Batsuuri Munkhbat1 , Sapaar Bayarmagnai1
, Sapaar Bayarmagnai1 , Bayasgalan Battsagaan2
, Bayasgalan Battsagaan2 and Urjinlkham Jagdagsuren1*
and Urjinlkham Jagdagsuren1*
1 School of Dentistry, Mongolian National University of Medical Sciences (S.Zorig street, Ulaanbaatar 14210, Mongolia)
2 School of Pharmacy, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia (S.Zorig street, Ulaanbaatar 14210, Mongolia)
Corresponding Author E-mail: urjinlkham@mnums.edu.mn
DOI : https://dx.doi.org/10.13005/bpj/2697
Abstract
Recent studies indicate that the incidence of oral mucosal diseases has increased worldwide due to predisposing factors, including unbalanced dietary intake, prolonged therapy with antibiotics, bad habits, and environmental pollution. Akhizunber, a novel herbal preparation delivered for the treatment of oral mucosal ulcers, was prepared from the medicinal herbs of Achillea asiatica Serg, leaves of Juniperus sabina L, and roots of Bergenia crassifolia (L) Fritsch in a ratio of 2:1:2, immersed for 1 week in 40% ethanol. Previous studies revealed effectiveness of Akhizunber in the treatment of oral aphthous stomatitis. Simultaneously, Akhizunber showed in vitro inhibitory effect on development of Candida albicans. For those reasons, we aimed to study effect of Akhizunber in the treatment of oral candidiasis. First, we studied in vitro effects of Akhizunber on biofilm formation by C. albicans on type I collagen cell desks, precoated with mucin. In a clinical study, a total of 50 patients diagnosed with oral candidiasis were participated. In the experimental group, oral administration of azole antifungal agents combined with local application of Akhizunber was performed. In biofilms grown under exposure to higher concentrations of Akhizunber, inhibitory effects on formation of hyphae from yeast cells were observed. The local treatment of oral lesions with Akhizunber combined with oral administration of antifungal agents accelerated healing of mucosal lesions by 3-5 days in comparison with the local use of povidone iodine. Oral administration of antifungal agents in combination with the local application of Akhizunber on mucosal lesions in combined therapy of patients with oral candidiasis showed high effectiveness and can be considered as an alternative treatment option.
Keywords
biofilms; Candida albicans; Fungi; herbal; oral candidiasis
Download this article as:| Copy the following to cite this article: Munkhbat B, Bayarmagnai S, Battsagaan B, Jagdagsuren U. The In Vitro and In Vivo Antifungal Activities of Akhizunber, and Therapeutic Effects Against Biofilm Forming Candida Isolates in Combination with Fluconazole. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Munkhbat B, Bayarmagnai S, Battsagaan B, Jagdagsuren U. The In Vitro and In Vivo Antifungal Activities of Akhizunber, and Therapeutic Effects Against Biofilm Forming Candida Isolates in Combination with Fluconazole. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3XomRb3 |
Introduction
Researchers pointed out that the incidence of oral mucosal diseases has recently increased due to the food and drug consumption, toxic habits, and as a result of environmental pollution. Akhizunber, a Mongolian herbal preparation invented for the treatment of oral mucosal ulcers, is prepared from the herbs of Achillea asiatica Serg, leaves of Juniperus sabina L and roots of Bergenia crassifolia L (Fritsch) in a ratio of 2: 1: 2 with 40% ethanol.
 |
Figure 1: Achillea aziatica. Serg
|
 |
Figure 2: Juniperus sabina L
|
 |
Figure 3: Bergenia crassifolia (L) Fritsch
|
For the complete extraction of biological compounds, appropriate particles of 2 mm were chosen, and 40% ethanol as a convenient extragent, and suitable extraction methods as USP, German pharmacopeial and Bosin methods were utilized. Criteria of effective extraction process are determined by the amount of tannin in the extract.
The preparation was named Akhizunber due to the first syllables of Latin names of these plants 1. Bergenia crassifolia L (Fritsch) (saxifragaceae) is used in traditional medicine as an anti-inflammatory and analgesic agent for the treatment of gastro-intestinal and oral diseases 2. A.asiatica Serg (Asteraceae) is used in the treatment of hemorrhages and stomach ulcer 3. Flowers of this herb are used in the treatment of headache, influenza and asthma. Essential oils of Juniperus sabina L (cupressaceae)have an antibacterial activity 4. Specimens of Bergenia collected from Gachuurt village in fall season, were identified as Bergenia crassifolia L (Fritsch), flowers and leaves of Achillea were prepared from Khairtkhaan Mountain in Tuv province, in months of June and July, and were determined as Achillea asiatica Serg, green branches of Juniperus were collected from Otgon soum, Zavkhan aimag in summer season, and were identified as Juniperus sabina L. A. asiatica Serg is a perennial herb with rhizome and it is found in the Khuvsgul, Khentei, Mongol-Daurian, Khovd and Mongolian Altai geographical ranges 5. It can be found in forest fringes and sandy terraces on western and eastern slopes of mountains. Bergenia crassifolia L (Fritsch) is a species of perennial herb found in Khentei, Khangai cedar forests in alpine belt 6-10 and Juniperus sabina L is a shrubby, extremely variable in shape herb, up to 1.5 m tall andfound onconiferous forests and forest edges 11-13.Previous studies showed presence of hamazulene, α-pinene, sabinene, limonene, flavonoids in Bergenia crassifolia 12 and some essential oils in Juniperus Sabina 13.
Effectiveness of Akhizunber preparation in the treatment of oral aphthous stomatitis were shown in a previous study. The mean healing period of minor aphthous sores treated with Akhizunber was 8.6±3.1 days. The application of Akhizunber resulted in a healing of aphtha without scarring.
In that study, major recurrent aphthous stomatitis was diagnosed in 16.4% of all cases of RAS. The healing period of these sores treated with Akhizunber was 15.3±2.9 days. The sores healed leaving tense scars. The healing period of herpetiform ulcers was 10.3±2.1 days.
In a previous study, Akhizunber showed regenerative effect on mucosal cells, proved by histomorphologic analyses. The preparation exhibited strong inhibitory activities on growth of S. aureus and C.albicans. However, antibacterial effects against E. coli and M. luteus were low.
Superficial and invasive candidiasis is a very common infectious disease 14-16, with Candida albicans as a dominant etiological agent 14,16,17. The shift from colonization to infection by Candida is related to local and systemic immune factors of the host organism, including as well as the administration of immunosuppressive agents and broad-spectrum antibiotics, and the use of dentures 18. The ability of C. albicans and other species of Candida to develop biofilms on inert surfaces or living tissues favors recalcitrant and chronic candidiasis associated, in many instances, with resistance to current antifungal therapy 18-20.
The aim of this study was to evaluate the in vitro and in vivo antifungal activities of Akhizunber, and therapeutic effects against biofilm forming Candida isolates in combination with fluconazole.
Materials and Methods
Microorganisms and growth conditions
In vitro study was performed at the Department of Oral Microbiology, Institute of Health Biosciences, the University of Tokushima Graduate School, Tokushima, Japan. C. albicans CAD1, a clinical isolate 31, was used in biofilm formation. Candida cells were vaccinated in heart of brain infusion broth (BHI, Difco, Becton Dickinson, Sparks, USA), and breeded under the aerobic conditions at 37°C for 18 h 32. After incubation, the cells were gathered in a stationary phase by circuitous at 3 000 × g for 10 min, and the pellets were laved with saline of phosphate-buffered (PBS, 0.01 M, pH 7.5). Prior to biofilm experiments, the yeast cells were resuspended in yeast nitrogen base (YNB)/100 mM glucose medium supplemented with 2.5 mM N-acetylglucosamine to a final concentration of 106 CFU/ml through adjusting the optical density of suspensions to 0.99 at 600 nm wavelength (Beckman DU- 520 UV-Visible spectrophotometer).
Formation of mucin pellicle
Prior to biofilm formation, type I collagen celldesks (Celltight C-1 Celldesk LF, MS-0113K, Sumitomo Bakelite Co. Ltd, Tokyo, Japan) were coated with mucin from bovine submaxillary glands (M3895 – Type I-S, Sigma-Aldrich Co., St. Louis, MO, USA) blended in phosphate-buffered saline (PBS, pH 7.2) at the final concentration of 0.5 mg/ml, as described before 31.
Biofilm formation and Akhizunber addition
Biofilms were grown on mucin-coated type I collagen celldesks located in the wells of flat-bottomed 24-well polystyrene cell culture plates (TPP, Switherland). For biofilm formation a method previously described 32,33 was adapted with minor modifications. Briefly, yeast cells were inoculated in YNB/100 mM glucose medium supplemented with 2.5 mM N-acetylglucosamine at a cell density of 106 CFU/ml and 1 ml of cell suspension was transferred into each well containing type I collagen celldesk. For initial attachment experiments, the plates were incubated aerobically in the presence of Akhizunber for 90 min while shaking in an orbital shaker at 75 × g to allow initial attachment of the cells. For biofilm formation, Akhizunber was added to celldesks after initial attachment phase. The spent medium was aspirated, the celldesks were gently rinsed twice with PBS (500 μl) to remove any loosely adhered cells and 1.0 ml of fresh YNB medium supplemented with different concentrations of Akhizunber was added into the wells. Then, the plates were incubated aerobically at 37° C for 24 hours. For control, the biofilms grown in YNB medium supplemented with ethanol was used. All experiments were performed in triplicate.
CFU counts
CFU counting assay was used to determine the quantity of viable adherent Candida cells present on each celldesk. After initial adhesion phase, each celldesk was gently rinsed with 500 μl of PBS, and treated with PBS containing 0.25% trypsin to enzymatically detach the adhered cells from the disc surface 34. After series of decimal dilutions with PBS, 100 μl of diluted cell suspensions were plated onto Sabouraud glucose agar and incubated at 37°C for 24 hours under the aerobic conditions. To investigate the effect of Akhizunber on biofilm formation, the plates incubated aerobically for 24 hours were used. Colony numbers were counted and expressed as CFU per disk using the following formula: CFU/disk = CFU number × 10 × dilution factor.
Scanning Electron Microscopy (SEM)
Biofilm structure was visualized by SEM. After biofilms were developed, the spent medium was aspirated, and the wells were laved gently with distilled water. Then, biofilms were fixed in 2.5% glutaraldehyde solution for 1 hour at 37°C. The wells were then rinsedthree times with PBS and the biofilms were dehydrated through series of graded incubations in various concentrations of ethanol, as described before 34. Dried samples were placed into a gold sputter coater. Gold-coated biofilms were viewed at 1000× and 3000× magnification using Miniscope TM-1000 scanning electron microscope (Hitachi High-Technologies Corp., Tokyo, Japan).
Research design
The clinical trial study was conducted on 50 patients who were admitted to the Central Dental Hospital of the School of Dentistry, Mongolian National University of Medical Sciences and diagnosed with oral candidiasis. Identification and differentiation of fungi, their resistance to antifungal agents were performed in Molecular Biology and Microbiology laboratory of “Gyals” center, Ulaanbaatar city, Mongolia. Patients were divided into couple groups, namely, a treatment group and a control group using single-blind method. The patients were examined, and the primary and secondary morphological elements on the oral mucosa were noticed, the shape, size, and position of the elements were determined, the assessment was given according to the oral examination sheets and noted on the card. In the treatment group, Akhizunber preparation was used (Akhizunber preparation was rinsed and soaked in cotton roll for 20 minutes), and in the control group, Povidone iodine solution was used (mouth was rinsed and a cotton roll was used for 20 minutes). After the start of the treatment, re-examination was carried out on 1, 2, 3, 4, 5, 6, 7 days, and if necessary, on 14 and 21 days, and the changes were noted on the research card, and the results of the treatment were monitored.
Statistical analysis
An unpaired t-test was carried out for continuous variables to compare the mean between two groups. For categorical variables, Pearson Chi-square test was conducted. Statistical significance was determined at a p- value lower than 0.05. STATA 14 program was used for statistical analysis.
Ethical statement
The study was approved by the Research Ethics Committee of the Mongolian National University of Medical Sciences on June 08, 2018 (№2018/3-10).
Results
Akhizunber showed an inhibitory effect on initial attachment and biofilm formation
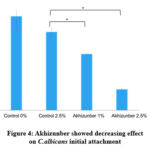 |
Figure 4: Akhizunber showed decreasing effect on C.albicans initial attachment
|
After addition of Akhizunber on C.albicans cultures, the viable cell numbers were counted on initial attachment and biofilm formation stages. Addition of Akhizunber at concentrations of 1% and 2.5% showed an inhibitory effect on initial attachment of C.albicans compared to control groups Fig (4).
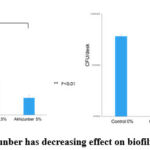 |
Figure 5(a, b): Akhizunber has decreasing effect on biofilm formation (24 h).
|
Further, Akhizunber in concentrations of 1.0%, 2.5% and 5.0% showed decreasing effect on C.albicans biofilm formation (Fig 2a and b). These results may suggest that Akhizunber plays an important role during Candida biofilm formation. Interestingly, C.albicans biofilms formed in the presence of a higher Akhizunber concentrations 2.5% were broken off during washing and the biofilm removability was the highest. Fig (5a, b).
SEM reveals variations in biofilm morphology
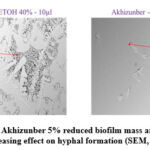 |
Figure 6: Akhizunber 5% reduced biofilm mass and showed decreasing effect on hyphal formation (SEM, ×100).
|
We tested the morphology of Candida biofilms formed on mucin-coated cell desks by SEM (Fig 6). The SEM images identified that the 48-h biofilms of C.albicans incubated with the presence of 40% ethanol were fully matured, consisting of a dense network of yeast and hyphae. In the biofilms formed by C.albicans in the presence of Akhizunber, a dramatic decrease in cell numbers was observed Fig (6).
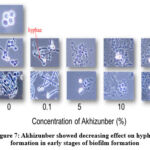 |
Figure 7: Akhizunber showed decreasing effect on hyphal formation in early stages of biofilm formation
|
Formation of Candida biofilms grown on type I collagen celldesks was affected by the presence of Akhizunber in the culture medium. The presence of Akhizunber in media showed an inhibitory effect on hyphae formation from yeast cells Fig (7).
Clinical trial results
Table 1: Age and gender characteristics of study participants
|
|
|
|
Age |
|
|
|
|
N (%) |
41-50 |
51-60 |
61-70 |
71-80 |
|
Male |
12 (24%) |
3 (25%) |
3 (25%) |
4 (33,3%) |
2 (16.7%) |
|
Female |
38 (76%) |
9 (23.6%) |
7 (18.4%) |
18 (47.4%) |
4 (10.6%) |
Overall, 50 patients diagnosed with oral candidiasis including 12 males (24%) and 38 females (76%) in the range of 41-80 years, were enrolled into this study. Among the study participants, 22 (44%) were in the age of 61-70 years, and 12 (24%) in the age of 41-50 years Table (1).
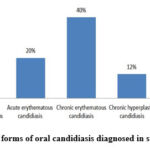 |
Figure 8: Clinical forms of oral candidiasis diagnosed in study participants.
|
Of the 50 participants enrolled, 20 (40.0%) were diagnosed with chronic hyperplastic form of oral candidiasis, 10 (20.0%) had acute pseudomembranous form, and 10 (20.0%) acute erythematous form of infection Fig (8).
Table 2: Duration of denture usage in study participants
|
Variables |
n |
Mean ±SD |
p- value |
|
Chronic |
30 |
5.46 ± 1.85 |
p< 0.05 |
|
Acute |
20 |
3.90 ± 2.88 |
For those with chronic candidiasis, the mean duration of denture usage was 5.5±1.8 years. However, the mean duration of denture usage for patients diagnosed with acute forms of candidiasis was 3.9±2.9 years Table (2), (p<0.05).
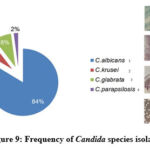 |
Figure 9: Frequency of Candida species isolated
|
Candida albicans was the most identified pathogen in our study groups, with the total incidence of 84%. The non-Candida albicans species isolated from cultures, were С. krusei 6%, С. tropicalis8%, and C. parapsilosis2% Fig (9).
Table 3: Antifungal susceptibility of Candida isolates
|
|
C. albicans |
C. glabrata |
C. krusei |
C. parapsilosis |
|
|
n= 42 |
n= 4 |
n= 3 |
n= 1 |
|
Flucanazole |
43% |
– |
33% |
– |
|
Voriconazole |
– |
– |
– |
– |
|
Caspofungin |
– |
– |
– |
– |
|
Micafungin |
– |
– |
– |
– |
|
Amрhotericin B |
9% |
– |
– |
– |
|
Flucytosine |
48% |
100% |
67% |
100% |
Antifungal susceptibility of Candida isolates was determined. Flucytosine was the most sensitive antifungal, followed by Flucanazole. C. albicans species were sensitive to Flucytosine (48%), Flucanazole (43%), and Amphotericin B (9%) Table (3).
Table 4: Treatment outcome comparisons
|
Healing period (day) |
Study groups |
Chi-square test |
||||
|
Akhizunber |
Povidone iodine |
|||||
|
n |
% |
n |
% |
value |
p- value |
|
|
3-5 |
15 |
60% |
0 |
0% |
24.990a |
0.001 |
|
6-10 |
10 |
40% |
15 |
60% |
||
|
Over 10 |
0 |
0% |
10 |
40% |
||
|
Total |
25 |
50% |
25 |
50% |
||
=24.99, p-value=0.001
After determining susceptibility of Candida isolates to antifungal agents, combined treatment of study groups, with oral administration of antifungal agents, combined with mouth rinsing and mucosal application of local agents was performed, and the treatment outcomes were analyzed Table (4). The mean healing period of 3-5 days was observed in 60% (p<0.001) of patients in a study group treated with local mouth rinsing and applications of Akhizunber, while in a control group treated with local Povidone iodine, all patients recovered in a period 6-10 days.
Discussion
Pseudomembranous candidiasis is characterized by presence of white or whitish-yellow creamy plaques on oral mucosal surface. These pseudo-membranes usually occur on labial and buccal mucosa, dorsum of tongue, hard and soft palate, and can easily be scraped off exposing an underlying erythematous mucosa 7,8,11,12. Acute form of pseudomembranous candidiasis is usually seen in infants with an immature immune system, and adults with predisposing factors, as nutritional deficiency, extremes of age, local immunosuppression (inhalation of steroid aerosols in asthmatics), immunodeficiency conditions such as in patients with HIV/AIDS 9,10.
Denture trauma due to unfitted removable dentures is one of the main etiological factors of chronic erythematous candidiasis and angular cheilitis. In our study, more than one third of patients were diagnosed with chronic erythematous and chronic hyperplastic forms of oral candidiasis 11,13.
Chronic hyperplastic candidiasis, so-called candidal leukoplakia usually occurs as homogeneous and plain plaque-like or nodular lesions found in various sites of oral mucosa 13. This form of candidiasis usually affects males in their middle-ages and associated with smoking. Angular cheilitis manifests as ulcerated fissures, affecting commissures of lips. Other microorganisms implicated are Staphylococcus aureus and Streptococci 31.
Rhomboid glossitis occurs as well-demarcated and diamond-shaped lesions around the midline of tongue dorsum 32. It usually seen in individuals who use inhalation-steroids and tobacco smokers 33,34.
Wearing of ill-fitting removable dentures is the one of the main predisposing factors to oral Candida infection. Denture related candida infections occur in almost 65% of denture wearers and the lesions are usually asymptomatic 35,36. It is believed that acrylic resin promotes adhesion of fungi to the surface and the space under denture base becomes microenvironment favorable to the development of microbial biofilm 37,39.
The anaerobic environment with reduced flow of saliva under the base impedes washout of adhered biofilm. Ill-fitting removable dentures may cause trauma to oral mucosa and increase the risk of penetration of sore spots by Candida cells. In our study, 70% of patients were removable partial denture wearers and 12% were used complete dentures. This may have played a crucial role in the development of infection in study participants 40.
After culturing microorganisms, differentiating and determining resistance of isolates to antifungal agents, we revealed that cultures are sensitive to Flucanazole and Flucytosine. Flucanazole is an antifungal agent of azole group that possesses fungistatic effect, with significant efficacy in the treatment of infections caused by C. albicans. Fluconazole and itraconazole are widely used in clinical practice because of their excellent pharmacokinetic properties, as good oral and intestinal absorption, and wide distribution in tissues. Furthermore, fluconazole maintains high salivary concentrations and most effective agent for treating moderate and severe oral candidiasis 41-47. In our study, the combined treatment of moderate and severe oral candidiasis included oral administration of Fluconazole for 1-3 days and 3-5 days, consequently. Numerous systematic reviews demonstrated that inappropriate diet, low physical activity, underlying disease, alcohol consumption as well as psychosocial stressors are significantly associated with high prevalence of oral candidiasis.
Conclusion
Akhizunber showed an inhibitory effect on C.albicans initial attachment and biofilm formation.Akhizunber may inhibit hyphae formation from yeast cells in biofilms of C.albicans.Oral administration of Fluconazole in combination with mouth rinsing with Akhizunber showed higher therapeutic effect.
Acknowledgement
The authors indebted to the staff of Department of Oral Microbiology, Graduate School of Oral Sciences, the University of Tokushima, and the Department of Pharmacology, School of Pharmacy, MNUMS for their assistance in the research work.
Conflict of Interest
The authors declare that they have no conflict of interest concerning this study.
Funding Sources
This research was supported by grants from Mongolian National University of Medical Sciences, Division for Science and Technology, Grant №17/01/05.
References
- Urjinlkham J, Batsuuri M, Choyjamts G, Oyunbat B. Clinical features of Recurrent Aphthous Stomatitis and therapeutic effects of “Akhizunber”. The International Journal of Oral Health ISSN 1738-8937: 12. 2016; 66.
- Alexander N.Sh, Olga N.P, Marina N.M, Valery G.M, Hildebert W. Bergenia crassifolia (L) Fritsch-Pharmacology and phytochemistry. Phytomedicine. 21. 2014; 1534-1542.
- Dorjsembe B, Julee H, Kim M, Dulamjav B, Jigjid T, Chu won Nh. Achillea asiatica extract and its active compounds induce cutaneous wound healing. Journal of Ethnopharmacology. 206. 12. 2017; 306-314.
- Shuwu X, Guoting L, Lijuan Q, Ruihua Zh, Ping Ch, Zhigang L, Jieyun Zh, Xiangjie G, Zhao L, Aying M, Yueying Q, Yan Zh. Podophyllotoxin Extractad from Juniperus sabina Fruit Inhibits Rat Sperm Maturation and Fertility by Promoting Epididymal Epithelial Cell Apoptosis. Evidence-Based Complementary and Alternative Medicine Volume ID 6958982: 2017; 14.
- Ligaa U, Davaasuren B, Ninjil N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine. Ulaanbaatar. JCK Printing. 2005; 333.
- Grubov V. Key to the Vascular plants of Mongolia. Ulaanbaatar. Gan printing (With an Atlas). 2008; 293.
- Ligaa U, Davaasuren B, Ninjil N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine. Ulaanbaatar. JCK Printing. 2005; 72.
- Grubov V. Key to the Vascular plants of Mongolia. Ulaanbaatar. Gan printing (With an Atlas). 2008; 163-393.
- Vivek V, Byahatti K, Vasantakumar P, Marina G, Souza D. Effect of Phenolic Compounds from Bergenia ciliate (Haw) Sternb. leaves on Experimental kidney stones Ancient Science of Life. 30. 1: 2010; 14-17.
- Bolshunova E.A, Lamazhapova G.P, Zhamsaranova S.D. Research of liposo malform of Bergenia crassifolia (L) Fritsch influence on formation of adaptation potential of the body. Bull. East Siberian State Univ. Technol. 4. 2010; 83-88.
- Nilufer O, Didem D, Alper G, Mustafa A, Fatma E. Comparative Analysis of Chemical Profile, Antioxidant, In- vitro and In-vivo Antidiabetic Activities of Juniperus foetidissima Willd and Juniperus sabina L. Iranian Journal of Pharmaceutical Research. 2017; 16. Special Issue: 64-74.
- Miceli N, Travato A, Dugo P, Cacciola F, Donato P, Marino A, Bellinghieri V, Barbera T, Guvenc A, Taviano M. Comparative analysis of flavonoid profile, antioxidant and antimicrobial activity of the berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009; 57. 6570-6577.
- Ligaa U, Davaasuren B, Ninjil N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine. Ulaanbaatar. JCK Printing. 2005; 65.
- McCarty T, White C, Pappas P. Candidemia and invasive candidiasis. Infect Dis Clin North Am. 2021; 35. p 389-413.
- Saito H, Shodo R, Yamazaki K, et al. The association between oral candidiasis and severity of chemoradiotherapy- induced dysphagia in head and neck cancer patients: a retrospective cohort study. Clin Transl Radiot Oncol. 2020; 31.p 13-18.
- Hu L, He C, Zhao C, et al. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb Pathog. 2019; 134. 103575.
- Miranda K, Marcos C, Mateo E, et al. Prevalence and antifungal susceptibility profiles of Candida glabrata, Candida parapsilosis and their close-related species in oral candidiasis. Arch Oral Biol. 2018; 95.p 100-107.
- Vila T, Sultan A, Montelongo D, et al. Oral candidiasis: a disease of opportunity. J Fungi (Basel Switzerland). 2020; 6. p 1-28.
- Cavalheiro M, Teixeira MC. Candida biofilms: threats, challenges, and promising strategies. Front Med. 2018; 5.p 1-15.
- Silva S, Rodrigues C, Araujo D, et al. Candida species biofilms antifungal resistance. J Fungi (Basel Switzerland). 2017; 3.8.
- Whaley S, Berkow E, Rybak J, et al. Azole antifungal resistance in Candida albicans and emerging non-albicans species. Front Microbiol. 2017; 7. p 1-12.
- Shi C, Liu J, Li W. et al. Expression of fluconazole resistance-associated genes in biofilm from 23 clinical isolates of Candida albicans. Brazilian J Microbiol. 2019; 50. p 157-163.
- Mukherjee P, Chandra J, Kuhn D, et al. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003; 71. p 4333-4340.
- Salari S, Khosravi A, Mousavi S, et al. Mechanism of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV -infected patients with oropharyngeal candidiasis, J Mycol Med. 2016; 26. p 35-41.
- Katagiri H, Fukui K, Nakamura K, et al. Systemic hematogenous dissemination of mouse oral candidiasis is induced by oral mucositis. Odontology. 2018; 106. p 389-397.
- Lu M, Li T, Wan J, et al. Antifungal effects of phyto-compounds on Candida species alone and in combination with fluconazole. Int J Antimicrob Agents. 2017; 49. p 125-136.
- Bailly C. Targets and pathways involved in the anti-tumor activity of citral and its stereo-isomers. Eur J Pharmacol. 2020; 871. 172945.
- Khan M, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemother.2012; 67. p 618-621.
- Freire J, Junior J, Silva D, et al. Antibiofilm activity of essential oils against Candida albicans strains isolated from users of dental prostheses. Evid Based Complement Altern Med. 2017; 7158756.
- Chatrath A, Gangwar R, Kumari P, et al. In vitro anti-biofilm activities of citral and thymol against Candida tropicalis. J Fung. 2019; 5. p 13.
- Grubov V. Key to the Vascular plants of Mongolia. Ulaanbaatar. Gan printing (With an Atlas). 2008; 28. 328.
- Satibaldiva D , Mursalieva B , Gemedjieva N, Sultanova N, Elibaeva N, Turatseva C. Study of the composition and content of biologically active substances of Bergenia crassifolia (L)Fitsch and Sanguisorba officinalis (L). Kaz NU. Bulletin. Biology series 1 (57). 2013; 82-87.
- Shatar S, Basics of forest pharmaceuticals and chemical technology Bergenia crassifolia L. Fritsch. Ulaanbaatar: Ekimto Printing. 2005; 103.
- Sardari F, Khalili P, Hakimi H, Mahmoudaghaei S, Abedi P, The Prevalence of denture stomatitis in cigarette and hookah smokers and opium addicts: Findings from rafsanjan cohort study. BMC Oral Health 2021; 455.
- Hirota K, Murakami K, Nemoto K and Miyake Y. Coating of a surface with 2-methacryloyloxyethyl phosphorylcholine (MPC) co-polymer significantly reduces retention of human pathogenic microorganisms. FEMS Microbiol Lett 248. 2005; 37-45.
- Li J, Hirota K, Yumoto H, Matsuo T, Miyake Y, Ichikawa T. Enchanced germicidal effects of pulsed UV-LED irradiation on biofilms. J Appl Micribiol 2010; 109. 2183-2190.
- Li J, Hirota K, Goto T, Yumoto H, Matsuo T, Miyake Y, Ichikawa T. Biofilm formation of Candida albicans on implant overdenture materials and its removal. J Dent 2012; 40. 686-692.
- Nur A, Hirota K, Yumoto H, Hirao K, Liu D, Takashi K, Murakami K, Matsuo T. Effects of extracellular DNA and DNA-binding protein on the development of a Streptococcus intermedius biofilm. J Appl Microbiol 2013; 115. 260-270.
- Zomorodian K, Haghighi N, Rajaee N, Pakshir K, Tarazooie B, Vojdani M. Assessment of Candida species coloniza -tion and denture-related stomatitis in complete denture wearers. Med Mycol 2011; 49. 208-11.
- Lu M, Yan H, Yu C, et al. Proton pump inhibitors act synergistically with fluconazole against resistant Candida albicans. Sci Rep. 202.10(1). p 498. doi:10.1038/s41598-019-57174-4.
- Xueqi Ch, Jiyong W, Lei S, Jing N, Shan S, Shujuan S. Antifungal Effects and Potential Mechanisms of Benserazide Hydrochloride Alone and in Combination with Fluconazole Against Candida albicans. Drug Design, Development and Therapy 2021; 15. p 4701-4711. https://doi.org/10.2147/DDDT. S336667.
- Katherine M, Cristina B, Aitzol P, Ivan C, Estibaliz M, Elena S, Lucila M, Guillermo O, Elena E. In vitro and in vivo anti- Candida activity of citral in combination with fluconazole. J of Oral Microbiology. 2022; 14. 2045813. https://doi.org/10.1080/20002297.2022.2045813.
- Aishwarya Jayan and Swati Gupta* Effective Combinations Against Efflux Pump Overexpressed on Azole Resistance Candida and Dermatophytes: A Systematic Review. Biomedical & Pharmacology Journal, March 2023. Vol. 16(1), p. 15-25. https://dx.doi.org/10.13005/bpj/2583
- Rashmeen Naaz , Shricharith Shetty , Sharad Chand , Nandakumar UP , Vinay BC and Bharath Raj KC * Prospective Observational Study on Prescribing Pattern of Antifungal Drugs among the 400 Out-patients in Department of Dermatology in a Tertiary Care Hospital Biomedical & Pharmacology Journal, March 2021. Vol. 14(1), p. 311-316. https://dx.doi.org/10.13005/bpj/2127
- Alessandro Leite Cavalcanti, Yeska Paola Costa Aguiar, Fabio Gomes Dos Santos, Alidianne Fabia Cabral Cavalcanti, Ricardo Dias De Castro. Susceptibility of Candida albicans and Candida non-albicans Strains to Essential Oils. Biomedical & Pharmacology Journal Vol. 10(3), 1101-1107 (2017) https://dx.doi.org/10.13005/bpj/1209
- Snazina Saeedi, Shamimul Hasan, Kuldeep Seema Singh Parmar. Conventional and Recent Diagnostic Aids in Oral Candidal Infections: A Brief Overview. Biomedical & Pharmacology Journal Vol. 10(1), 419-426 (2017). https://dx.doi.org/10.13005/bpj/1124
- Ali Mikaeili, Msoud Modarresi, Mansa Maleki. Anti-Candida Effects of Clotrimazole and Fluconazole against Isolated C. albicans from Patinets of Candidiasis Admitted in Medical Mycology Lab of Special Clinic of Kermanshah University of Medical Sciences, 2014. Biomedical & Pharmacology Journal Vol. 8(2), 931-935 (2015). https://dx.doi.org/10.13005/bpj/844.








