Sushil Chittrarasan1,2 , Arunkumar Radhakrishnan1,3
, Arunkumar Radhakrishnan1,3 , Tanuja Lella4
, Tanuja Lella4 , Lakshitha Niyatee Rao K1,5
, Lakshitha Niyatee Rao K1,5 , Padmaja Sugumar1
, Padmaja Sugumar1 , Srivignesh Ravi1,6
, Srivignesh Ravi1,6  and Abinaya Elango1,3*
and Abinaya Elango1,3*
1Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpattu, TamilNadu, India.
2Department of Pharmacology, Karpagam Faculty of Medical Sciences and Research, Coimbatore, TamilNadu, India.
3Centre for Herbal Pharmacology and Environmental Sustainability, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chengalpattu, TamilNadu, India.
4Department of Pharmacology, Sree Balaji Medical College and Hospital, Bharath Institute of Higher Education & Research, Chromepet, Chennai, Tamilnadu, India.
5Department of Pharmacology, M.S Ramaiah medical college, Bangalore, Karnataka, India.
6Department of Pharmacology, KMCH Institute of Health Sciences and Research, 99A, Avinashi road, Coimbatore, TamilNadu, India
Corresponding Author E-mail: dr.abi.smc@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2752
Abstract
Objective: The objective was to evaluate the efficacy and safety of methanolic leaf extract of Prosopis juliflora (PJ) in Lipopolysaccharide (LPS) induced SIRS (Systemic Inflammatory Response Syndrome) in rats using clinical, hematological, biochemical parameters, inflammatory markers, histopathological examination of vital organs and mortality. Materials and Methods: 60 male Wistar albino rats were divided into 4 groups with 18 rats each in groups 1, 2 &3 and 6 rats in group 4. Rats in group 1 were not given any treatment and served as inflammation (SIRS) control while rats in group 2 received Hydrocortisone 5 mg/kg IV 12th hourly and rats in group 3, received methanolic leaf extract of PJ2 mg/kg 12th hourly orally from day 1 to day 3. SIRS was induced in groups 1 to 3 on day 4 with single intraperitoneal injection of LPS. The rats in groups 1-3 were divided into subgroups- A, B and C, that were sacrificed on day 5, 6 and 7, ie., 24, 48 and 72 hours after LPS injection respectively. The treatment was continued with hydrocortisone and PJ leaf extract in groups 2 and 3 till the animals were sacrificed. The laboratory assessments were carried out at the time of sacrificing the animals. Group 4 animals did not receive any treatment and were sacrificed on day 4 to provide presumptive baseline data. The data were statistically compared using repeated measures ANOVA within the groups and one-way ANOVA between the groups. Results: The results showed that PJ leaf extract exhibited anti-inflammatory activity in terms of improvement in body temperature, total WBC count and all the inflammatory markers and the data was statistically significant for all parameters (p<0.05). Conclusion: Based on the results, it can be concluded that PJ has a potential therapeutic role in SIRS.
Keywords
Inflammation; Lipopolysaccharide; Prosopis juliflora; SIRS
Download this article as:| Copy the following to cite this article: Chittrarasan S, Radhakrishnan A, Lella T, Rao K. L. N, Sugumar P, Ravi S, Elango A. Assessment of Efficacy and Safety of Methanolic Leaf Extract of Prosopis Juliflora in Lipopolysaccharide Induced Systemic Inflammatory Response Syndrome (SIRS) in Wistar Albino Rats. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Chittrarasan S, Radhakrishnan A, Lella T, Rao K. L. N, Sugumar P, Ravi S, Elango A. Assessment of Efficacy and Safety of Methanolic Leaf Extract of Prosopis Juliflora in Lipopolysaccharide Induced Systemic Inflammatory Response Syndrome (SIRS) in Wistar Albino Rats. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3PRtJLM |
Introduction
Systemic Inflammatory Response Syndrome (SIRS) is an exaggerated defense response exerted by the body as a result of noxious or harmful stimuli such as infection, trauma, acute inflammation, ischemia or reperfusion and surgery, in order to find and get rid of the source of insult or stimuli. SIRS involves the release of certain inflammatory mediators and acute phase reactants that modulate hematological, autonomic, endocrine and immunological alterations in the body. Though the surge in acute phase reactants is defensive, a dysregulated cytokine storm would result in a severe inflammation causing reversible or irreversible end organ dysfunction and death.1
“The American College of Chest Physicians/Society of Critical Care Medicine-sponsored sepsis definitions consensus conference” held in 1991 in Chicago, Illinois established a set of clinical parameters to identify those subjects with SIRS easily in any clinical setting. SIRS is objectively defined by satisfaction of any two of the following criteria: “1. Body temperature over 38 or under 36 degrees Celsius, 2. Heart rate greater than 90 beats/minute, 3. Respiratory rate greater than 20 breaths/minute or partial pressure of CO2 less than 32 mmHg, 4. Leukocyte count greater than 12000 or less than 4000 /microliters or over 10% immature forms or bands”.2
Whenever an inflammation is induced by an infectious or noninfectious stimuli, complex immune responses set in, involving the humoral and cell mediated immunity, complement system and cytokines. The pro inflammatory cascade is mediated by the cytokines IL-1and TNF-alpha, that induce NF-kB leading to the release of IL-6, IL-8 and INF gamma and the compensatory anti-inflammatory drive is mediated by IL-4 and IL-10. If the balance between the pro inflammatory and anti-inflammatory pathways shifts in favor of pro-inflammatory, SIRS sets in. (1) If not treated, the ongoing inflammation could result in multiorgan failure and sometimes death. Hence, early diagnosis of SIRS, identification of its etiology and prompt intervention is very crucial to prevent multi organ dysfunction.
The mortality due to SIRS and sepsis is more than 35%. In addition to hemodynamic support, antibiotics and steroids are used in the management of SIRS.1 In diseases like COVID- 19 (Corona Virus Disease-19), SIRS can be a life- threatening complication. Although, steroids can help in this situation, there is no specific treatment for SIRS of varying etiologies including COVID-19. There is always a need for potential therapeutic options that could combat the inflammation in SIRS and prevent organ dysfunction.
The World Health Organization (WHO) reports that 88% of countries in the world use traditional medicines for various ailments and it has been an integral resource for years and mainstay for a few countries where they do not have proper access to the conventional medicine (3). Traditional medicines have gained popularity during the pandemic in reducing the symptoms associated with COVID-19 infection and inflammation. Few plants such as Nigella sativa (black cumin), Gymnanthemum amygdalinium and Azadirachta indica were evaluated for their efficacy in COVID-19 associated inflammation due to their anti-inflammatory, antiviral and immune modulatory properties.4 Similarly, Kabasurakudineer (KSK), a herbal concoction with several ingredients have gained popularity in India during COVID-19 and the Government of India promoted the use of KSK for prophylaxis and treatment of COVID-19, as it has antipyretic, anti-inflammatory, antioxidant and immunomodulatory properties.5-9
Prosopis juliflora (PJ), an invasive plant that belongs to the family Fabaceae, can grow in variety of soils and varied climatic conditions. The plant is available abundantly in Tamil Nadu. The phytoconstituents present in P.juliflora include phenols, tannins, steroids, terpenoids, flavonoids and alkaloids and various parts of the plant including seeds, fruits and leaves have been reported to have potent anti-inflammatory, antioxidant, anti-bacterial, anti-ulcer, anti-fungal and anticancer properties.10 There are no adequate data available in the literature evaluating the effectiveness of PJ in severe forms of inflammation. With this background, we wanted to evaluate the efficacy of PJ leaf extract in LPS induced SIRS in rats in comparison to the steroid, Hydrocortisone.
Objectives
The primary objective was to evaluate the efficacy of methanolic leaves extract of P.juliflora in LPS induced SIRS and the secondary objective was to evaluate the safety of methanolic leaves extract of P.juliflora in LPS induced SIRS in Wistar albino rats.
Outcomes
Primary outcomes
To evaluate the efficacy of methanolic leaves extract of P.juliflora in LPS induced SIRS with the following:
Body temperature
Total WBC count
Inflammatory markers- Interleukin-6 (IL-6), interleukin-10 (IL-10), Tumor necrosis factor (TNF-α), Interferon gamma (IFN-γ), Ferritin and C-reactive protein (CRP)
Histopathological assessment of Liver, Kidney and Brain
Secondary outcomes
To evaluate the safety of methanolic leaves extract of P.juliflora in LPS induced SIRS with the following:
Complete Blood Count (CBC)
Renal Function Tests (RFT) – Urea, creatinine
Liver Function Tests (LFT) – Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Total bilirubin and Direct bilirubin
Mortality
Material and methods
Drugs and chemicals
Lipopolysaccharide (Escherichia coli 0111: B4) was obtained from Sigma aldrich for inducing SIRS
P.juliflora was identified and certified by authorized botanist
Hydrocortisone was commercially obtained from nearby Chemist shop
Kits
Estimation of rat plasma IL-6, IL-10, TNF-α and INF-γ was done using commercially available ELISA kits as per the standard operating procedures.
Dose selection
The doses of Hydrocortisone and PJ were selected based on the previous literature reports that evaluated the anti-inflammatory activity of these interventions.11, 12
Experimental design
The study was initiated after obtaining approval from the Institutional Animal Ethics Committee (Ref no: IAEC1/Proposal:59/A.Lr/Dt:05.03.2021). 60 male Wistar albino rats, 6 to 8 weeks old, weighing 200-250 grams were used in this experiment. The animals were housed for about 14 days out of which the duration of experiment was 7 days. The rats were given standard pellet feed and kept in polypropylene cages (3 rats in each cage) with 12 hours light-dark cycle. Bedding, temperature and humidity were maintained as per the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines.
The rats were divided into 4 groups with 18 rats in groups 1, 2 & 3 and 6 rats in group 4. Groups 1-3 were further divided into 3 subgroups- A, B and C. Rats in group 1 were not given any treatment and served as inflammation (SIRS) control while in groups 2 and 3, the animals received Hydrocortisone 5 mg/kg IV 12th hourly and methanolic leaf extract of Prosopis juliflora 2 mg/kg 12th hourly respectively from day 1 to day 3.
SIRS was induced in all the rats of groups 1 to 3 on day 4 with single intraperitoneal injection of LPS (procedure explained in detail in next section). The rats in the subgroups A, B and C of groups 1,2 and 3 were sacrificed on day 5, 6 and 7, ie., 24, 48 and 72 hours after LPS injection respectively.
Group 4 animals served as normal control as SIRS was not induced and no treatment was given. They were sacrificed on day 4 to get control data for all parameters (presumptive baseline data indicating the pre induction levels). The treatment was continued with hydrocortisone and PJ leaf extract in groups 2 and 3 till the animals were sacrificed. The detailed treatment schedule from day 1 to day 7 in all the groups is provided in table 1.
Table 1: Treatment Schedule
|
Groups |
Subgroups |
Treatment |
Sacrifice |
||
|
Prior to LPS injection (day 1-3) |
LPS injection (day 4) |
After LPS injection (day 5-7) |
(After LPS injection) |
||
|
Group 1 (Inflammation control) (n=18) |
Subgroup A (n=6) |
No treatment |
No treatment |
No treatment |
At 24hrs (Day 5) |
|
Subgroup B (n=6) |
No treatment |
No treatment |
No treatment |
At 48hrs (Day 6) |
|
|
Subgroup C (n=6) |
No treatment |
No treatment |
No treatment |
At 72hrs (Day 7) |
|
|
Group 2 (standard treatment) (n=18) |
Subgroup A (n=6) |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly for 24hrs, IV |
At 24hrs (Day 5) |
|
Subgroup B (n=6) |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly for 48hrs, IV |
At 48hrs (Day 6) |
|
|
Subgroup C (n=6) |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly, IV |
Hydrocortisone 5mg/kg, 12th hourly for 72hrs, IV |
At 72hrs (Day 7) |
|
|
Group 3 (P.juliflora) (n=18) |
Subgroup A (n=6) |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly for 24hrs, oral |
At 24hrs (Day 5) |
|
Subgroup B (n=6) |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly for 48hrs, oral |
At 48hrs (Day 6) |
|
|
Subgroup C (n=6) |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly, oral |
P.juliflora leaf extract 2mg/kg, 12th hourly for 72hrs, oral |
At 72hrs (Day 7) |
|
|
Group 4 (normal control) (n=6) |
— |
No treatment |
No treatment |
No treatment |
On day 4, ie when SIRS was induced in other groups, these animals were sacrificed to get control data |
Note: n=number of rats
Induction of systemic inflammatory response syndrome
Among the various animal models for systemic inflammatory response syndrome in rats, we used LPS for inducing SIRS. Lipopolysaccharide (Escherichia coli 0111: B4) 5mg/kg was dissolved in 0.1 ml/20gm body weight PBS (Phosphate buffered saline), and given intraperitoneally to the rats of groups 1, 2 and 3.13
Extract preparation
P.juliflora plant was identified and certified by the authorized botanist before starting the experiment. The leaves of P.juliflora (3kg) were washed, shade dried for 3 days and grounded to fine powder, mixed with 80% methanol at 20% (w/v) concentration with continuous mixing in rotary evaporator for 24 hours at room temperature. The extract was then filtered through Whatman no:4 filter paper, and the excess solvent were removed using rotary evaporator at 55oC. The extract was stored at 4ºC for the experiment.14
Study assessments
After sacrificing the animals at their respective time points as detailed in Table 1, the following parameters were assessed:
CBC (complete Blood Count)
Renal Function Tests (RFT) – Urea, creatinine
Liver Function Tests (LFT) – Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Total bilirubin and Direct bilirubin
Inflammatory markers- Interleukin (IL)-6, Interleukin (IL)-10, Tumor necrosis factor (TNF)-α, Interferon gamma (IFN)-γ, Ferritin and C-reactive protein (CRP)
Histopathological examination of liver, kidney and brain
Statistical analysis
The data were analyzed using GraphPad Instat software (version 3.1). Quantitative data were summarized as mean ± standard deviation. Inferential statistics was done using repeated measures ANOVA (Analysis of Variance) for within group comparison and one-way ANOVA for between group comparison, followed by Post hoc analysis using Tukey’s multiple comparison test. P value <0.05 was considered significant.
Results
All the rats in groups 1-3 developed SIRS, as evidenced by increased body temperature and total WBC count, as per the SIRS criteria.1 There was no mortality in any of the groups. The clinical, haematological, biochemical and inflammatory markers observed in the study were statistically analysed within the groups and between the groups and the results are discussed parameter wise in the following section. Statistically significant p values are denoted by * in the tables and graphs.
Body temperature
Within group analysis of the body temperature was carried out only in subgroup C of groups 1, 2 and 3 as it had the data at all time points. Between group analysis was carried out by comparing the subgroup C of groups 1, 2 and 3.
In contrast to inflammation control (group 1), where the rats had elevated body temperature till the end (72 hours), hydrocortisone and PJ extract treated rats showed significant decrease in the body temperature. The body temperature of hydrocortisone treated rats was significantly low at 24 hours after LPS injection when compared to inflammation control, but it was not significantly reduced with PJ in comparison to group 1 (inflammation control). At 48 hours and 72 hours, the reduction in body temperature was similar in both hydrocortisone and PJ treated rats (Table 2).
Table 2: Body temperature
|
|
Prior to LPS Injection |
After LPS Injection |
P value |
||||||
|
Groups |
Sub groups |
Day 1 (Mean± SD) |
Day 2 (Mean± SD) |
Day 3 (Mean± SD) |
At 24 hours (Mean± SD) |
At 48 hours (Mean± SD) |
At 72 hours (Mean± SD) |
Within group comparison (Repeated measures ANOVA) |
Inter-Group comparison (One way ANOVA) |
|
Group 1- Inflammation Control |
A |
35.82 ± 0.19 |
36.17 ± 0.12 |
35.12 ± 0.17 |
40.05 ± 0.09 |
– |
– |
– |
<0.0001* |
|
B |
36.09 ± 0.22 |
35.26 ± 0.2 |
36.19 ± 0.13 |
39.12 ± 0.12 |
38.64 ± 0.18 |
– |
– |
||
|
C |
35.18 ± 0.09 |
35.94 ± 0.24 |
36.05 ± 0.34 |
39.25 ± 0.14 |
39.15 ± 0.08 |
39.12 ± 0.22 |
<0.0001* |
||
|
Group 2- Hydrocortisone |
A |
36.22 ± 0.17 |
35.67 ± 0.16 |
35.16 ± 0.17 |
37.75 ± 0.12 |
– |
– |
– |
|
|
B |
35.01 ± 0.11 |
35.06 ± 0.13 |
36.08 ± 0.19 |
37.92 ± 0.06 |
37.44 ± 0.34 |
– |
– |
||
|
C |
35.66 ± 0.06 |
35.65 ± 0.18 |
36.01 ± 0.25 |
37.93 ± 0.15 |
37.58 ± 0.26 |
35.87 ± 0.23 |
<0.0001* |
||
|
Group 3- PJ extract |
A |
35.12 ± 0.16 |
35.19 ± 0.18 |
35.42 ± 0.11 |
39.98 ± 0.06 |
– |
– |
– |
|
|
B |
36.26 ± 0.09 |
36.62 ± 0.28 |
36.04 ± 0.25 |
39.02 ± 0.08 |
37.81 ± 0.12 |
– |
– |
||
|
C |
36.12 ± 0.14 |
36.07 ± 0.23 |
36.08 ± 0.21 |
39.36 ± 0.14 |
37.92 ± 0.07 |
37.06 ± 0.29 |
<0.0001* |
||
|
Group 4- Control (pre induction) |
– |
35.62 ± 0.12 |
36.09 ± 0.13 |
35.12 ± 0.17 |
– |
– |
– |
– |
|
Blood parameters
In this study, there is no baseline data in the subgroups. Hence, the animals in group 4 were scarified at the time of LPS injection in other groups and the hematological, inflammatory and biochemical parameters were measured. This data was considered as presumptive baseline data indicating the pre induction levels for statistical purpose to assess whether 24 hours post LPS data satisfies induction of inflammation. Further, the data of subgroups (A, B & C) in each group were comparatively analyzed to check whether the parameters improve or worsen.
At 24 hours post LPS injection
All the blood parameters showed significant increase or decrease at 24 hours compared to presumptive baseline data (group 4), in favor of inflammation in groups 1, 2 and 3, except ferritin which did not increase significantly in hydrocortisone treated (group 2) rats at 24 hours post LPS injection.
Subgroups (24, 48 and 72 hours post LPS injection)
Inflammation control (Group 1):
All the inflammatory and blood parameters showed significant increase from 24 hours to 72 hours, except Hb which showed significant decline at 72 hours compared to 24 hours. These observations were in favor sustained inflammation till 72 hours.
Observations in group 2 (Hydrocortisone) and 3 (PJ):
Hematological parameters – Significant improvement in Hb and significant reduction in WBC with hydrocortisone and PJ extract were noted at 72 hours. Though there is improvement in Hb level at 72 hours, it has not attained the preinduction levels in both treatments whereas the total WBC count returned to preinduction levels in both hydrocortisone and PJ at 72 hours (Figure 1 and Table 3).
Renal function tests – Urea levels showed statistically significant increase after induction of inflammation (24, 48 and 72 hours) in group 2 & 3. However, there was no clinical significance as the values were within the normal range. Creatinine levels were increased after 24 hours of LPS injection in group 1, 2 & 3, but there was significant reduction in the creatinine levels at 72 hours in groups 2 & 3 and the reduction was similar in both, though the values did not reduce to the preinduction levels (Figures 2 & 3). Creatinine levels did not reduce in group 1, inflammation control.
Liver function tests – Liver enzymes showed significant decline at 72 hours with both hydrocortisone and PJ in group 2 and 3, after an initial increase at 24 hours. AST and ALT decreased to preinduction levels at 72 hours with hydrocortisone but not with PJ. ALP returned to normal at 48 hours with hydrocortisone and at 72 hours with PJ. There was no clinically significant change noted with total and direct bilirubin (Figures 4-6, Table 4). Improvement in LFT was not noted in group 1.
Inflammatory markers – All the inflammatory markers (CRP, ferritin, IL-6, IL-10, TNF-α and INF-γ) showed significant reduction at 72 hours with both hydrocortisone and PJ. In hydrocortisone group, ferritin was not significantly elevated at 24 hours. IL-6 & IL-10 reduced to normal (preinduction levels) at 48 hours and TNF-α & INF-γ returned to normal at 72 hours. In PJ group, IL-10 was reduced to preinduction level at 48 hours, while Ferritin, IL-6, TNF-α and INF-γ became normal at 72 hours. Though CRP showed significant decline at 72 hours with both treatments, it did not reduce to the preinduction levels (Figures 7-10, Table 5).
Histopathological assessment
The animals in inflammation control (group 1) had inflammatory changes in liver, kidney and brain at 72 hours post LPS injection. The animals treated with hydrocortisone and PJ extract had regenerative changes in liver and kidney after 72 hours post LPS injection. But brain had better regenerative changes in Hydrocortisone group at 72 hours compared to PJ group which still had minimal inflammatory changes. The histopathological changes are presented in figure 11.
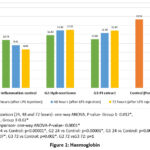 |
Figure 1: Haemoglobin |
Table 3: Total WBC count
|
Groups |
Subgroups |
WBC (cell/smm3) (Mean±SD) |
P value (Inter Subgroup) (One way ANOVA) |
P value (Inter group) (One way ANOVA) |
|
Group 1- Inflammation Control |
24 hrs |
12633.33 ± 1139.59 |
0.0004* |
P<0.0001* |
|
48 hrs |
14566.66 ± 1216.04 |
|||
|
72 hrs |
15800.00 ± 704.27 |
|||
|
Group 2- Hydrocortisone |
24 hrs |
13866.66 ± 1184.34 |
0.004* |
|
|
48 hrs |
13002.01 ± 1300.38 |
|||
|
72 hrs |
11283.33 ± 930.41 |
|||
|
Group 3- PJ extract |
24 hrs |
13916.66 ± 1792.67 |
0.02* |
|
|
48 hrs |
13066.66 ± 628.22 |
|||
|
72 hrs |
11033.33 ± 742.06 |
|||
|
Group 4- Control (Pre induction) |
NA |
9833.33 ± 2593.58 |
NA |
|
|
Post hoc: G1 24 vs Control: p=0.02*, G2 24 vs Control: p=0.0001*, G3 24 vs Control: p=0.0001*, G2 72 vs Control: p=0.69, G3 72 vs Control: p=0.86, G2 48 vs G3 48: p=1, G2 72 vs G3 72: p=1 |
||||
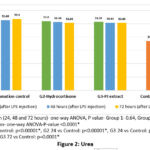 |
Figure 2: Urea |
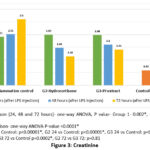 |
Figure 3: Creatinine |
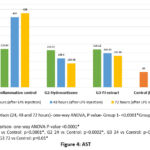 |
Figure 4: AST |
 |
Figure 5: ALT |
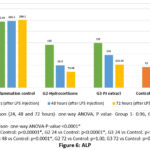 |
Figure 6: ALP |
Table 4: Bilirubin
|
Groups |
Subgroup |
Total bilirubin (mg/dl) (Mean±SD) |
P value (Inter Subgroup comparison) |
P value (Inter group comparison) |
Direct bilirubin (mg/dl) (Mean±SD) |
P value (Inter Subgroup) (One way ANOVA) |
P value (Inter group) (One way ANOVA) |
|
Group 1- Inflammation Control |
24 hrs |
0.50 ± 0.09 |
0.77 |
0.03* |
0.12 ± 0.06 |
0.74 |
0.46 |
|
48 hrs |
0.51 ± 0.07 |
0.10 ± 0.03 |
|||||
|
72 hrs |
0.53 ± 0.06 |
0.11 ± 0.04 |
|||||
|
Group 2- Hydrocortisone |
24 hrs |
0.45 ± 0.06 |
0.63 |
0.09 ± 0.03 |
0.65 |
||
|
48 hrs |
0.42 ± 0.07 |
0.08 ± 0.02 |
|||||
|
72 hrs |
0.41± 0.09 |
0.08 ± 0.01 |
|||||
|
Group 3- PJ extract |
24 hrs |
0.49 ± 0.09 |
0.38 |
0.10 ± .05 |
0.76 |
||
|
48 hrs |
0.47 ± 0.07 |
0.09 ± .04 |
|||||
|
72 hrs |
0.42 ± 0.10 |
0.08 ± 0.05 |
|||||
|
Group 4- Control (Pre induction) |
NA |
0.32 ± 0.06 |
NA |
0.06 ± 0.02 |
NA |
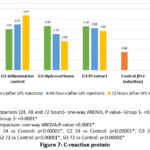 |
Figure 7: C-reactive protein |
 |
Figure 8: Ferritin |
 |
Figure 9: IL-6 |
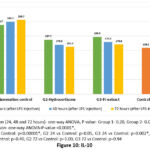 |
Figure 10: IL-10 |
Table 5: TNF- α & INF- γ
|
Groups |
Subgroups |
TNF-α |
INF-γ |
||||
|
TNF-α (pg/ml) (Mean±SD) |
P value (Inter Sub Group) |
P value (Inter group) |
INF-γ (pg/ml) (Mean±SD) |
P value (Inter Sub Group) (One way ANOVA) |
P value (Inter group) (One way ANOVA) |
||
|
Group 1- Inflammation Control |
24 hrs |
238.23 ± 16.98 |
0.37 |
<0.0001* |
240.52 ± 15.38 |
0.30 |
<0.0001* |
|
48 hrs |
249.88 ± 22.54 |
251.86 ± 20.98 |
|||||
|
72 hrs |
253.92 ± 18.21 |
256.88 ± 17.64 |
|||||
|
Group 2- Hydrocortisone |
24 hrs |
187.87 ± 11.09 |
<0.0001* |
188.32 ±11.25 |
<0.0001* |
||
|
48 hrs |
168.92 ± 12.11 |
164.56 ± 12.87 |
|||||
|
72 hrs |
138.86 ± 10.99 |
136.52 ± 10.12 |
|||||
|
Group 3- PJ extract |
24 hrs |
187.85 ± 10.97 |
0.04* |
188.36± 10.88 |
0.02* |
||
|
48 hrs |
179.98 ± 13.22 |
178.04± 12.45 |
|||||
|
72 hrs |
168.67 ± 12.14 |
166.92± 12.98 |
|||||
|
Group 4- Control (Pre induction) |
NA |
112.87 ± 10.21 |
NA |
132.48 ± 11.24 |
NA |
||
Post hoc analysis
TNF- α
G1 24 vs Control: p<0.00001*, G2 24 vs Control: p<0.00001*, G3 24 vs Control: p<0.00001*, G2 48 vs Control: p<0.00001*, G2 72 vs Control: p=0.07, G3 72 vs Control: p<0.00001*, G2 48 vs G3 48: p=0.94, G2 72 vs G3 72: p=0.02*
INF- γ
G1 24 vs Control: p<0.00001*, G2 24 vs Control: p<0.00001*, G3 24 vs Control: p<0.00001*, G2 48 vs Control: p=0.007*, G2 72 vs Control: p=1.00, G3 48 vs Control: p<0.00001*, G3 72 vs Control: p=0.003*, G2 48 vs G3 48: p=0.80, G2 72 vs G3 72: p=0.01*
 |
Figure 11: Histopathology of vital organs (at 72 hours after LPS injection) Discussion: |
Discussion
Traditional medicines have been used for treating variety of ailments including acute and chronic inflammatory conditions.15, 16 In this study, we evaluated the efficacy and safety of methanolic extract of PJ in comparison to Hydrocortisone. We studied a few clinical parameters, biochemical &haematological assessments and inflammatory markers in all the groups. The treatment was started prior to induction of SIRS and continued for up to 24, 48 and 72 hours. All the rats in groups 1-3 developed SIRS, as evident from increase in the body temperature and total WBC count at 24 hours after LPS injection, both of which are important in diagnosing SIRS.
Increase in body temperature is a critical criterion in SIRS. The inflammation control group showed hyperpyrexia which persisted till 72 hours (end of the study) after SIRS induction. Hydrocortisone treated rats exhibited gradual reduction in the body temperature at 48 and 72 hours, compared to 24 hours after LPS injection. Though not highly significant, PJ treated animals also showed improvement in fever. The ethanolic extract of PJ was evaluated for its anti-pyretic activity by Gopinath S.M et al., in 2013, in brewer’s yeast inducted hyperthermia in rats and they observed that PJ extract at doses of 250 and 500 mg/kg exhibited potent anti-pyretic activity, similar to the standard drug paracetamol (150 mg/kg).17 Our findings in the present study are consistent with Gopinath S.M et al., where PJ could be used in hyperpyrexia.
Anemia is one among the most significant characteristics of systemic inflammation. Hepcidin is a hormone that regulates iron and is produced by hepatocytes. The cytokines that are increased in excess amounts, as a result of systemic inflammation tend to increase hepcidin levels. Hepcidin inhibits the absorption of iron in the duodenum and the release of iron from the macrophages in the spleen, which causes anaemia.18 Hb level was significantly reduced in inflammation control as a result of SIRS, which is also associated with increased levels of inflammatory cytokines in the blood, but treatment with hydrocortisone and PJ extract improved the Hb, comparable to control. This could be due to the reduction in the levels of cytokines in the treatment groups.
It is a known fact that WBC count should be <4000 or >12000 in SIRS.1 In our study, we observed an elevated WBC count >12000 in the inflammation control throughout the study at 24, 48 and 72 hours, and the treatment groups at 24 hours. Hydrocortisone reduced the WBC count significantly at 72 hours (TC reduced to less than 12000). In PJ treated rats, statistically significant difference was not seen, though the total count at 48 and 72 hours was numerically less when compared to 24 hours.
When the renal function tests (Urea and Creatinine) were analysed, both the paraments showed significant increase in inflammation control group, which could be due to damage to the kidney as a result of SIRS. The treatment with hydrocortisone and PJ did not significantly reduce urea levels. But the creatinine levels in the treatment groups showed significant reduction at 72 hours after LPS injection ie. Hydrocortisone and PJ reduced the creatinine levels significantly, which indicates that PJ exerts protective effect against SIRS induced renal damage similar to Hydrocortisone, which could be due to the antioxidant and anti-inflammatory properties.10
Derangement of liver enzymes is common in sepsis and SIRS, as liver has two opposite roles. It acts not only as a source of inflammatory mediators but also a target organ for inflammatory cytokines mediated damage. Progressive liver damage can occur in SIRS as a part of MODS, sometimes leading to fulminant hepatic failure. Increase in bilirubin levels is not usually proportionate to the enzyme levels and isolated hyperbilirubinemia is a rare finding in SIRS.19 LPS induced SIRS in mice models showed elevated alanine transaminase enzymes starting from 2 hours and peaking in 24 hours.13 In our study, we could observe an increase in total bilirubin after inducing SIRS. Though the values are statistically significant, it is not having much of clinical relevance as the values fall within the acceptable range. The liver enzymes (AST, ALT and ALP) were elevated in the inflammation control but in hydrocortisone and PJ treated groups, there was a significant reduction at 72 hours compared to 24 hours after LPS injection. PJ leaf extract, in addition to anti-inflammatory activity, has free radical scavenging action and protects against liver damage by reducing the expression of pro-inflammatory cytokines in the liver.11
Serum ferritin is an acute phase reactant, and the levels correlate with the degree of acute and chronic inflammation. It may be used as biomarker of uncontrolled inflammation and to assess the effectiveness of an intervention in inflammation.20 In our study, we observed elevation in the ferritin levels after injecting LPS in inflammation control and PJ groups. Ferritin level was similar to control in hydrocortisone treated rats. Hydrocortisone and PJ treated groups showed reduced ferritin levels at 72 hours compared to 24 hours post LPS injection. Though PJ group did not have similar ferritin levels at 24, 48 and 72 hours as that of Hydrocortisone, within group analysis showed that the levels were significantly reduced at 72 hours compared to 24 hours.
Serum CRP can be used as an early marker of inflammation in SIRS patients, as it increases within 24 hours of onset of SIRS.21 Similar to ferritin, CRP levels elevated after LPS injection in all the groups at 24 hours, which gradually decreased at 48 hours and 72 hours. Hydrocortisone started reducing the CRP levels at 48 hours as compared to PJ, where the reduction was highly significant at 72 hours after induction of SIRS.
With regard to the inflammatory cytokines analysed in our study (IL-6, IL-10, TNF-α and INF-γ), all the inflammatory markers were elevated after SIRS induction. However, treatment with hydrocortisone and PJ reduced the levels of these pro-inflammatory cytokines. The anti-inflammatory effect of PJ was proven in carrageenan paw edema model in rats, where the PJ leaf extract given in the doses of 100, 200 and 400 mg/kg showed potent anti-inflammatory activity in terms of reduction in the paw volume.22 The findings of Choudhary PK et al., are supported by our current research, where PJ leaf extract has shown favourable effect on the pro-inflammatory markers, in SIRS. Thus, PJ leaf extract may be considered as a potential therapeutic option in acute inflammation.
The in-hospital mortality rate in sepsis is about 36.9% and the duration of SIRS before the onset of organ failure is found to be an important prognostic factor in sepsis.23 Hence, prompt intervention and early treatment of SIRS is essential in reducing the morbidity and mortality in sepsis. In addition to ensuring hemodynamic stability with IV fluids, vasopressors and inotropes may be used in some cases. If SIRS is due to sepsis, broad spectrum antibiotics would be used. Steroids in low doses such as 200 to 300 mg of hydrocortisone or other equivalent improve the survival rate in SIRS and sepsis. The cytokines including IL-1 and TNF-alpha are the early mediators of SIRS and their role is of paramount importance in shifting towards a pro-inflammatory drive. These two mediators in turn stimulate NF-KB which will induce massive release of other cytokines like IL-6, IL-8 and interferon gamma. In order to antagonize the effect of these pro-inflammatory cytokines, a compensatory anti-inflammatory response syndrome (CARS) is activated, which is mediated by the release of anti-inflammatory cytokines such as IL-4 and IL-10. The balance between SIRS and CARS decides the rate of inflammation and organ damage.1 Hence, any treatment option that is able to combat the active inflammation in SIRS by inhibiting the pro inflammatory cytokines could be used in addition to other supportive treatment in SIRS.
In this study, we were able to get potential information with regard to the efficacy of PJ towards reducing the clinical symptoms and inflammatory markers in SIRS. With the advent of COVID-19, focus has been shifted towards the traditional medicine and AYUSH treatments for ameliorating the symptoms associated with infection and inflammation.
PJ leaf extract exhibited favourable effect on the clinical symptoms, liver enzymes, kidney function and inflammatory markers in SIRS. It was safe at the dose used and hence could be considered for further evaluation in severe acute and chronic inflammatory conditions. The major limitation of this study is that the biochemical, haematological and inflammatory markers were analyzed only once at different time points of sacrifice ie., 24, 48 and 72 hours in the sub-groups. Baseline and end of study comparison in the same animal was not done, as we need more than 5 ml of blood to do all these assessments, which is not possible with tail vein or retro orbital puncture when the animal is alive. The required amount of blood was collected through cardiac puncture after sacrificing the animals. More precise data could have been obtained if before-after comparisons were done. But still, this study was able to generate data to show the efficacy of PJ in SIRS as we had inflammation control and normal control rats, which we used for comparing the data.
Conclusion
Based on the results observed in this study, it was noted that PJ exhibited anti-inflammatory activity at a dose of 4mg/kg/day in terms of improvement in body temperature, total WBC count and inflammatory markers. In addition, PJ was found to have a protective role against SIRS induced renal and liver damage, as indicated by the improvement in creatinine and liver enzymes. No mortality was seen in any of the groups. The effect of PJ on most of the parameters assessed was comparable to hydrocortisone. Hence, it can be concluded that PJ has a potential therapeutic role in severe acute inflammatory conditions like SIRS.
Acknowledgement
The authors thankfully acknowledge the Indian Council of Medical Research (ICMR), India for providing financial assistance to this research study. The authors also thank Chettinad Academy of Research and Education for supporting them in conducting this research study.
Conflict of Interest
There is no conflict of interest.
Funding Sources
The research was partially funded by ICMR under “Financial support for MD/MS/DM/MCh/DNB/DrNB/MDS thesis” scheme [Ref no: 3/2/Dec-2020/PG-Thesis-HRD (30)].
References
- Chakraborty RK, Burns B. Systemic inflammatory response syndrome. 2019.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-55.
CrossRef - Available from: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine.
- Singh S, Singh TG, Mahajan K, Dhiman S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. Journal of Pharmacy and Pharmacology. 2020;72(10):1306-27.
CrossRef - Neethu D. Anti-inflammatory, antipyretic and antibacterial study of Kabasura kudineer choornam: KMCH College of Pharmacy, Coimbatore; 2017.
- Kumar KNS, Divya KG, Mattummal R, Erni B, Sathiyarajeswaran P, Kanakavalli K. Pharmacological actions of contents of kabasura kudineer-a siddha formulation for fever with respiratory illness. Indian Journal of Pharmaceutical Education and Research. 2021;55(1):36-55.
CrossRef - Jose SP, Ratheesh M, Sheethal S, Rajan S, Saji S, Narayanan V, et al. Anti-inflammatory effect of Kaba Sura Kudineer (AYUSH approved COVID-19 drug)-A Siddha poly-herbal formulation against lipopolysaccharide induced inflammatory response in RAW-264.7 macrophages cells. Journal of Ethnopharmacology. 2022;283:114738.
CrossRef - Basha SS, Tripathi D, Koora S, Satyanarayana K, Jayaraman S. Molecular docking analysis of potential compounds from an Indian medicinal soup” kabasura kudineer” extract with IL-6. Bioinformation. 2021;17(5):568.
CrossRef - Divya K, Pathak J, Reddy RKM. In vivo efficacy and safety of Siddha medicine Kabasura Kudineer among COVID 19 infected Syrian golden hamsters. 2021.
CrossRef - Ukande MD, Shaikh S, Murthy K, Shete R. Review on Pharmacological potentials of Prosopis juliflora. Journal of Drug Delivery and Therapeutics. 2019;9(4-s):755-60.
CrossRef - Hassan SM, Taha AM, Eldahshan OA, Sayed AA, Salem AM. Modulatory effect of Prosopis juliflora leaves on hepatic fibrogenic and fibrolytic alterations induced in rats by thioacetamide. Biomedicine & Pharmacotherapy. 2019;115:108788.
CrossRef - Xinli Y, Suyun Q. Effect of different doses of hydrocortisone on rat with septic encephalopathy. Biomedical Research. 2017;28(6):2535-40.
- Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. Journal of biomedical science. 2017;24(1):1-17.
CrossRef - Saleh I, Abu-Dieyeh MH. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Scientific Reports. 2021;11(1):7871.
CrossRef - Ghasemian M, Owlia S, Owlia MB. Review of anti-inflammatory herbal medicines. Advances in Pharmacological and Pharmaceutical Sciences. 2016;2016.
CrossRef - Ruckmani A, Meti V, Vijayashree R, Arunkumar R, Konda VR, Prabhu L, et al. Anti-rheumatoid activity of ethanolic extract of Sesamum indicum seed extract in Freund’s complete adjuvant induced arthritis in Wistar albino rats. Journal of Traditional and Complementary Medicine. 2018;8(3):377-86.
CrossRef - Gopinath S, Reddy JM, Dayanand K, Shankar A. To evaluate the antipyretic activity of Prosopis juliflora ethanolic extract in brewer’s yeast induced hyperthermia in rats. 2013.
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta haematologica. 2009;122(2-3):78-86.
CrossRef - Szabo G, Romics L, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clinics in liver disease. 2002;6(4):1045-66.
CrossRef - Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. International immunology. 2017;29(9):401-9.
CrossRef - Sierra R, Rello J, Bailén MA, Benítez E, Gordillo A, León C, et al. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensive care medicine. 2004;30:2038-45.
CrossRef - Choudhary P, Nagori B. Oral Prosopis juliflora treatment ameliorates inflammatory responses against carrageenan induced paw edema in rats. Journal of Scientific and Innovative Research. 2013;2(5):888-92.
- Sugita H, Kinoshita Y, Baba H. The duration of SIRS before organ failure is a significant prognostic factor of sepsis. International journal of emergency medicine. 2012;5(1):1-7.
CrossRef








