Akash Samanta1 , Nupur Kataria1
, Nupur Kataria1 , Kiran Dobhal1*
, Kiran Dobhal1* , Naveen Chandra Joshi 2
, Naveen Chandra Joshi 2 , M.P Singh3
, M.P Singh3 , Shalu Verma1
, Shalu Verma1 , Jyotsana Suyal1
, Jyotsana Suyal1 and Vikash Jakhmola1
and Vikash Jakhmola1
1Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University-Dehradun, India.
2Division of Research and Innovation (DRI), Uttaranchal University -Dehradun, India.
3School of Agriculture, Uttaranchal University-Dehradun, India.
Corresponding Author E-mail: kiran.thapliyal2728@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2700
Abstract
Fatty acid, present in edible oil, is a key constituent in our diet. The iodine number is a measure of the amount of unsaturated fatty acid in fat and oil. Iodine is a trace element that is required by humans for normal biological function. The iodine value (IV) of four edible oils was determined in this study: castor oil, peppermint oil, almond oil, and coconut oil. Iodine is a wonderful reagent for converting the unsaturation into the saturation of fat and oil. The purported technique offered a reliable and rapid determination of IV. The Wijs, or iodine monochloride, potassium iodate, and American Oil Chemists' Society's (AOCS) Fourier transform infrared spectroscopy (FT-IR) are all used to determine IV. Both Wijs and potassium iodate are iodometry-based titrations, whereas the AOCS method is applied through FT-IR. C=C stretching in the range of 1635.48cm-1-1652.77 cm-1, C=O band in the range of 1744.23 cm-1- 1747.49 cm-1, C-H stretching in the range of 2923.9 cm-1- 2925.85 cm-1, O-H stretching in the range of 3448 cm-1- 3472 cm-1 were observed in different dilution for identification of unsaturated fatty acid in numerous oils through FT-IR. All methods are satisfactory; meanwhile, the potassium iodate method is safer than the Wijs method experimentally and more economical than the AOCS method. IV for castor oil, peppermint oil, almond oil, and coconut oil were computed at 84.67 I2/100g,5.56 I2/100gm,99.09 I2/100gm,8.21 I2/100gm along with the deviation by three methods.
Keywords
Almond oil; castor oil; Edible oil; FT-IR spectroscopy; Wijs method
Download this article as:| Copy the following to cite this article: Samanta A, Kataria N, Dobhal K, Joshi N. C, Singh M. P, Verma S, Suyal J, Jakhmola V. Wijs, Potassium Iodate, and AOCS Official Method to Determine the Iodine Value (IV) of Fat and Oil. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Samanta A, Kataria N, Dobhal K, Joshi N. C, Singh M. P, Verma S, Suyal J, Jakhmola V. Wijs, Potassium Iodate, and AOCS Official Method to Determine the Iodine Value (IV) of Fat and Oil. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/42HPsJj |
Introduction
Food adulteration is a global phenomenon that is causing public health hazards. Edible oil is one of the most unavoidable components of human food and is an outstanding nourishing source for muscle and tissue development. Edible oils are mixtures of triglycerides. 1,2,3 The iodine content of fats is one way to determine the amount of unsaturation in fatty acids. 4,5 The iodine value (IV) is the amount of iodine taken per 100 g of fat or oil. In cases of high iodine values, more unsaturation is present. Fatty acids are integral components of lipids and are composed of a carboxyl (-COOH) group and long hydrocarbon chains. 6,7
Material and Method
Apparatus
All glassware was calibrated and washed to the desired room condition.
Reagents and solutions
AR-grade chemicals were used in experiments. All the communal reagents were purchased from commercial supplies and used without any further decontamination. Wijs reagent or Iodine Monochloride (ICI) in glacial ethanolic acid; CAS no.7790-99-0 from Sisco Research Lab. Pvt. Ltd., Potassium Iodide (KI) CAS Number 7681-11-1from Thermo Fisher Scientific India Pvt. Ltd., Potassium Iodate (KIO3); CAS no. 7758-05-6 from Thomas Baker (Chemicals) Pvt. Ltd., Soluble Starch; CAS No. 9005-84-9from Avantor Performance Materials, India limited, Sodium thiosulfate (Na2S2O3); CAS Number 10102-17-7 from Sigma-Aldrich, Cyclohexane(C6H6), Glacial Acetic acid (CH3COOH), Sulfuric acid(H2SO4), Carbon tetrachloride (CCl4), and distilled water were utilized during the experiment.
Determination of iodine value through Wijs solution or hanus method
Wijs’ method is a modified version of Hubls’ method. This is based on the iodometric titration method, in which iodine is produced indirectly and reacts with the unsaturation compound.8,9.
Preparation of oil sample
By adding 25 mL of Wijs reagents, an aliquot of the oil sample (0.1 gm) and an equal portion of cyclohexane and glacial ethanolic acid are properly mixed and covered with aluminum foil and swirled to ensure a homogeneous mixture. Then kept the solution in the dark for about 30 minutes. In another Erlenmeyer flask, the blank sample was prepared under identical conditions.
Standardization of sodium thiosulphate solution
20 mL of potassium iodate solution was mixed with 1 g of potassium iodide in distilled water. Instantly add about 1 mL sulfuric acid and immediately titrate the dark brown solution with a 0.1 M sodium thiosulfate solution until a straw-colored solution is observed. After that, add about 4 mL of starch solution without interrupting the titration; continue until the purple colour just disappears and the solution becomes transparent.
Procedure
Mix an equal portion of cyclohexane and glacial acetic acid and add 25 mL of Wijs solution; close it with aluminum foil after mixing properly. Kept this one in the dark for about 30 minutes. After that, add 20 mL of a 10% potassium iodide solution to both the sample and the blank. Both solutions were diluted with distilled water to the desired concentration. Experiments were conducted in a dark place to protect the Wijs solution, which got oxidized in the sunlight. Next, titrate the blank and sample with a standardized 0.1 M sodium thiosulfate solution. The process continues until the solution displays a pale yellow colour. As an indicator, add 2 mL of a 1% starch solution. The titration continued indefinitely until the blue colour was no longer visible. Take note of the burette’s lower meniscus reading and calculate the IV of these samples.
Determination of iodine value through potassium iodate
Potassium iodate determines the number of double bonds in an oil or fat fraction by an iodometric method. 10
Preparation of potassium iodate solution
In this method, first, we prepared 0.1 M potassium iodate in glacial ethanoic acid. Then, in an amber-color bottle, combine 2.1 g of potassium iodate with 100 mL of glacial acetic acid, which must be thoroughly mixed. The mixture was kept at room temperature in a dark place for 1-2 hours.
Preparation of Sample
Place a precisely measured (0.1 g) oil sample in a clean, dry iodine flask. After that, add a 10 mL standard solution of potassium iodate to the sample flask, which has been previously prepared and allowed to stand for 2 hours in a dark place. In another iodine flask, the sample blank was prepared under the same conditions.
Procedure
Potassium iodate was taken in the iodine flask and allowed to stand for 2 hours in a dark place. Afterward, add 10% potassium iodide (10 mL) and 1 mM sulfuric acid (10 mL) to the sample, as well as a blank, and mix properly. Dilute up to the mark with distilled water. Next, titrate the blank and sample with a standardised 0.1 M sodium thiosulfate solution by using a 1% starch solution (1-2 mL) as an indicator. Titrate until the blue colour fades, then record the ridding of the burette’s lower meniscus.11,12,13
FT-IR Spectroscopy Experiment
The Nicolet Summit LITE (Serial No. BFJ2010008) FT-IR model with a LiTaO3 detector from the Central Instrumentation Facility (CIF), Uttaranchal University-Dehradun (India), is used for the AOCS method execution. Different oil samples were diluted in different solutions for maximum interpretation. Oil samples were diluted in 0.2% (w/v) and 0.05% (w/v) solutions of carbon tetrachloride, and the spectra were interpreted, which means more transparency and clearer graphs. After diluting these solutions, we used the potassium bromide method to detect the oil samples’ functional groups. 14,15,16
Result
The iodine value, or iodine number, is the therapeutic window of edible oil, which expresses the quantity of unsaturated fatty acids present. Here we used different methods for determining the iodine value: the Wijs method and potassium iodate; manual methods are depicted in Figure 1.
 |
Figure 1: Iodine value determination |
The basis of the official method of Wijs’ Method is a simple determination of the iodine number or iodine absorbed by the sample via the blank and sample determinations. A different set of oil samples was titrated with a sodium thiosulfate solution. Table 1.
Table 1: Measurement of iodine value by the Wijs method
|
Oil sample |
Avg. Net volume of Sodium thiosulphate in Burette |
|
IV (I2/gm) |
Reported value. (I2/gm) |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
Avg. |
|||
|
Castor Oil |
81.71 |
80.05 |
82.44 |
80.38 |
80.47 |
81.83 |
81.15 |
81.08 |
81-91 |
|
Peppermint Oil |
85.67 |
87.88 |
87.65 |
86.86 |
86.98 |
87.53 |
87.10 |
5.58 |
5-6 |
|
Almond Oil |
78.71 |
80.05 |
80.94 |
80.18 |
79.11 |
79.67 |
79.78 |
98.47 |
93-105 |
|
Coconut Oil |
86.77 |
86.78 |
86.65 |
86.81 |
86.66 |
86.87 |
86.76 |
9.89 |
7-11 |
|
Blank |
87.67 |
87.88 |
88.23 |
85.66 |
85.98 |
89.83 |
87.54 |
– |
– |
Potassium iodate titration is a versatile technique to detect IV. The iodine values of each vegetable oil were computed in six trials by potassium iodate, where they displayed good reproducibility. Table 2.
Table 2: Measurement of Iodine Value by the Potassium Iodate Method
|
Oil sample + Potassium iodate |
Avg. Net volume of Sodium thiosulphate in Burette |
|
IV (I2/gm) |
Reported value. (I2/gm) |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
Avg. |
|||
|
Castor Oil |
6.82 |
6.85 |
6.76 |
6.68 |
6.05 |
6.8 |
6.66 |
84.51 |
81-91 |
|
Peppermint Oil |
15.88 |
15.91 |
15.87 |
15.98 |
15.85 |
15.89 |
15.90 |
5.71 |
5-6 |
|
Almond Oil |
10.03 |
10.23 |
10.64 |
10.44 |
10.55 |
10.85 |
10.46 |
99 |
93-105 |
|
Coconut Oil |
15.33 |
15.84 |
15.99 |
15.86 |
15.55 |
15.75 |
15.72 |
7.99 |
7-11 |
|
Blank |
15.23 |
16.01 |
16.22 |
17.88 |
16.11 |
16.65 |
16.35 |
|
|
The next method is the AOCS method through FT-IR spectroscopy. In FT-IR, the infrared absorption spectra or range of frequent unsaturated fatty acid derivatives have been identified. The absorption bands characteristic of the unsaturated linkages is shown and discussed in Table 3 and Figure 2-9.
Table 3: Characteristics of FT-IR spectra of different oil samples in cm-1
|
|
Dilution in 0.2% CCl4 |
Figure No. |
|||
|
Oil sample |
C=C stretching |
C-H stretching |
C=O band |
O-H band |
|
|
Castor Oil |
1652.773 |
2923.573 |
1747.492 |
3448.19 |
2 |
|
Peppermint Oil |
1652.666 |
2923.9 |
1747.37 |
3460.406 |
3 |
|
Almond Oil |
1635.48 |
2925.744 |
1747.37 |
3472.496 |
4 |
|
Coconut Oil |
1652.666 |
2925.003 |
1747.5 |
3445.425 |
5 |
|
Oil sample |
Dilution in 0.05 % CCl4 |
Figure No. |
|||
|
C=C stretching |
C-H stretching |
C=O band |
O-H band |
||
|
Castor Oil |
1652.666 |
2925.859 |
_ |
3454.557 |
6 |
|
Peppermint Oil |
1643.326 |
2923.9 |
1750.37 |
3456.767 |
7 |
|
Almond Oil |
1635.492 |
2925.62 |
1747.635 |
3462.446 |
8 |
|
Coconut Oil |
1645.171 |
2925.432 |
1744.232 |
3451.14 |
9 |
 |
Figure 2: FT-IR spectra of castor oil in 0.2% dilution with carbon tetrachloride |
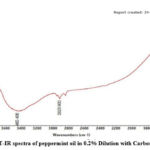 |
Figure 3: FT-IR spectra of peppermint oil in 0.2% Dilution with Carbon tetrachloride |
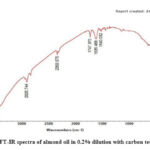 |
Figure 4: FT-IR spectra of almond oil in 0.2% dilution with carbon tetrachloride |
 |
Figure 5: FT-IR spectra of coconut oil in 0.2% Dilution with Carbon tetrachloride |
 |
Figure 6: FT-IR spectra of castor oil in 0.05% Dilution with Carbon tetrachloride |
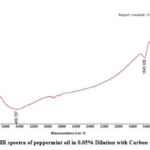 |
Figure 7: FT-IR spectra of peppermint oil in 0.05% Dilution with Carbon tetrachloride |
 |
Figure 8: FT-IR spectra of almond oil in 0.05% Dilution with Carbon tetrachloride |
 |
Figure 9: FT-IR spectra of coconut oil in 0.05% Dilution with Carbon tetrachloride |
Further calculations were computed as per the AOCS official method. Table 4.
Table 4: Computation of IV through the AOCS method
|
S. No |
Samples |
IVAOCS |
IVIR |
Standard Deviation |
|
1 |
Castor Oil |
87.984 |
88.436 |
0.5 |
|
2 |
Peppermint Oil |
4.653 |
5.396 |
0.7 |
|
3 |
Almond Oil |
99.405 |
99.807 |
0.4 |
|
4 |
Coconut Oil |
6.022 |
6.77 |
0.5 |
All three methods were satisfactory and produced the result in precise form. Figure 10 showed the conclusion of all the observations.
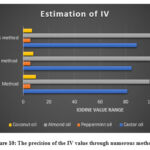 |
Figure 10: The precision of the IV value through numerous methods |
Discussion
The Wijs method is based on the iodometry titration method; iodine is induced indirectly by the reaction of sodium thiosulfate solution with potassium iodide. Iodine is a very active atom that attracts unsaturated fatty acids in fat and oil. IV reported through the Wijs method was 81.08 I2/gm, 5.58 I2/gm, 98.47 I2/gm, and 9.89 I2/gm for castor oil, peppermint oil, almond oil, and coconut oil, respectively, which are compatible with previously reported standards. Table 1. The potassium iodate method is also a kind of iodometry titration; meanwhile, it is easier to conduct, safer, and more economically relevant than the Wijs and AOCS methods for measurement of unsaturation in fats and oils. IV reported through the potassium iodate method was 84.51 I2/gm, 5.71 I2/gm, 99 I2/gm, and 7.99 I2/gm for castor oil, peppermint oil, almond oil, and coconut oil, respectively, which are compatible with previously reported standards. Table 2. The AOCS method by FT-IR is an advanced method to evaluate the unsaturation in fat and oil. The unsaturation level was reported in the range of 1635 cm1to 1652 cm1. Two major functional groups were present in the triglyceride molecule: the carbonyl group (C=O) and the double bond group (C=C). Intriguingly, these two groups play a major role in this AOCS study. There was a decent relationship between IVIR and IVAOCS, as well as a linear connection. A linear regression of the optimized validation on FT-IR results, obtained for the predicted IVs against their standard IVs, yields the following equation: IV p = 0.9965IVa + 0.76, where IV p is the IV predicted by FT-IR and IV a is the IV determined by the AOCS method, with R2 = 0.9995. Indicating the consistency of IVs is determined using the current technique and the AOCS reference technique for different types of oil samples with the calibration standard. Table 4 showed the present method has a decent performance in predicting the iodine value of edible oil samples.
Conclusion
The purpose of our research was to identify the iodine value of oils with simple and efficient techniques. Based on the analysis, it can be concluded that FT-IR spectroscopy is a simple, rapid, and reagent-free method for the determination of the iodine number of edible oils. This method is applicable to a variety of edible oils ranging from the lowest IV to the highest IV without any calibration. Using the data from this study, we came to the following conclusion: the usage of a new reagent, i.e., potassium iodate, for the determination of iodine value is precise and agrees with the results of the standard method of determining iodine value, which is the Hanus method. It cuts down on the number of samples, reagents, and solvents used. But this new reagent, potassium iodate, only identify the compounds that have less unsaturation present. Both these methods are time-consuming and costly, so we also determine the iodine value with the FT-IR method, which is advanced, precise, fast, and simple. The FT-IR analytical spectrum was simple and required a small volume of reagent and sample. By employing the FT-IR technique, the chemical cost is negligible as compared to the AOCS standard method. The double bonds of unsaturated fatty acids have absorption bands in desirable spectrum regions, which is why FT-IR spectroscopy is suitable for determining the IV of fats and oils. Based on our result, our study concludes that FT-IR spectroscopy offers a good supplement or alternative method for the determination of the iodine value of vegetable oils. IV observation explores the content of polyunsaturated fatty acids (PUFA), viz., linoleic acid, linolenic acid, etc. These four oils employed in the investigation are rich in PUFA and exert beneficial effects. This method can be employed for future purposes in the quantitative measurement of PUFA as conjugated and nonconjugated dienes and trienes in different edible oils.
Acknowledgment
Authors conveyed special thanks to Mr. Jitender Joshi, Chancellor, and Prof. (Dr.) Dharam Buddhi, Vice Chancellor of Uttaranchal University-Dehradun encourages publishing this research work. We are grateful to the Division of Research and Innovation (DRI) and Central Instrumentation Facility (CIF), Uttaranchal University-Dehradun, India for providing all facilities during the experimental work.
Conflicts of Interest
There is no conflict of interest.
Funding Sources
This work is done under the Seed Money Project, funded by the Division of Research and Innovation (DRI), Uttaranchal University Dehradun (India), Uttaranchal University-Dehradun, Uttarakhand, India. The grant number of the funding source is UU/DRI/SM/2022/15.
References
- Kratz M, Gülbahçe E, von Eckardstein A, et al. Dietary mono- and polyunsaturated fatty acids similarly affect LDL size in healthy men and women. J Nutr. 2002;132(4):715-718. doi:10.1093/jn/132.4.715
CrossRef - Sachdeva V, Roy A, Bharadvaja N. Current Prospects of Nutraceuticals: A Review. Curr Pharm Biotechnol. 2020;21(10):884-896. doi:10.2174/1389201021666200130113441
CrossRef - Zhang Y, Zhuang P, Wu F, et al. Cooking oil/fat consumption and deaths from cardiometabolic diseases and other causes: prospective analysis of 521,120 individuals. BMC Med. 2021;19(1):92. Published 2021 Apr 15. doi:10.1186/s12916-021-01961-2
CrossRef - Bai G, Ma CG, Chen XW, Hu YY, Guo SJ. Thermal degradation of stigmasterol under the deodorisation temperature exposure alone and in edible corn oil. Food Chem. 2022; 370:131030. doi: 10.1016/j.foodchem.2021.131030.
CrossRef - Timilsina H, Modi B, Basnyat R. Phytochemical, Antimicrobial and Ethnobotanical Study of Calotropis gigantea. Journal of Health and Allied Sciences. 2020;10(2):23-27. doi:10.37107/jhas.136.
CrossRef - V. Amrutkar S, R. Patil A, K. Ishikar S. A Review on Herbal Soap. Research Journal of Topical and Cosmetic Sciences. Published online June 30, 2022:49-54. doi:10.52711/2321-5844.2022.00008
CrossRef - Mikulcová V, Kašpárková V, Humpolíček P, Buňková L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules. 2017;22(5):700. Published 2017 Apr 27. doi:10.3390/molecules22050700l of applied pharmacy.2018;10: 1-4.
CrossRef - Peamaroon N, Jakmunee J, Moonrungsee N. A Simple Colorimetric Procedure for the Determination of Iodine Value of Vegetable Oils Using a Smartphone Camera. Journal of Analysis and Testing. 2021;5(4):379-386. doi:10.1007/s41664-021-00168-x.
CrossRef - Said F, Amer MM, Ahmed AKS, Said AA. Method for the determination of iodine value. Journal of Pharmacy and Pharmacology. 1964;16(3):210-211. doi:10.1111/j.2042-7158.1964.tb07446.x.
CrossRef - Gong T, Zhang X. Determination of iodide, iodate and organo-iodine in waters with a new total organic iodine measurement approach. Water Research. 2013;47(17):6660-6669. doi:10.1016/j.watres.2013.08.039
CrossRef - Pereira AC, Rocha FR. Liquid-liquid microextraction in a multicommuted flow system for direct spectrophotometric determination of iodine value in biodiesel. Anal Chim Acta. 2014;829:28-32. doi:10.1016/j.aca.2014.04.049
CrossRef - Xu L, Zhu X, Yu X, Huyan Z, Wang X. Rapid and Simultaneous Determination of the Iodine Value and Saponification Number of Edible Oils by FTIR Spectroscopy. European Journal of Lipid Science and Technology. 2018;120(4):1700396. doi:10.1002/ejlt.201700396.
CrossRef - Yildiz Y. Iodine Value in Partially Hydrogenated Castor Oil (Ricinus Oil) as determined by AOCS Official Method Cd 1-25 (Wijs’ Method). Advances in Clinical Toxicology. 2020;5(3). doi:10.23880/act-16000190
CrossRef - Ramraje GR, Patil SD, Patil PH, Pawar AR. A brief review on: Separation techniques chromatography. Asian Journal of Pharmaceutical Analysis. 2020;10(4):231-238. doi:10.5958/2231-5675.2020.00041.1
CrossRef - Nandkhile N, Vanpure S, Khetmalis P, Bhondwe R. Spectroscopic Analysis and ADME Studies of Phytochemicals in Methanolic Leaves Extract of Sonneratia apetala Buch.-Ham. Research Journal of Pharmacognosy and Phytochemistry. Published online July 16, 2022:155-162. doi:10.52711/0975-4385.2022.00029
CrossRef - Rana R, Kumar A, Bhatia R. Impact of infra red spectroscopy in quantitative estimation: An update. Asian Journal of Pharmaceutical Analysis. 2020;10(4):218-230. doi:10.5958/2231-5675.2020.00040.x
CrossRef







