Saida S. Ncibi1* , Aymen, M. Madkhali2,3
, Aymen, M. Madkhali2,3 , Magbool E. Oraiby4
, Magbool E. Oraiby4 , Jamilah A. Almalki1, Hussein A. Khadashi6, Abdullah A. Mobarki5
, Jamilah A. Almalki1, Hussein A. Khadashi6, Abdullah A. Mobarki5 , Syam Mohan6
, Syam Mohan6 and Hassan A. Hamali2
and Hassan A. Hamali2
1Department of Biology, College of Science, Jazan University, KSA.
2Department of Medical Laboratory Technology, Faculty of Applied Medical Science, Jazan University, Jazan, KSA.
3Medical Research Center, Jazan University, Jazan, Kingdom of Saudi Arabia.
4Poison control center Jazan, KSA.
5Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Jazan University, Jazan, Kingdom of Saudi Arabia
6Substance Abuse and Toxicology Research center, Jazan University Jazan, KSA.
Corresponding Author E-mail:sncibi@jazanu.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/2723
Abstract
Artemisia judaica, Ruta graveolens, and Suaeda monoica, indigenous plants to Jazan, second smallest region of Saudi Arabia, have several uses in the local folk medicine. This research aims to study the chemical composition of their methanol extracts and to explore some related biological activities. The different extracts Gas Chromatography Mass Spectroscopy profiling revealed the occurrence of many compounds within these extracts. Besides, this study revealed varied and selective antibacterial activities of these extracts. Ruta graveolens methanol extract was effective in inhibiting the growth of all tested microorganisms. Furthermore, they exhibit an interesting cytotoxic effect on human breast cancer cell lines, especially Artemisia judaica methanol extract. These findings suggested that Artemisia judaica (Asteraceae), Ruta graveolens (Rutaceae), and Suaeda monoica (Chenopodiaceae) could be natural sources for the discovery of new drugs.
Keywords
Antibacterial; Antioxidant; Artemisia Judaica; Cytotoxic; Suaeda Monoica; Ruta Graveolens
Download this article as:| Copy the following to cite this article: Ncibi S, S, Madkhali A. M, Oraiby M. E, Almalki J. A, Khadashi H. A, Mobarki A. A, Mohan S, Hamali H. A. Antioxidant, Antibacterial and Cytotoxic Activities of Artemisia judaica, Ruta graveolens and Suaeda monoica from Saudi Arabia. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Ncibi S, S, Madkhali A. M, Oraiby M. E, Almalki J. A, Khadashi H. A, Mobarki A. A, Mohan S, Hamali H. A. Antioxidant, Antibacterial and Cytotoxic Activities of Artemisia judaica, Ruta graveolens and Suaeda monoica from Saudi Arabia. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3RD2XYH |
Introduction
During the last decades, several researchers reported the effectiveness of Plants’ bioactive substances to fight communicable and non-communicable diseases1, warranting their further exploitation as sources of new drugs2.
Jazan, a coastal region on the Red Sea, southwestern area of Saudi Arabia; has a rich flora used in the local folk medicine, amongst Artemisia judaica (Asteraceae), Ruta graveolens (Rutaceae), and Suaeda monoica (Chenopodiaceae)3.
According to previous studies, Artemisia species are widely spread and frequently used in traditional medicine to reduce phlegm, relieve cough, stop and minimize pain, induce sweat dieresis, and activate blood circulation4–5. Ruta graveolens has various properties; antioxidant, antiepileptic, anti-pyretic, anti-cancer, anti-microbial (including fungal, bacterial and parasitic), as well as, diuretic, purgative, hepatoprotective, and hypotensive6. Besides, it is a toxin antidote and an insect repellent. Suaeda monoica treat sore throat, microbial infections, rheumatoid arthritis, asthma, snake bites, skin disease, ulceration, and toxic hepatitis1.
This current study aims to analyze the chemical composition of Artemisia judaica, Ruta graveolens, and Suaeda monoica methanol extracts and investigate their antioxidant, antibacterial, and cytotoxic activities.
Materials and Methods
Chemicals, bacteria, and cell lines
Chemicals, MCF-7 cell line, and bacteria strains were provided from Sigma Aldrich, Germany, American Type Culture Collection and theCentre of Biotechnology of Sfax, Tunisia, and, respectively.
Plant material and extract preparation
Dried collected plants were grinded. Powdered matter (500 g) was extracted by continuous mixing in 1 L methanol (95%) for 72 h at room temperature with occasional shaking. Finally, methanol of the filtrate was evaporated and methanol extracts residues were stored at 4°C.
Phytochemical screening
To carry out the phytochemical screening, aqueous solutions of different extracts were prepared as follows: In 10 ml distilled water, 100 mg of each extract was dissolved using a laboratory sonicator (30 min at 37°C).
Total phenolic contents
The Folin–Ciocalteu method was usedto measure the total phenolic contents of the different extracts7. Briefly, 2.5 ml of 10% Folin–Ciocalteu reagent (v/v) and 2.0 ml of 7.5% Na2CO3 was mixed with 0.5 ml of each plant aqueous solution. Gallic acid was used as a standard phenol in this assay. After 40 min incubation at 45°C period, the mixture absorbance was measured at 765 nm. Unless otherwise stated, all tests of the analysis were performed in triplicates. Total phenolic contents were expressed as gallic acid equivalents (eq GA mg/g dry weight of the different extracts).
Total flavonoid contents
Total flavonoid contents of the extracts were determined using the formation of the flavonoid–aluminum complex procedure8. 1 ml aluminum chloride solution (2%) was added to 1 ml of each aqueous sample. Absorbance of the final mixture was measured at 430 nm after 15 min incubation at room temperature. Rutin was used to determine the standard curve. Results were presented as rutin equivalents (mg RE/g dry weight).
Estimation of antioxidant property
Free radical-scavenging activity assay
DPPH test was carried out to measure the free radical-scavenging activity9. 1 ml of a 0.1 mM methanolic solution of DPPH were added to 1 ml of the aqueous solutions (50–300 µg/ml). After 30 min incubation in darkness at 27°C, sample absorbance was determined at 517 nm. The standard of this assay was the Ascorbic acid.
Ferric reducing power test
Chu et al. (2000) method was adopted to determine the Ferric reducing anti-oxidative power (FRAP) of the different extracts10. A mixture of 2.5 ml of potassium phosphate buffer (0.1 M, pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide was prepared. Then, 1.0 ml from varying concentrations (50–500 µg/ml) of plant extract solution was added to this mixture, followed by incubation 20 min at 50°C. Next, 2.5 ml of trichloroacetic acid (10% [w/v]). After that, 2.5 ml of water and 0.5 ml of FeCl3 (0.1% [w/v]) were added to 2.5 ml of the reaction mixture. Incubation of this solution 30 min at 28°C the color developed and its absorbance at 700 nm was determined. Standards for this experiment were ascorbic acid and ranitidine.
Gas chromatography mass spectroscopy (GC-MS) analysis
A GC-MS analysis for the four methanol extracts were conducted to identify the bioactive compounds11. The model of the GC-MS used device was QP 2010 Plus, Shimadzu, Tokyo, Japan. The used column was VF-5ms fused silica capillary, its length 30 mm, 0.25 mm ID, and 0.25 𝜇m df. Electron impact mode at 70eV was adopted to ionize samples’ components. In that experiment, the carrier gas used is helium (99.99%). The flow was 0.96 ml/min and the injection volume was 2.0 µI. Temperature was 250°C at the injector and 280°C at ion-source. Total GC running time is 36 min. Retention time, retention indices, and mass spectra were adopted to identify the compounds. For the mass spectra, values were fixed at a scan interval of 0.5 seconds and fragments from 40 m/z–450 m/z.
The spectrum of the known compound stored in the database of National Institute of Standard and Technology (NIST) library were used as references to ascertain the name, molecular weight, and structure of the components of the extracts. Mass spectra analysis was done using Software version 2.71. To calculate the relative percentage amount of each compound, its average peak area was compared to the total area.
Cell line and cell cultures
To grow and maintain the cell line, RPMI-1640 medium (pH 7.4) supplemented with FBS (10%), penicillin (100 U/ml), and streptomycin (100 g/ml), was selected. Incubator temperature was fixed at 37°C with 90% humidity and 5% CO2. The CO2 incubator was from New Brunswick Scientific. DMSO was used to dissolve the extracts used in this study (DMSO < 0.05% in media). Control cell cultures received only DMSO.
Cell viability assay
In vitro cytotoxicitywas determined using the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) microculture tetrazolium viability assay12. Unless otherwise stated, sample analyses were carried out in triplicates. Briefly, various samples with various concentrations (highest was 200 µg/ml) were incubated for 72 h at 37°C. Untreated cells and blank cell-free were used as controls. After the addition of 5mg/ml MTT to each well, a second incubation for further 4 h after was done. Finally, to solubilize the formazan crystals100 µl of DMSO were added to each well. The final mixture absorbance was read at 490 nm (BioTek microtiter plate reader, Winooski, VT, USA).
Cell proliferation inhibitory rate were calculated using the following formula:

Extracts cytotoxicity on the cancer cell lines (IC50) expressed the sample concentration that reduced the treated cells account by 50% with respect to control cells.
Antibacterial activities
In this study, DMSO was used to dissolve the different plant extracts, the initial concentration was 30 mg/ml. Agar-well diffusion method was adopted to carry out the antibacterial activities, as previously presented13.
Onto the surface of agar plates, 106 cfu/ml for bacteria were inoculated (200 μl fresh cell suspension). Then, using a sterile Pasteur pipettes, 6 mm diameter wells were banged in the inoculated agar medium. After that, 100 μl of each extract solution (30 mg/ml) were poured to these wells. To allow the diffusion of the extracts in the agar, plates were kept 4 h at 4°C. After 24 h incubation at 37°C, inhibition zone diameters (IZD) was performed to evaluate the antibacterial activity. In this assay, Gentamicin (150 μg/ml) was used as positive control. The average values were taken from running the samples in triplicates.
Minimum inhibitory concentration (MIC) is defined as the lowest concentration that completely inhibits bacteria growth (microorganism). In this study, micro-well dilution method (125–2,5 mg/ml) was conducted to determine extracts MIC values. Bacterium suspensions (106 CFU/ml) were incubated, in plates, 24 h at 37°C. To determine extracts MIC values, 40 μl of MTT (0.5 mg/ml dissolved in distilled water) were added to the wells, followed by an incubation 30 min at 37°C. In this experiment, red-purple color development indicated that the bacteria were biologically active.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 22.0 software (SPSS Inc., IBM, Chicago, Illinois, USA) was used to perform the statistical analysis study. Average value of the triplicates was calculated. Results are represented as means ± standard deviation (SD). Comparison between samples are carried out using ANOVA test followed by a post hoc. p value < 0.05 was considered statistically significant.
Results and Discussion
Worldwide and throughout the human history, medicinal plants were a normative basis for the maintenance of good health14. The used herbal formulations (crude extract, tincture, teas, poultices, powders, and others) differ according to the intended aim1. In order to understand their healthiness mechanism and to rationalize plants use, several researches investigated plants’ secondary metabolites in depth during the last decades.
Amongst these compounds, phenolic compounds – phenolic acids and flavonoids – have been of great interest in research 15–17.Scientific investigations proved that these natural phytochemicals have antioxidant, anti-microbial, and anticancer activities2, 18, 19.
This study is dealing with Artemisia judaica (Asteraceae), Ruta graveolens (Rutaceae), and Suaeda monoica (Chenopodiaceae). Folk medicine in Saudi Arabia cited the use of these plants to treat different ailments1.
Practitioners used Artemisia judaica to treat many disorders, such as gastro-intestinal disorders, poor eyesight, cardiovascular disease, risk of atherosclerosis, skin disorders, cancer and arthritis 20–21.
Ruta graveolens has anti-inflammatory and immune stimulant properties. It treats hypertension, cramps to hysteria, edema visual impairment as well as malaria and sclerosis 22-23.
Suaeda monoica possesses a curative action for hepatitis and protects liver against paracetamol-induced injury24. Furthermore, this plant helps healing wounds25.
Table 1 presents TPC and TFC of the different extracts. According to this study, RGME has the highest level of phenols and flavonoids compared to SMME and AJME. These amounts are different compared to the results of other studies. Asgharian (Asgharian et al., 2020) reported different amounts of phenols and flavonoids within R. graveolens hydroalcholic extract, TPC were 14.1 ± 0.47 mg GAE/g and TFC were 15.8 ± 0.19 mg rutin equivalent/g26. Concerning Artemisia Judaica, Hashem (Hashem et al., 2013) recorded TPC 726.8 mg GAE/g and TFC 705.73 mg rutin equivalent/ug fresh weight27. In addition, other studies proved that Suaeda monoica contains high amounts of phenols and flavonoids28–29. During experiment conduction, several factors such as extraction conditions and used solvent can lead to such variance. Allam (Allam et al., 2019) suggested that Methanol extract contains the greatest amount of phenolics and flavonoids in Artemisia Judaica30.
Table 1: TPC and TFC are presented, respectively, as gallic acid equivalents (eq GA mg /g dry weight of the different extracts) and rutin equivalents (eq R mg /g dry weight of the different extracts) of Artemisia judaica methanol extract (AJME), Ruta graveolens methanol extract (RGME), Suaeda monoica methanol extract (SMME). Three independent runs data are displayed as mean ± standard deviation (SD).
|
TPC (µg G A/mg dry plant) |
TFC (µg R/mg dry plant) |
|
|
RGME |
89,03±3,6 |
3,34±0,9 |
|
AJME |
51,39±4,2 |
2,41±0,78 |
|
SMME |
34,54±2,75 |
3,056±0,61 |
DPPH and FRAP tests were adopted to estimate the antioxidant activities of these extracts. The DPPH assay deals with the abilities in plants’ methanol extracts to donate hydrogen to the DPPH radical, which leads to bleaching of the DPPH solution. Figure1 presents changes in the free radical scavenging abilities of methanol extracts of the different plants based on their percent inhibition. Results show that RGME has an important scavenging activity tha is similar to ascorbic acid. However, Artemisia judaica methanol extract (AJME) and Suaeda monoica methanol extract (SMME) produced significantly lower scavenging activities than ascorbic acid.
 |
Figure 1: DPPH free radical scavenging activities of AJME, RGME, SMME, and ascorbic acid. Three independent runs results are displayed as mean ± standard deviation (SD). |
One of the assays that has the ability to measure the total antioxidants levels in plant is the FRAP assay, where samples with antioxidant compounds had the ability to reduce Fe(III) in potassium ferricyanide to Fe(II) and thus to change the final solution color; yellow to light green.
Figure 2 represents the ferric reducing antioxidant activities of different extracts. Results show that activities of all extracts are significantly lower than the activity of ascorbic acid. However, SMME activity is significantly higher than ranitidine, RGME, and AJME activities.
 |
Figure 2: Ferric Reducing Antioxidant power of AJME, RGME, SMME, ascorbic acid, and ranitidine. |
In this study, the GC-MS analysis of the different extracts revealed the occurrence of a large number of compounds 196, 120, and 342 from AJME, RGME, and SMME, respectively. Compounds that have high similarity (≥90%) with the GC library are represented in tables 2, 3, and 4. The identified compounds are presented with their retention time (RT), similarity, percentage, and their reported biological activities.
GC-MS analyses revealed that these extracts contain several components that could be important in many fields: medicine, food industry, cosmetic industry, and others. This richness justifies their antioxidant, antibacterial, and cytotoxic activities.
These extracts are rich in phytosterols that have great interest in research due to their nutritional, biological, and medicinal activities, including anti-hypocholesterolemic, anti-inflammatory, anti-oxidative, and anti-tumor.
AJME contains 35 compounds with similarities higher than 90% with the GC library (Table 2). Some of them have anti-microbial activities. Others are antioxidant, hepatoprotective, and immunomodulatory agents31–33. Furthermore, several compounds have insecticidal activities or fragrance compounds34. Moreover, artemisinin, an essential compound of this plant extract, treats malaria according to many studies35.
Table 2: Details of phytocompounds detected by GC-MS analysis of Artemisia judaica methanol extract (AJME).
|
|
Compound |
Retention time |
Percentage |
Similarity |
Biological activities |
|
1 |
Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R)- |
5.092 |
1.44 |
97 |
curing dental composites36 |
|
2 |
1,7-Octadiene-3,6-diol, 2,6-dimethyl- |
6.11 |
0.01 |
90 |
Fatty alcohol |
|
3 |
alpha-Fenchyl acetate |
6.26 |
0.22 |
97 |
Fragrance Compounds |
|
4 |
Eugenol |
6.903 |
0.03 |
90 |
Immunomodulatory |
|
5 |
DL-Proline, 5-oxo-, methyl ester |
7.154 |
0.06 |
93 |
Antibacterial and antifungal |
|
6 |
2-Propenoic acid, 3-phenyl- |
7.536 |
0.08 |
91 |
hepatoprotective agents |
|
7 |
Davana ether |
8.133 |
0.76 |
94 |
hepatoprotective agents |
|
8 |
Dodecanoic acid (CAS) |
8.215 |
0.03 |
90 |
Lauric Acid |
|
9 |
1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- |
8.568 |
0.22 |
95 |
Anti-microbial potential |
|
10 |
Davanone |
8.761 |
0.34 |
92 |
hepatoprotective agents |
|
11 |
Isopropyl dodecanoate |
8.79 |
0.2 |
91 |
|
|
12 |
1,2-Benzenedicarboxylic acid, diethyl ester |
8.878 |
0.17 |
95 |
Antimicrobial37 |
|
13 |
2-Naphthalenemethanol, decahydro-.alpha |
9.556 |
0.19 |
90 |
|
|
14 |
Mome Inositol |
10.143 |
1.28 |
90 |
|
|
15 |
7–Ethyl–1,4-dimethylazulene. |
10.172 |
1.09 |
94 |
Chamazulene |
|
16 |
Hexadecanoic acid, methyl ester |
11.2 |
0.46 |
94 |
Insecticidal, |
|
17 |
n-Hexadecanoic acid |
11.486 |
2.94 |
95 |
Insecticidal |
|
18 |
Heptadecanoic acid |
12.108 |
0.13 |
92 |
Anti-cancer38 |
|
19 |
Cyclooctacosane |
12.289 |
0.11 |
95 |
|
|
20 |
Octadecanoic acid |
12.795 |
0.61 |
96 |
|
|
21 |
Matricarin |
14.395 |
1.15 |
90 |
Sedative |
|
22 |
Grossmisine |
14.436 |
0.34 |
92 |
Insect repellent39 |
|
23 |
Dihydroartemisinin, 10-O-(t-butyloxy)- |
14.862 |
0.46 |
90 |
|
|
24 |
1-Heptacosanol |
15.814 |
0.19 |
98 |
|
|
25 |
Heneicosane |
16.386 |
0.02 |
90 |
|
|
26 |
13-Docosenamide, (Z)- |
16.518 |
0.09 |
94 |
|
|
27 |
2,6,10,14,18,22-Tetracosahexaene |
16.58 |
0.09 |
95 |
Antimicrobial37 |
|
28 |
alpha.-Tocopherol-.beta.-D-mannoside |
20.194 |
0.04 |
91 |
Antimicrobial37 |
|
29 |
Stigmasterol |
22.68 |
0.83 |
93 |
Antimicrobial37 |
|
30 |
Obtusifoliol |
23.476 |
0.15 |
94 |
Anticancer : |
|
31 |
Stigmast-5-en-3-ol, (3.beta.)- |
23.913 |
1.02 |
91 |
|
|
32 |
Artemetin |
24.357 |
1.47 |
93 |
Anti-edematogenic |
|
33 |
9,19-Cyclolanost-23-ene-3,25-diol, (3.beta.,23E)- |
25.312 |
0.06 |
91 |
Pesticides37 |
|
34 |
Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R)- |
5.092 |
1.44 |
97 |
|
|
35 |
1,7-Octadiene-3,6-diol, 2,6-dimethyl- |
6.11 |
0.01 |
90 |
|
RGME contains 22 compounds that have similarity with GC library higher than 90% (Table 3). Some of them have neuroprotective, antioxidant, and anti-microbial effects40. Besides, Scopoletin is a pharmacologically active coumarin derivative compound found in various plant Ruta species.
Table 3: Details of phytocompounds detected by GC-MS analysis of Ruta graveolens methanol extract (RGME).
|
|
Compound |
Retention time |
Percentage |
Similarity |
Biological activities |
|
1 |
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
5.012 |
1.43 |
96 |
Antioxidant41 |
|
2 |
1-Pyrrolidineethanamine |
5.535 |
0.51 |
95 |
anti-anginal drug |
|
3 |
1,2,3-Propanetriol, monoacetate |
5.881 |
0.62 |
93 |
Anti-cancer42 |
|
4 |
2-Undecanone (CAS) |
6.242 |
0.45 |
93 |
Anti-inflammatory |
|
5 |
DL-Proline, 5-oxo-, methyl ester |
7.15 |
0.08 |
89 |
Antimicrobial |
|
6 |
2-Acetoxytetradecane |
7.379 |
0.2 |
90 |
Antibacterial43 |
|
7 |
Diethyl Phthalate |
8.886 |
0.68 |
98 |
|
|
8 |
4-(3,4-Methylenedioxyphenyl)-2-butanone |
9.083 |
0.41 |
95 |
Food flavor |
|
9 |
7H-Furo[3,2-g][1]benzopyran-7-one |
11.016 |
3.1 |
95 |
|
|
10 |
n-Hexadecanoic acid |
11.46 |
1.84 |
96 |
Palmitic Acid |
|
11 |
2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy- (CAS) |
11.818 |
1 |
91 |
Scopoletin |
|
12 |
Xanthotoxin |
12.335 |
0.27 |
93 |
Neuroprotective44 |
|
13 |
Phytol |
12.424 |
1.07 |
97 |
Precursor of vitamin E |
|
14 |
Methoxsalen |
12.514 |
3 |
92 |
Xanthotoxin |
|
15 |
9,12,15-Octadecatrienoic acid, (Z,Z,Z)- |
12.651 |
4.53 |
97 |
Linolenic acid |
|
16 |
7H-Furo[3,2-g][1]benzopyran-7-one, 2,3-dihydro-2-(1-hydroxy-1-methylethyl)-, (S)- (CAS) |
14.07 |
0.4 |
91 |
— |
|
17 |
1-Octadecanol (CAS) |
14.617 |
1.37 |
90 |
Antimicrobial |
|
18 |
Acetamiprid |
14.84 |
0.59 |
94 |
Insecticide |
|
19 |
dl-.alpha.-Tocopherol |
20.201 |
1.68 |
95 |
Antimicrobial37 |
|
20 |
Ergost-5-en-3-ol, (3.beta.,24R)- (CAS) |
22.24 |
1.26 |
95 |
Campesterol |
|
21 |
Neophytadiene |
23.627 |
0.78 |
90 |
Anti-inflammatory |
|
22 |
Stigmast-5-en-3-ol, (3.beta.)- (CAS) |
23.887 |
1.13 |
91 |
Phytodterol |
SMME has 19 compounds that are similar to GC library, higher than 90%, including hydroxyamphetamine (Table 4). Hydroxyamphetamine is an indirect-acting adrenergic agonist. Its main effect is to induce the release of norepinephrine from the nerve ending, which indirectly initiates muscle contraction. Previous studies cited that Suaeda monoica flavonoids, saponins, alkaloids, polyphenols, resins, tannins, and coumarins are therapeutic phytoconstituents 45–47.
Table 4: Details of phytocompounds detected by GC-MS analysis of Suaeda monoica methanol extract (SMME).
|
|
Compound |
Retention time |
Percentage |
Similarity |
Biological activities |
|
1 |
Benzeneacetic acid |
5,845 |
0,37 |
90 |
antitumor |
|
2 |
1,3-BENZENEDIOL |
6,256 |
0,26 |
95 |
catechol |
|
3 |
1H-Indole (CAS) |
6,494 |
0,06 |
89 |
|
|
4 |
2-Methoxy-4-vinylphenol |
6,567 |
1,9 |
93 |
Aromatic substance |
|
5 |
Phenol, 2,6-dimethoxy- |
6,883 |
0,46 |
95 |
Anti-fungal Anti-helminthic |
|
6 |
2,4-Imidazolidinedione (CAS) |
6,924 |
0,24 |
94 |
Antioxidant48 |
|
7 |
DL-Proline, 5-oxo-, methyl ester |
7,152 |
0,33 |
90 |
|
|
8 |
p-Hydroxyamphetamine |
7,95 |
1 |
95 |
|
|
9 |
1,2-Benzenedicarboxylic acid, diethyl ester (CAS) |
8,885 |
4,14 |
96 |
|
|
10 |
Neophytadiene |
10,56 |
0,51 |
94 |
|
|
11 |
Hexadecanoic acid, methyl ester (CAS) |
11,199 |
2,73 |
96 |
|
|
12 |
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- (CAS) |
12,375 |
0,41 |
91 |
|
|
13 |
Phytol |
12,415 |
1,34 |
96 |
|
|
14 |
9,12,15-Octadecatrienoic acid, (Z,Z,Z)- |
12,631 |
3,45 |
92 |
|
|
15 |
n-Tetracosanol-1 |
14,605 |
0,46 |
90 |
Lignoceryl alcohol |
|
16 |
1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester |
14,862 |
0,69 |
94 |
Anti-microbial |
|
17 |
1-Docosanol (CAS) |
19,568 |
0,25 |
90 |
antiviral |
|
18 |
Vitamin E |
20,189 |
4,85 |
95 |
|
|
19 |
Stigmasterol |
22,634 |
3,02 |
94 |
|
Stigmasterol, an essential biochemical present in the three extracts, protects against ketamine-induced psychotic symptoms49. Additionally, stigmast-5-en-3-ol increases glucose intake and overcomes insulin resistance50.
On another hand, these extracts contain substances that could be toxic to animals and humans. Diethyl phthalate, octadecanol, heptadecane, Tricosenoic acid are among these toxic compounds. They are interested in several industrial fields. Dihydrobenzofuran, known as coumaran, is an acetylcholinesterase inhibitor and can be used as a biofumigant51.
Several gram-positive and gram-negative bacterial strains were used to test the potential antibacterial activities of the different extracts.
Tables 5 and 6 present the antibacterial activity results of this study. Table 5 shows the inhibition IZD, while Table 6 shows the MIC values. Gentamicin (150 μg/ml), antibiotic that affects all used bacterial strain, is the positive control in this research.
Table 5: Anti-microbial activities of the different extracts (30mg/ml) and gentamycin (150 µg/ml). Inhibition zones are presented as mean ± SD. The extracts were Artemisia judaica methanol extract (AJME), Ruta graveolens methanol extract (RGME), Suaeda monoica methanol extract (SMME).
|
|
E. coli |
P. aeruginosa |
S. typhimurium |
M. luteus |
S. aureus |
B. cereus |
|
Gentamicin |
20±1,1 |
18±0,8 |
20±1 |
19±1,5 |
25±0,7 |
20±1 |
|
RGME |
20±0,9 |
22±1 |
20±0,9 |
24±1,2 |
32±0,6 |
18±1,6 |
|
AJME |
18±2 |
18±1.3 |
ND |
ND |
ND |
14±2 |
|
SMME |
24±0,5 |
ND |
ND |
18±2 |
10±1 |
ND |
Table 6: Minimum inhibition concentrations of the different extracts. The extracts were Artemisia judaica methanol extract (AJME), Ruta graveolens methanol extract (RGME), Suaeda monoica methanol extract (SMME).
|
|
E. coli |
P. aeruginosa |
S. typhimurium |
M. luteus |
S. aureus |
B. cereus |
|
RGME |
6.25 |
12.5 |
12.5 |
3.12 |
1.55 |
12.5 |
|
AJME |
12.5 |
12.5 |
— |
— |
— |
25 |
|
SMME |
12.5 |
— |
— |
12.5 |
25 |
– |
Our current results showed the existence of varied and selective anti-microbial activities of the different extracts. RGME was effective in inhibiting the growth of all tested microorganisms. However, only E. coli appeared to be sensitive to SMME.
According to AlMousa (2021), the active fraction of the ethyl acetate Artemisia judaica extract as an anti-microbial effect is significantly observed using disc diffusion assays (at 30 µ/mL) against P. aeruginosa, S. aureus, F. solani, and A. niger52.
A recent study by Helal et al. (2019) reported that Ruta graveolens extract showed total lack of anti-microbial activity against E. coli and S. typhimurium40. However,essential oils of this plant have high antibacterial activity on S. aureus, K. pneumoniae, P. aeruginosa, and E. coli with MIC values 3.5–3.9 μg/ml, 4.5–5.2 μg/ml, 5.8–6.3 μg/ml, and 7.5–7.94 μg/ml, respectively53. In addition, according to previous research, Suaeda monoica leaves are a potential good source of antibacterial agents29.
This research carried out MTT assay to investigate AJME, RGME, and SMME cytotoxic activities on the MCF-7 cell line. Current results showed that Artemisia judaica (Asteraceae), Ruta graveolens (Rutaceae), and Suaeda monoica (Chenopodiaceae) are natural sources of cytotoxic agents. AJME has the highest cytotoxic potential (IC50135.7 ± 17.51) against MCF-7 cell line compared to SMME and RGME (cytotoxic potential of SMME and RGME are 176.07 ± 14.39; 285.22 ± 24.1, respectively).
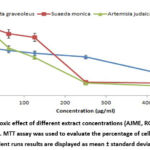 |
Figure 3: Cytotoxic effect of different extract concentrations (AJME, RGME, SMME) on MCF-7 cell line. MTT assay was used to evaluate the percentage of cell viability. |
Several researchers confirmed these results, Nasr et al., (2020); proved that Artemisia judaica methanol extract exhibited anti-proliferation activity against cell lines, including MCF-7, HepG2 Liver cells, and LoVo colon cells53.
Additionally, Varamini et al., (2009) study pointed the high cytotoxic activity of R. graveolens ethanol extract against different human tumor cell lines (such as; Burkitt’s lymphoma cell lines RAJI, RAMOS, prostate adenocarcinoma cell line (LNCap-FGC-10), cell lung carcinoma (Mehr-80) with IC(50); 24.3 microg/ml, 35.2 microg/ml, 27.6 microg/ml, 46.2 microg/ml, respectivelly54.
Conclusion
In conclusion, this study demonstrated that the methanol extracts of Artemisia judaica (Asteraceae), Ruta graveolens (Rutaceae), and Suaeda monoica (Chenopodiaceae), indigenous plants in the Jazan Province of Saudi Arabia, contain various metabolites that have important antioxidant, antibacterial, and cytotoxic activities. Thus, further research are recommended to isolate and purify their active compounds.
Acknowledgement
Researchers would like to thank Jazan University membership and Scientific Research deanship for their support and efforts to succeed this project.
Conflict of Interests
None to declare.
Funding source
This research was supported by a grant (000153/7/37) from Jazan University, Saudi Arabia.
References
- Sofowora, A., Ogunbodede, E., & Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 10.5:210-229 (2013)
- Parvez, M. K., Al-Dosari, M. S., Rehman, M. T., Alajmi, M. F., Alqahtani, A. S., & AlSaid, M. S. New terpenic and phenolic compounds from Suaeda monoica reverse oxidative and apoptotic damages in human endothelial cells. Saudi Pharmaceutical Journal 29:1102-1111 (2021)
- Rahman, M.A., Mossa J.S., Al-Said, M.S., & Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 75:149-61(2004)
- Al-Wahaibi, L.H.N., Mahmood, A., Khan M., & Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arabian Journal of Chemistry. 13: 2053-2065 (2020)
- Abu-Darwish, M.S., Cabral, C., Gonçalves, M.J., Cruz, M.T., Zulfiqar, A., Khan, I.A., Efferth, T., & Salgueiro, L. J. Ethnopharmacol. 191:161-168 (2016)
- Ghramh, H. A., Ibrahim, E. H., Kilnay, M., Ahmad, Z., Alhag, S. K., Khan, K. A., Taha, R., & Asiri, F. M. Silver Nanoparticle Production by Ruta graveolens and Testing Its Safety, Bioactivity, Immune Modulation, Anticancer, and Insecticidal Potentials. Bioinorganic Chemistry and Applications. 2020:11. (2020).
- Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. Methods in Enzymology. 299:152–178 (1999)
- Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., & Vidal, N. Food Chemistry, 97:654–660 (2006)
- Grzegorczyk-Karolak, I., Matkowski, A., & Wysokinska H. Food Chemistry. 104:536–541. (2007).
- Chu, Y.H., Chang, C.L., & Hsu, H.F. J. Sci. Food Agric.80:561–566 (2000)
- Fatima, N., Rizwan, M., Hobani, Y. H., Marwan, A. E., Kumar, B. V., Al Sunosi, R., Abdulwahab, S. I., Areeshi, M. Y., Alvi, A., & Oriaby, M. E. Journal of Pharmacognosy and Phytochemistry. 6:197-204 (2017)
- Syam, S., Abdelwahab, S. I., Al-Mamary, M. A., & Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules (Basel, Switzerland). 17:6179-6195 (2012)
- Tagg, J. R., & McGiven, A. R. Appl. Microbiol. 21:943 (1971)
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn Rev. 6(11):1-5. (2012). doi: 10.4103/0973-7847.95849.
- Rahman, M. A., Mossa, J. S., Al-Said, M. S., & Al-Yahya, M. A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 75:149-161 (2004)
- Tounekti, T., Mahdhi, M., & Khemira, H. Ethnobotanical study of indigenous medicinal plants of jazan region, saudi arabia.Evidence – Based Complementary and Alternative Medicine. 2019:45 (2019)
- Alqethami, A., & Aldhebiani, A. Y. Medicinal plants used in Jeddah, Saudi Arabia: Phytochemical screening. Saudi Journal of Biological Sciences. 28:805-812 (2021)
- Almousa, A., Hassan, M., Abdallah, H., & Abo-Dahab, N. Anti-microbial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia. Journal of King Saud University – Science. 33(1) (2021). https://doi-org.sdl.idm.oclc.org/10.1016/j.jksus.2021.101462
- Elansary, H. O., Szopa, A., Kubica, P., O. El-Ansary, D., Ekiert, H.; A. Al-Mana, F. Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Anti-microbial and Anticancer Activities. Processes8(3):283 (2020) https://doi-org.sdl.idm.oclc.org/10.3390/PR8050531
- Janackovic, P., Novakovic, J., Soković, M., Vujisic, L., Giweli, A., Dajic, S.Z., & Marin, P. Composition and anti-microbial activity of essential oils of Artemisia judaica, A. herba-alba and A. arborescens from Libya. Archives of Biological Sciences. 67:455-466 (2015)
- Abd-Elhady, H. Insecticidal Activity and Chemical Composition of Essential Oil from Artemisia Judaica L. Against Callosobruchus Maculatus (F.) (Coleoptera: Bruchidae). Journal of Plant Protection Research. 52:347-352 (2012)
- Reethi, K., Kuttan, G., & Kuttan, R. Anti-Tumour activity of Ruta graveolens extract. Asian Pacific journal of cancer prevention. 7:439-43 (2006)
- Mahmoud, E. A., Elansary, H. O., & El-Ansary, D. O., Al-Mana, F. A. Elevated Bioactivity of Ruta graveolens against Cancer Cells and Microbes Using Seaweeds. Processes. 8:75 (2020)
- Elsharabasy, F. S., Metwally, N. S., Mahmoud, A. H., Soliman, M. S., Youness, E. R., Farrag, A. H., & Arafa, S. Phytoconstituents and Hepatoprotective Effect of Suaeda monoica Forssk and Suaeda Pruinosa Lange. Biomed Pharmacol J. 12(1):117-129 (2019)
- Padmakumar, K., & Ayyakkannu, K. Antiviral activity of marine plants. Indian Journal of Virology. 13:33-6 (1997)
- Asgharian, S., Hojjati, M. R., Ahrari, M., Bijad, E., Deris, F., & Lorigooini, Z. Ruta graveolens and rutin, as its major compound: investigating their effect on spatial memory and passive avoidance memory in rats. Pharm Biol. 58(1):447-453 (2020)
- Hashem, H., Abdel Rahman, A. R., Kassem, H., & Aziz, N. Bio-Herbicidal potential of desert plants Artemisia judaica L., Asphodelus microcarpus Salzm. and Viv. and Solanum nigrum L. against Portulaca oleracea and Phalaris minor. Egypt. J. Exp. Biol. (Bot.). 15(1): 99-109 (2019)
- Muthazhagan, K., Thirunavukkarasu, P., Ramanathan T., & Kannan, D. Studies on Phytochemical Screening, Antimicrobial and Anti Radical Scavenging Effect Coastal Salt Mash Plant of a Suaeda monoica. Research Journal of Phytochemistry, 8:102-111 (2014)
- Lincy, P., Paulpriya, K., & Mohan, V.R. Pharma science monitor an international journal of pharmaceutical sciences pharmacochemical characterization and antibacterial activity of Suaeda monoica leaf forssk ex gmel (Chenopodiaceae). Pharma Science Monitor. 4:3947-3963 (2013)
- Allam, H., Benamar, H., Ben Mansour, R., Ksouri, R. & Bennaceur M. Phenolic Composition, Antioxidant, and Antibacterial Activities of Artemisia Judaica Subsp. Sahariensis, Journal of Herbs, Spices & Medicinal Plants, 25(4):347-362 (2019)
- Abubakar, M. N., & Majinda, R. R. T. GC–MS analysis and preliminary anti-microbial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines. 3(1):3p (2016)
- Patra, J. K., Das, G., & Baek, K. H. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible Seaweed, Laminaria japonica L. Molecules. 20:12093-12113 (2015)
- Ziaei, A., Ramezani, M., Wright, L., Paetz, C., & Schneider, A.Z. Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytother. Res. 25:557-562 (2011)
- Al-Wahaibi, L. H. N., Mahmood, A., Khan, M., & Alkhathlan, H. Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arabian Journal of Chemistry. 13(1):2053-2065 (2020)
- Kshirsagar, S., Rao, R. Antiviral and Immunomodulation Effects of Artemisia. Medicina (Kaunas, Lithuania). 57. (2021). 10.3390/medicina57030217.
- Dos Santos, D. C., da Silva Barboza, A., Schneider, L. R., Cuevas-Suárez, C. E., Ribeiro, J. S., & Damian, M. F., Campos, A. D., Lund, R. G. Anti-microbial and physical properties of experimental endodontic sealers containing vegetable extracts. Sci Rep. 11(1):6450 (2021)
- Ravi, R., Zulkrnin, N. S. H., Rozhan, N. N., Yusoff, N. R. N., Rasat, M. S. M., Ahmad, M. I., Hamzah, Z., Ishak, I. H., Mohd Amin, M. F. Evaluation of Two Different Solvents for Azolla pinnata Extracts on Chemical Compositions and Larvicidal Activity against Aedes albopictus (Diptera: Culicidae). Journal of Chemistry, 2018:8 pages (2018)
- Xu, C., Wu, P., Gao, J., Zhang, L., Ma, T., Ma, B., Yang, S., Shao, G., Yu, Y., Huang, X., Yang, X., Zhang, B. Heptadecanoic acid inhibits cell proliferation in PC 9 non small cell lung cancer cells with acquired gefitinib resistance. Oncol Rep. 41(6):3499-3507 (2019)
- Adekenov, S. M. & Mukhametzhanova, G. M. & Gayane A., & Juraj, H. Insect repellent and feeding deterrent activity of natural sesquiterpene lactones and their derivatives. Czech Chemical Society Symposium Series. 13:177-184 (2015)
- Helal, I. M., El-Bessoumy, A., Al-Bataineh, E., Joseph, M. R. P, Rajagopalan, P., Chandramoorthy, H.C., & Ben Hadj, A. S. Anti-microbial Efficiency of Essential Oils from Traditional Medicinal Plants of Asir Region, Saudi Arabia, over Drug Resistant Isolates. Biomed Res Int. 2019:9 pages (2019)
- Čechovská, L., Cejpek, K., Konečný, M., & Velisek, J. On the role of 2,3-dihydro-3,5-dihydroxy-6-methyl-(4H)-pyran-4-one in antioxidant capacity of prunes. European Food Research and Technology. 233:367-376 (2011)
- Casuga, F. P., Castillo A. L., & Corpuz M. J. A. T. Bioactive Compounds and Cytotoxicity of Ethyl Acetate Extract From Broussonetia luzonica (Moraceae) Blanco Leaves against Hepatocellular Carcinoma (Hepg2) Cell Lines. Pharmacognosy Journal. 8(5):497-501 (2016)
- Chibani, S., Bouratoua, A., Kabouche, A., Laggoune, S., Semra, Z., Smati, F. & Kabouche, Z. Composition and antibacterial activity of the essential oil of Ruta chalepensis subsp. angustifolia from Algeria. Der Pharmacia Lettre. 5(5):252-255 (2013)
- Kurach, L., Kulczycka-Mamona, S., Kowalczyk, J., Skalicka-Woźniak, K., Boguszewska-Czubara, A., El Sayed, N., Osmani, M., Iwaniak, K., & Budzyńska, B. Mechanisms of the Procognitive Effects of Xanthotoxin and Umbelliferone on LPS-Induced Amnesia in Mice. Int J Mol Sci. 22(4):1779 (2021)
- Kokpal, V., Miles, D. H., Payne, A. M., & Chittarwong, V. Chemical constituents and bioactive compounds from mangrove plants. Stud. Nat. Prod. Chem. 7:175–199 (1990)
- Lakshmi, K. P., & Narsimha, Rao G. M. Anti-microbial activity of Suaeda monoica (Forsst ex Geml) against Human and plant pathogens. Res. J. Pharm. Biol. Chem. Sci. 4:680–685 (2013)
- Muthazhagan, K., Thirunavukkarasu, P., Ramanathan, T., & Kannan, D. Studies on phytochemical screening, anti-microbial and antiradical scavenging effect of a coastal salt mash plant Suaeda monoica. Res. J. Phytochem. 8:102–111 (2014).
- Berczyński, P., Duchnik, E., Kruk, I., Piechowska, T., Aboul-Enein, H.Y., Bozdağ-Dündar, O., & Ceylan-Unlusoy, M. 6-Methyl 3-chromonyl 2,4-thiazolidinedione/2,4-imidazolidinedione/2-thioxo-imidazolidine-4-one compounds: novel scavengers of reactive oxygen species. Luminescence. 29(4):367-73 (2014)
- Yadav, M., Parle, M., Jindal, D. K., & Dhingr, S. Pharmacological Reports. 70:591–599 (2018)
- Sujatha, S., Anand, S., Sangeetha, K. N., Shilpa, K., Lakshmi, J., Balakrishnan, A., & Lakshmi, B. S. International Journal of Diabetes Mellitus. 2:101–109 (2010).
- Rajashekar, Y., & Kumar, H. V., & Ravindra, K. & Nandagopal, B. Isolation and characterization of biofumigant from leaves of Lantana camara for control of stored grain pests. Industrial Crops and Products. 51:224-228 (2013).
- Almousa, A., Hassan, M., Abdallah, H., & Abo-Dahab, N. Anti-microbial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia. Journal of King Saud University – Science. 33(1) (2021).
- Nasr, F., Noman, O., Mothana, R., Alqahtani, A., & Al-Mishari, A. Cytotoxic, anti-microbial and antioxidant activities and phytochemical analysis of Artemisia judaica and A. sieberi in Saudi Arabia. African journal of pharmacy and pharmacology. 14:278-284 (2020)
- Varamini, P., Soltani, M., & Ghaderi, A. Cell cycle analysis and cytotoxic potential of Ruta graveolens against human tumor cell lines. Neoplasma. 56(6):490-3 (2009)
Abbreviations
AJME: Artemisia judaica Methanol Extract;
RGME: Ruta graveolens Methanol Extract;
SMME: Suaeda monoica Methanol Extract;
TPC: total phenolic contents;
TFC: total flavonoids contents;
DPPH: 2,2-diphenyl-1-picrylhydrazyl;
FRAP: Ferric reducing anti-oxidative power ;
GCMS: Gas Chromatography Mass Spectroscopy ;
MTT: (3-(4, 5–2-yl)-2, 5-diphenyltetrazolium bromide;
OD: Optical density;
MIC: minimum inhibitory concentration;
IZD: inhibition zone diameters.








