuclear cells (BN) |
5.4 |
4.6 |
11.9 |
6.6 |
8.4 |
0.00002* |
|
Changes in total |
15.5 |
29.1 |
27.9 |
34.4 |
10.2 |
0.0001* |
In the table are shown the mean and standard deviation number of micronuclei cells (MN), pycnotic nuclei cells (Pyk), karyorrhectic cells (KR), cells that underwent karyolysis (KL), binuclear cells (BN) and the changes in total of the cells. The mean and standard deviation is shown for the samples taken before the treatment, (base line) first column and after the first treatment (after treatment) second column. Third column represents the differences of the above-mentioned changes before and after treatment. The last column shows the p values obtained the Student’s t-test, with ns presenting1 ‘not significant’ and (*) being p<0.05.
Table 3: Differences of degenerative modifications in exfoliative epithelial cells of buccal mucosa according to the age.
|
Type of degenerative changes |
Age 45-61 |
Age 61-80 |
|
|
|
|
Differences Mean |
Differences Mean |
T-test |
p |
|
Micronuclei (MN) |
0.10 |
0.00 |
— |
NT |
|
Pycnotic Nuclei (Pyk) |
11.00 |
10.40 |
1.0 |
0.36 |
|
Karyorrhectic cells (KR) |
16.59 |
17.00 |
0.64 |
0.51 |
|
Karyolysis (KL) |
18.29 |
24.00 |
0.6 |
0.55 |
|
Binuclear cells (BN) |
9.36 |
8.00 |
2.6 |
0.12 |
|
Changes in total |
8.20 |
13.24 |
0.6 |
0.53 |
The table represents the mean number of micronuclei cells (MN), pycnotic nuclei cells (Pyk), karyorrhectic cells (KR), cells that underwent karyolysis (KL), binuclear cells (BN) and the changes in total of the cells according to the age of the patients divided in two groups. The last column shows the p values obtained the Student’s t-test.
Discussion
Micronuclei are fragments of chromosomes or entire chromosomes, which failed to reach the poles of the division axis during mitosis, and remain encapsulated as a separate nucleus during the process of telophase. The chromosome losses or the dysfunction of the mitotic spindle caused by aneugenic mechanisms, could be detected if approached with micronucleus testing 4.
The oral cavity epithelium undergoes constant regeneration through the continuous production of new cells in the basal layer by mitosis and the migration of these new cells to the surface will replace the old ones. However, the basal layer contains stem cells which during nuclear division, can express genetic damages (breakage or loss of chromosomes) such as MN. These newly produced cells may/or may not contain MNs, eventually differentiated into the spiny cell layer. Moreover, some of these cells can slip into cells with fragmented nuclei (karyorrhectic cells), pyknotic nuclei, condensed chromatin, or even karyolysis which is the complete loss of the nuclear material 5. Sometimes, though rarely, cells may become stuck at the binuclear stage or may show bud-shaped nuclei, which is known as the so-called “broken eggs” in buccal mucosal cells, as a gene amplification biomarker. These biomarkers could be detected both in lymphocytes and in buccal cells, which represent a broader assessment of genome damage than MN alone, in the context of cytotoxicity and cytostatic effects 6.
Casartelli et al. analyzed the frequency of MN in buccal exfoliative cells in normal mucosa, in precancerous lesions and in squamous cell carcinoma 7. They concluded that the MN levels correspond to the progression of the neoplastic disease since they have shown a gradual increase of this biomarker in the steps starting normal mucosa to precancerous lesions and finally to carcinoma. Published biomonitoring studies with evaluated MN in the buccal mucosa have analyzed the effect of many factors, including the influence of environmental and workplace exposure, chemoprevention, radiotherapy, the influence of life habits, the influence of tumors and other diseases 8.
There are some types of cells which contain two nuclei within, and these types of cells are called binuclear. These types of cells are often seen in cancer and they can be caused by different factors. If during cell division the cell division groove begins to regress then the cell joins again, causing the chromosomes not to separate 9. These cells can also appear during the failure of cytokinesis, in which case the cell division loop is not formed at all, thus causing the remaining of both nuclei in one single cell. Pyknosis or karyopyknosis represents the irreversible condensation of chromatin in the nucleus of cells undergoing necrosis. This is followed by karyorrhexis or fragmentation of the nucleus 10. Karyorrhexis is the destructive fragmentation of the nucleus of the dying cell, in which the chromatin is distributed irregularly throughout the cytoplasm. This is usually followed by karyolysis 11. The last, represents the complete dissolution of chromatin by the activity of the enzyme DNase in the cells that are dying. In apoptosis, after karyorrhexis the nucleus is usually dissolved into parts called the apoptotic bodies 12.
Exposure of cells to cytotoxic substances can result in different outcomes for those cells. Cells can undergo necrosis, during which they lose their cell membrane integrity and die rapidly as a result of cell lysis. Cells can stop their active growth and division (decreased cell viability), or they can activate the genetic program that controls cell death (apoptosis). Interestingly, some cells unergo rapid necrosis, which means that there is not enough time for them to activate apoptotic mechanisms, therefore, they do not show markers of apoptosis 13.
Breast cancer is the most commonly diagnosed cancer in women worldwide, in 2008 alone there were 1.38 million new cases diagnosed (about 23% of the total in women alone, and about 11% of the total, both sexes combined). The incidence rate of breast carcinoma in women is highest in Western Europe and lowest in East and Central Africa 14. Even though breast cancer is a disease mainly attributed to women, it also occurs in men. However, breast cancer in man is rare as it presents 1% of the total breast cancer cases and 1% of cancer cases in men. This makes it difficult in approaching this disease in man in terms of therapy as well as diagnosis 15. Thus, due to limited data in male breast cancer, the treatment is primarily based on those used for women 16.
The third-generation therapeutic regimens for breast carcinoma chemotherapy follows:
AC-paclitaxel: Doxorubicin and cyclophosphamide 60 mg/m2 and 600 mg/m2 respectively, both IV, on day 1 every 3 weeks for 4 cycles, followed by paclitaxel 80 mg/m2 by 1-h IV infusion weekly for 12 weeks (more effective than AC) 17 or
TAC: Docetaxel 75 mg/m2, doxorubicin 500 mg/m2 and cyclophosphamide 500 mg/m2 all IV, on day 1 every 3 weeks for 6 cycles (more effective than FAC) 18,19 or
FEC-docetaxel: 5-FU 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2 all IV, on day 1 every 3 weeks for 3 cycles, followed by docetaxel 100 mg/m2 IV every 3 weeks for 3 cycles (more effective than 6 cycles of FEC) 20 orFEC-paclitaxel: 5-FU 600 mg/m2, epiru
bicin 90 mg/m2, and cyclophosphamide 600 mg/m2 all IV, on day 1 every 3 weeks for 4 cycles, followed by 3 weeks without treatment; continued with paclitaxel 100 mg/m2 IV weekly for 8 cycles (more effective than 6 cycles of FEC) 21,22.
On the other hand, as a result of the application of cytotoxic drugs, the number of binuclear cells has decreased, while the karyolytic cell number has increased. In the future, these parameters can be used as cytotoxic markers in the studies of different drugs 23. In a previous study regarding acute modifications of the nuclei of buccal cells in chemotherapy treated cancer patients, our group found a significant increase in the frequency of karyorrhexis, karyolysis and pyknosis compared to the control group. Moreover, the frequency of karyorrhexis was significantly higher after chemotherapy treatment, compared to the pre-treatment period (8.8± 3.0 vs. 3.6±4.9, p<0.006). Accordingly with the obtained results, we have found that the acute therapy does not induce damages in the chromosome, but in some cases, it may have cytotoxic effect 24.
In the present study, the average number of pyknotic changes (p<0.0002), karyorrhexis (p<0.0001), karyolysis (p<0.0002), and of the binuclear cells (p<0.00003) had a significant increase. A significant increase of all changes in the nuclei of the exfoliating cells of the buccal mucosa was found in male patients with breast carcinoma treated with chemotherapy as well(p<0.0002). However, we observed that age is not a factor that significantly influenced the frequency of nuclear alterations after chemotherapy in male patients with breast carcinoma.
Conclusion
The number of modifications in the nuclei of buccal cells of the mouth could serve as a detection test or important marker for the evaluation of the cytotoxicity of cytostatic drugs in male patients with breast carcinoma. These results show differences after one single chemotherapy use, however further studies would be of benefit to examine the differences after other cycles of chemotherapy and better evaluate the precision of the test.
The results show that all protocols applied to the treatment of patients with breast carcinoma have induction in cell cytotoxicity, but have not given induction in chromosomal changes and formation of micronuclei.
Some patients may have been affected by the time of disease detection (delay for various reasons).
Acknowledgments
We thank all study participants.
Conflict of Interest
No conflict of Interest
Funding source
No funding source
References
- Fenech M, Holland N, Chang WP, Zeiger E, Bonassi S. The HUman MicroNucleus Project–An international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res. 1999;428(1-2):271-283. doi:10.1016/s1383-5742(99)00053-8
CrossRef - Lucero L, Pastor S, Suárez S, et al. Cytogenetic biomonitoring of Spanish greenhouse workers exposed to pesticides: micronuclei analysis in peripheral blood lymphocytes and buccal epithelial cells. Mutat Res. 2000;464(2):255-262. doi:10.1016/s1383-5718(99)00200-4
CrossRef - Speit G, Schmid O. Local genotoxic effects of formaldehyde in humans measured by the micronucleus test with exfoliated epithelial cells. Mutat Res. 2006;613(1):1-9. doi:10.1016/j.mrrev.2006.02.002
CrossRef - Kirsch-Volders M, Vanhauwaert A, De Boeck M, Decordier I. Importance of detecting numerical versus structural chromosome aberrations. Mutat Res. 2002;504(1-2):137-148. doi:10.1016/s0027-5107(02)00087-8
CrossRef - Torres-Bugarín O, Zavala-Cerna MG, Nava A, Flores-García A, Ramos-Ibarra ML. Potential uses, limitations, and basic procedures of micronuclei and nuclear abnormalities in buccal cells. Dis Markers. 2014;2014:956835. doi:10.1155/2014/956835
CrossRef - Kassie F, Darroudi F, Kundi M, Schulte-Hermann R, Knasmüller S. Khat (Catha edulis) consumption causes genotoxic effects in humans. Int J Cancer. 2001;92(3):329-332. doi:10.1002/ijc.1195
CrossRef - Casartelli G, Bonatti S, De Ferrari M, et al. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol. 2000;22(6):486-492.
- Holland N, Bolognesi C, Kirsch-Volders M, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659(1-2):93-108. doi:10.1016/j.mrrev.2008.03.007
CrossRef - Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437(7061):1038-1042. doi:10.1038/nature03958
CrossRef - Kumar V, Abbas AK, Fausto N, Mitchell R. Robbins Basic Pathology. Elsevier Health Sciences; 2007.
- Zamzami N, Kroemer G. Condensed matter in cell death. Nature. 1999;401(6749):127-128. doi:10.1038/43591
CrossRef - Cotran RS, Kumar V, Collins T, Robbins SL. Robbins Pathologic Basis of Disease. Saunders; 1999.
- Riss TL, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2(1):51-62. doi:10.1089/154065804322966315
CrossRef - Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi:10.1002/ijc.25516
CrossRef - Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. 2019;173(1):37-48. doi:10.1007/s10549-018-4921-9
CrossRef - Zehr KR. Diagnosis and Treatment of Breast Cancer in Men. Radiol Technol. 2019;91(1):51M-61M.
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663-1671. doi:10.1056/NEJMoa0707056
CrossRef - Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302-2313. doi:10.1056/NEJMoa043681
CrossRef - Swain SM, Jeong JH, Geyer CE, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053-2065. doi:10.1056/NEJMoa0909638
CrossRef - Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(36):5664-5671. doi:10.1200/JCO.2006.07.3916
CrossRef - Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects o
f chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet Lond Engl. 2005;365(9472):1687-1717. doi:10.1016/S0140-6736(05)66544-0
CrossRef - Martín M, Rodríguez-Lescure A, Ruiz A, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100(11):805-814. doi:10.1093/jnci/djn151
CrossRef - Torres-Bugarín O, Ventura-Aguilar A, Zamora-Perez A, et al. Evaluation of cisplatin + 5-FU, carboplatin + 5-FU, and ifosfamide + epirubicine regimens using the micronuclei test and nuclear abnormalities in the buccal mucosa. Mutat Res. 2003;539(1-2):177-186. doi:10.1016/s1383-5718(03)00163-3
CrossRef - Alidema F, Kryeziu-Alidema M. Chemotherapy effects on acute alterations in the nuclei of buccal mucosa cells at patients with breast cancer | Instituti i Shëndetit Publik. Accessed September 9, 2023. https://www.ishp.gov.al/chemotherapy-effects-on-acute-alterations-in-the-nuclei-of-buccal-mucosa-cells-at-patients-with-breast-cancer/








