Manuscript accepted on :11-02-2022
Published online on: 12-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Surendra K Swarnkar
Second Review by: Dr. Sherif Ramzy
Final Approval by: Dr. H Fai Poon
Nagaraju Kancherla1 , Anusha Dhakshinmoothi2
, Anusha Dhakshinmoothi2 , K Chitra3
, K Chitra3 , Jayasree Palla4*
, Jayasree Palla4* and Ravi Babu Komaram1
and Ravi Babu Komaram1
1Department of Pharmacology, GSL Medical College and General Hospital, Rajamahendravaram, Andhra Pradesh, India
2Department of Pharmacology Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India.
3Department of Pharmaceutical Chemistry, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India.
4Department of Community medicine, GSL Medical College and General Hospital, Rajamahendravaram, Andhra Pradesh, India.
Corresponding Author E-mail:jayasreeobg@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2677
Abstract
Background and objective: Failure of natural homeostasis of healthy cell due to hyperproliferative nature of cancer, resulting in stimulation of various genes which are intensively participated in cell cycle, survival, angiogenesis and metastasis. Our study focused on the in-vitro antioxidant and cytotoxic potentials of the whole plant fractions of Cayratia auriculata, a medicinal plant belongs to Vitaceae family. Materials and Methods: The whole plant material was shade dried and powdered, fractions were prepared by using soxhlet extraction technique with the ascending order of polarity such as hexane < chloroform, < ethyl acetate < methanol. Screening for phytoconstituents in fractions was carried with standard biochemical instigations. Quantitative investigation was done by using different assays such as total phenolic content (TFC), ferric reducing antioxidant power (FRAP) assay, nitric oxide scavenging activity, 2,2 -Diphenyl-1-picrylhydrazyl (DPPH) assay and total antioxidant activity (TAC) to reveal antioxidant capacity. In-vitro cytotoxicity activity on A549 lung cancer cell line was evaluated by (3-(4, 5-dimethyl thiazole-2yl)-2, 5-diphenyl tetrazolium bromide) MTT assay Results: Phytochemical analysis of all four fractions showed the existence of varying degree of secondary bioactive metabolites but methanol fraction exhibited richness in phytoconstituents. Methanol fraction revealed good total phenolic content, potent antioxidant potential in FRAP, DPPH, Nitric oxide scavenging activity and total antioxidant activity in contrast to other tested fractions. MTT assay revealed that methanol fraction C. auriculata has strongest cytotoxic effect towards (A549) lung cancer cell line with an inhibitory concentration (IC50) value of 115.14 µg/ml. Conclusion: The results of present study indicate that different fractions of C. auriculata showed the existence of varying degree of phytochemicals, total phenolic content and dose dependent antioxidant activity. Methanolic fraction revealed richness in phytochemicals, total phenolic content, potent antioxidant, and anticancer property (in- vitro).
Keywords
Antioxidant; Anticancer; Cayratia Auriculata; Methanol fraction; Phytochemical
Download this article as:| Copy the following to cite this article: Kancherla N, Dhakshinmoothi A, Chitra K, Palla J, Komaram R. B. Preliminary Screening for In-Vitro Antioxidant and Anticancer Potentials in Whole Plant Fractions of Cayratia Auriculata (Vitaceae). Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Kancherla N, Dhakshinmoothi A, Chitra K, Palla J, Komaram R. B. Preliminary Screening for In-Vitro Antioxidant and Anticancer Potentials in Whole Plant Fractions of Cayratia Auriculata (Vitaceae). Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3MizIaK |
Introduction
Failure of natural homeostasis of healthy cell due to hyperproliferative nature of cancer, resulting in stimulation of various genes which are intensively participated in cell cycle, survival, angiogenesis and metastasis. It makes a path to global tombstoning, the finest reason for the morbidity and mortality across the world1. Modalities that are currently involving in the cancer treatment are radiation, hormonal, chemotherapy and also surgical interventions. Even though great effective improvement exhibited by all the above therapies but finally ends with serious toxic and hazardous side effects2. As the part of cancer treatment, chemotherapeutic agents such as vinblastine, vincristine and other drugs nearly 60% are contributed by natural substances or their by products, recognized by FDA (Food and Drug Administration) 3. In present cancer treatment, the role of chemotherapeutic agents has been limited due to emergence of drug resistance, multiple side effects and expensiveness. This makes a key role for researchers in persistent investigation of novel medicinal plants possessing potent anticancer activities and minimal side effects with least investment, enormously increasing in present era4.
Establishment of anticancer properties of various species of medicinal plants, which plays vital role in herbal medicine, commonly used in low economic countries, was extensively revealed by a vast number of researchers5-6. In view of worldwide cancer drugs sales during the year 2000, it was noticed that 14 drugs, out of 35 top cancer drugs were obtained from natural resources or their derivatives. Thus, it gives an important lead to investigate plenty of novel natural substances from medicinal plants7. It has been observing that there is a tremendous increase in attraction towards free radical and their derived products in recent years. The well known reactive species of oxygen (ROS) and nitrogen derived oxidising elements include hydrogen peroxide (H2O2), superoxide (O2−) anion, reactive hydroxyl radical (OH−), peroxyl radical (ROO−) and also peroxynitrite anion radical(ONOO), nitric oxide radical (NO) respectively8.
In present scenario majority of physiological disorders and diseases are the outcomes of oxidative stress, where disproportion symmetry between biosynthesis and neutralization of pre oxidants, concluded by current revealed results. Free radicals play a key role in initiation of oxidative stress, which ends with damage of endogenous biomolecules such as nucleic acids, lipids and proteins by pairing with them in order to attain stability. The above modifications in oxidative stress are greatly responsible for the initiation and development of countless physiological disorders like neurodegenerative, cardiovascular, gastrointestinal, respiratory and also particularly involved in various types of malignancies9.
It is most familiar that excellent antioxidant and anticancer potentials exhibited by plant rich phytoconstituents like tannins, phenolic compounds and flavonoids. Some studies reported fine linear relationship between increased life expectancy and decreased incidence of cancer mortality as well as coronary heart disease due to high dietary supplements of natural antioxidants10. Several natural polyphenolic compounds derived from plant origin or their crude fractions in in-vitro studies showed significantly higher antioxidant activities when compared to standard antioxidants like vitamin C as well as vitamin E. Well balanced consumption of vegetables and fruits, having high content of antioxidants notably lower the risk of numerous malignancies indicating that for the prevention of spread of cancer, antioxidants from natural sources could be efficient tools11.
Cayratia auriculata is belongs to family of Vitaceae, native of south Indian species. Cyphostemma auriculatum (Roxb) is the gamble synonym for C. auriculata (Roxb). It is well grown in dry evergreen to dry deciduous forests, climber in nature, commonly found in Myanmar, Bangladesh, West Bengal, Orissa12 Bihar, Rajasthan13 Gujarat, Madhya Pradesh14 Maharashtra15 Goa, Karnataka, Andhra Pradesh16-17 Tamilnadu18 and Kerala. Ethnobotanical review of C. auriculata suggested that one of the generally used plant in folklore medicine to treat cardiac disorders, rheumatism, intestinal worm infestations,19 dog bite, wound abscess, cough, malignancies, hydrocele, purulent wounds, wound healing , and also as blood purifier, earache as tonic,20 as astringent. This plant has also a great role treating animal’s diarrhoea and bloody dysentery21. Phytochemical analysis suggests that, vitaceae family species contains phenols, saponins tannins, alkaloids, flavonoids, steroids, stilbenoids and terpenoids22.Available literature survey on C. auriculata does not have antioxidant and anticancer potentials, hence the current study aimed to evaluate those properties (in-vitro evaluation).
Materials and Methods
Collection of plant material
Collection of plant material of C. auriculata was done in 2018, during the month of August from the Eastern Ghats of Visakhapatnam (Araku valley), A.P. India and got identified by Prof. S.B. Padal, Botanist, Department of Botany, Andhra University, Visakhapatnam, A. P., India. Plant herbarium was arranged and kept at Botany Department Herbarium; Andhra University, India. A voucher specimen (AU-BDH-022228) was deposited at department of Botany, Andhra University (AU), Visakhapatnam (Vizag), A. P., India. The collected study fresh plant material was cleaned with distilled water for the removal of unnecessary mud and derbies, allowed shade dried at room temperature for 14days. The dried whole plant material made into small pieces, converted into fine crude powdered form and stored in air tight container in a refrigerator at 4oC till used for further analysis.
Preparation of Plant Fraction
Thirty (30) grams of whole plant crude powder used for the preparation different fractions with the help of soxhlet extraction technique applying the procedure of Jensen 23 with various solvents in ascending order of their polarity viz. hexane, chloroform, followed by ethyl acetate and finally methanol. Fresh crude powder was used for extraction, filtration was done by Whatman No. 1 (42) filter paper. Each fraction was concentrated by using rotary vacuum evaporator and allowed to dry by using water bath to evaporate left- over solvent.
Preliminary Phytochemical analysis
The presence preliminary phytoconstituents determination was carried out with freshly prepared all four crude fractions of C. auriculata by with standard procedures for presence of numerous Phytoconstituents like glycosides, saponins, carbohydrates ,alkaloids, steroids, anthraquinones, Coumarins , terpenoids , flavonoids , proteins, phlobatannins and tannins 24.
Estimation of Total Phenolic Content (TFC) – (Folin-Ciocalteu method)
Estimation of the total phenolic content of all present study fractions of C. auriculata were carried out by (FCR) Folin Ciocalteu reagent described by Singleton V.L and Rossi J.A (1965)25. Gallic acid taken as the standard, dilutions varying from 50 to 1000 (μg/ml) were used to obtain calibration curve of standard. All the readings of this experiment were taken as triplicate and outcomes were communicated in terms of mg of GAE/g of tested fraction (mg of gallic acid equivalents).
Determination of antioxidant activity by using in vitro methods
FRAP (Ferric reducing antioxidant power) Assay
Estimation of reducing power antioxidant potentials of various fractions of C. auriculata was determined by utilizing ferric reducing antioxidant power test described by Oyaizu et al (1986)26. FRAP values were determined by estimating the absorbance of coloured product at 700 nm utilizing UV-Visible Spectrophotometer.
Nitric oxide radical scavenging activity
Antioxidant potential of test compounds by nitric oxide radical scavenging property determined as per the method described by Chakraborthy GS (2009)27. The optical density of resultant the color product, due to diazotization of the nitrite radical by sulphanilamide, followed by successive coupling taking place with Naphthylethylene diamine dihydrochloride was measured at 546nm utilizing UV-Visible Spectrophotometer. Percentage (%) of nitrite radical reducing capacity was determined with the below formula. Nitric oxide scavenged (%) = Abs (control) – Abs (test)/ Abs (control) × 100, where Abs (control) = absorbance of control sample and Abs (test) = absorbance of the tested samples (fractions/standard). The scavenging of capacity of testes fractions and standard were determined in relative to control, calibration curves were prepared and IC50 calculated.
Determination of antioxidant activity by 2,2 -Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capacity assay
Assessment free radical reduction potential of various crude fractions of C. auriculata carried out by using stable free radical scavenger (DPPH) 2, 2-diphenyl-1-picrylhydrazyl in relation to free radical-reducing potential or the capability of hydrogen donating property by the protocol followed by Braca et al. with minor alterations28. Optical density recorded at 517nm by UV-Visible Spectrophotometer. Standard selected for this experiment was vitamin C and methanol used as methanol. The percentage (%) of DPPH scavenging effect was expressed by using the formula: DPPH scavenging capacity (%) = [(Abs(c) –Abs (t))/ Abs(c)] × 100. In this (Abs(c)) is the control sample absorbance, and (Abs(t)) is the test sample or standard absorbance. The IC50 value represents the concentration, at which inhibition 50% of the DPPH free radical by the antioxidants (test sample or standard) also calculated in addition to percentage of inhibition.
Determination of Total Antioxidant Capacity (TAC)
Total antioxidant potential of crude fractions C. auriculata was analysed with phosphomolybdate assay, taking ascorbic acid as standard, adhering the protocol explained by Prieto et al29. The absorbance was recorded with UV-VIS spectrophotometer at 695nm.
In vitro cytotoxicity by MTT assay
The MTTassay dependent on colorimetric rule. In short, mitochondrial succinate dehydrogenase accountable for decrease of MTT molecule 3-(4, 5-dimethythiazol-2-yl)- 2, 5-diphenyl tetrazolium bromide (yellowish colour) to an insoluble, coloured formazan product (dark purple).The cells are treated with DMSO to the production of formazan after solubilisation of cells, which is estimated spectrophotometrically (570 nm) described by Denizot F et al30. Methanol crude fraction at various strengths was added to cells for example, 12.5, 25, 50, 100, 150, 200 µg/mL and allowed for 24 hrs incubation. The plates containing reaction mixture were allowed for incubation at 37ºC for extra 2-4 h. The formazan cells were broken down in 100μL DMSO and the optical density was taken spectrometrically at 570 nm. The morphological alterations of untreated i.e., control and the treated cells were seen under microscope magnifying lens after 24 h and captured. Entire the experimental readings were taken in triple manner. The level of (%) growth inhibition was expressed by the following in equation.
In this , C-(abs)= control absorbance, T(abs)]= test absorbance
A chart was constructed with concentration of fraction on x-axis and absorbance on y-axis. The IC50 (the concentration that needed to kill half of cells in biologically active cells after a 48 hours exposed to fraction) values were determined from the graph.
Results and Discussion
Preliminary Phytochemical analysis
In the pilot qualitative phyto-chemical investigation of four present study plant fractions of C. auriculata viz. methanol, ethyl acetate, chloroform and hexane revealed the existence of wide range of phytoconstituents. The abundant amount (+++) of tannins and flavonoids, moderate amount (++) of saponins, terpenoids and traces (+) of alkaloids, anthraquinones, carbohydrates, coumarins, glycosides, proteins, phlobatannins and steroids are found in methanol fraction. Ethyl acetate fraction revealed the existence of moderate amount (++) terpenoids, tannins and flavonoids, traces (+) of alkaloids, anthraquinones, carbohydrates, glycosides, proteins, phlobatannins and absence (-) of coumarins and steroids. Moderate amount (++) of flavonoids, traces of (+) alkaloids, carbohydrates, proteins, phlobatannins, saponins, tannins, terpenoids and absence (-) of anthraquinones, coumarins, glycosides and steroids were revealed by chloroform fraction. Hexane fraction showed the existence of traces (+) of proteins, alkaloids, saponins phlobatannins, carbohydrates terpenoids, tannins and absence (-) of flavonoids, anthraquinones, coumarins, glycosides, proteins, steroids (table 1). Presence of above listed phytoconstituents in vitaceae family species in different fractions reported by several authors31-32. It was suggested from the literature various phytoconstituents present in plants are responsible for their diversity of biological activities, which in turn plays a crucial role in treating several pathological conditions viz. high content of tannins in plant are responsible for their anti-inflammatory, anti diarrhoeal and antioxidant activity, anti-diuretic property exhibited by alkaloids. Anti-inflammatory, antioxidant, antimicrobial, anti-spasmodic and antifungal properties were exhibited by flavonoids, phenols and saponins 33- 34. Antioxidant properties exhibited by plant derived proteins; Plants are one of major source of proteins which are the important human organic molecules apart from carbohydrates and lipids 35- 36. It is familiar that various polyphenolic substances, which are structural resemblance of flavonoids, phenolic acids, and tannins, which have excellent antioxidant and anticancer activities, are inherently present in varying degree in most of plant materials. Plant derived phytoconstituents acts as lead molecules in the drug invention, design and development 37.
Table 1: Results of Qualitative Preliminary Phytochemical Analysis of the Hexane, Chloroform, Ethyl Acetate and Methanol Fractions of Cayratia Auriculata: (-) = Absence, (+) = Traces, (++) = Moderate, (+++) = Abundant of Phytoconstituents.
| Phytoconstituents | Methanol Fraction | Ethyl acetate Fraction | Chloroform Fraction | Hexane Fraction |
| Alkaloids | + | + | + | + |
| Anthraquinones | + | + | — | — |
| Carbohydrates | + | + | + | + |
| Coumarins | + | — | — | — |
| Flavonoids | +++ | ++ | ++ | + |
| Glycosides | + | + | — | — |
| Proteins | + | + | + | — |
| Phlobatannins | + | + | + | + |
| Saponins | ++ | + | + | + |
| Steroids | + | — | — | — |
| Tannins | +++ | ++ | + | + |
| Terpenoids | ++ | ++ | + | + |
Quantitative determination of total phenolic content (Folin-Ciocalteu Assay).
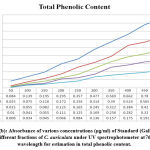
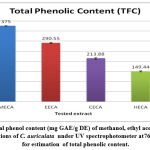
In the current examination, total level of phenols of various concentrates of C. auriculata was determined by the Folin–Ciocalteu reagent method and expressed as mg GAE/g dry fraction. Based on the results obtained from present study it is conformed that all fractions of C. auriculata possessing considerable levels of phenols, among the other fractions, methanol fraction exhibited high level of phenolic content. Total phenolic content in methanol fraction is 375±1.11mg GAE/g dry fraction, followed by ethyl acetate fraction (290.55±2.22)mg/g GAE/g dry fraction, chloroform (213.88±3.33)mg/g GAE/g dry fraction, hexane fraction (149.44±2.77) mg/g GAE/g dry fraction Fig.1 (a, b, &c).Generally many medicinal plants having high content antioxidants like phenols and ploy phenols. Several literature surveys proved that there is good relationship between phenolics content and their antioxidant properties38. The level of phenolic content of plant fractions are significantly responsible for antioxidant properties .It is well known that redox properties exhibited by the phenolic compounds are account for their antioxidant activity, which plays a vital role decomposing peroxides and free radical neutralizing reactions39. The results strongly provides the evidence that some the pharmacological properties of this plant are because of the presence of abundant amount of valuable polyphenols and phenolic molecules. The hydroxyl groups present phenols and polyphenols are responsible for free radical scavenging and antioxidant properties, depending on this phenomenon for rapid evaluation of scavenging properties of free radicals and antioxidant activities crude fractions, content of total phenols used as a standard reference40-41.
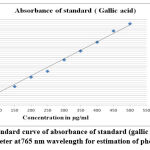 |
Figure 1(a): Standard curve of absorbance of standard (gallic acid) under UV spectrophotometer at 765 nm wavelength for estimation of phenolic contents. |
Determination of antioxidant activity by using in vitro methods
FRAP (Ferric ion reducing antioxidant power) Assay
Donation of hydrogen molecule is the important property of reducing compounds to exhibit their antioxidant capacity, which in turn resulting in the breakage of free radical chain reactions42. The outcomes revealed from the current study as shown in Fig.2. The absorbance of C. auriculata as well as standard antioxidant i.e. ascorbic acid fairly increased with increasing concentration, which is the resultant of varying shades of green chromophore (Fe2+-TPTZ complex) formation. Among four the four fractions viz. methanol, ethyl acetate, chloroform and hexane, methanol fraction revealed highest reduction capability than other fractions. The basic reason for ferric reducing power capacity because of the existence of phenol and polyphenolic compounds in various fractions in crude form of plants, antioxidant capacity by FRAP assay is a well known and the most significant indicator for the assessment of antioxidant potential of plant fractions 43.
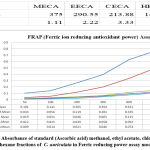 |
Figure 2: Absorbance of standard (Ascorbic acid) methanol, ethyl acetate, chloroform and hexane fractions of C. auriculata in Ferric reducing power assay model. |
Nitric oxide scavenging activity
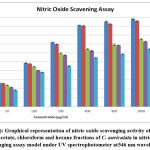
Homeostasis of many physiological functions, nitric oxide molecule (NO) plays a crucial role, which is produced endogenously from endothelial cells (EDRF) endothelial derived relaxing factor, neurons, macrophages and other cells44. Many diseases exhibited positive linear relationship with increased levels of (NO) nitric oxide45. From the results obtained of the present, it was noticed that methanolic fraction of C. auriculata (MECA) revealed higher inhibition capacity when compared with other fractions, but which was low when compared with ascorbic acid (standard). The maximum nitric oxide scavenging activity of crude fractions of C. auriculata viz. methanol, ethyl acetate, chloroform and hexane were 95.77%, 75.37%, 72.73% and 64.46% with IC50 values 88.9µg/ml, 268.72µg/ml, 331.52µg/ml and 515.84µg/ml respectively. The maximum nitric oxide scavenging activity of standard (ascorbic acid) 98.06% while IC50 value 71.11µg/ml, these values are closely similar to methanol fraction Fig.3 (a&b). Several authors reported that polyphenolic compounds and flavonoids are exhibiting their antioxidant property by scavenging the nitric oxide free radicals46- 48. From the above studies it may be concluded that the presence of flavonoids and polyphenols are responsible for nitric oxide scavenging affect which in turn responsible for antioxidant property of plant fractions.
Determination of antioxidant activity by 2, 2 -Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capacity assay
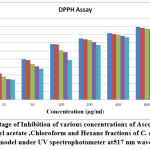
Methanol extricate showed the most extreme level of restraint among all the four concentrates, i.e., 81.88% followed by 69.54% of ethyl a acetate fraction, 58.02% of chloroform remove, 45.67% of hexane concentrate, and which slight lower than that of standard ascorbic acid i.e., 91.67% Fig.4(a,b,&c). The current outcomes suggest that the concentrates are obviously adequate free extreme scroungers and likely have the ability to stifle autoxidation of unsaturated fats and lipids, which might be positive in the treatment of a few infections where peroxidation of lipids is a transcendent system in their pathogenesis 49.
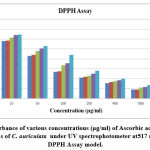 |
Figure 4(a): Absorbance of various concentrations (μg/ml) of Ascorbic acid (Standard) and different fractions of C. auriculata under UV spectrophotometer at 517 nm wavelength in DPPH Assay model. |
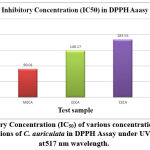 |
Figure 4(c): Inhibitory Concentration (IC50) of various concentrations of Ascorbic acid (standard) and fractions of C. auriculata in DPPH Assay under UV spectrophotometer at 517 nm wavelength. |
Estimation of capacity total antioxidant activity
The capacity of total antioxidant activity depended on the principle of decrease of Mo (VI) molecule to Mo (V) by the tested compounds at acidic pH, followed by the formation complex of varying shades Mo (V) /green phosphate with a peak optical density measured at 695 nm. Both water-dissolvable and fat-solvent cell antioxidants (total antioxidant activity) were assessed by this phenomenon 50. In the current study methanol fraction showed the higher antioxidant capacity between the concentrates, i.e., 238 mg AA/g dried plant fraction, 134.66 mg AA/g of ethyl acetate crude fraction, 71.33 mg AA/g of chloroform fraction and hexane fraction value is 51.33 mg AA/g dried plant fraction hexane Fig.5 (a & b).The basic reason of Crude fractions of plant materials exhibiting antioxidant property because of the abundance presence of flavonoids, phenol and polyphenolic compounds.
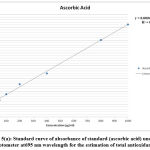 |
Figure 5(a): Standard curve of absorbance of standard (ascorbic acid) under UV Spectrophotometer at 695 nm wavelength for the estimation of total antioxidant capacity. |
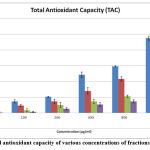 |
Figure 5(b): Total antioxidant capacity of various concentrations of fractions of C. auriculata |
In- Vitro Anticancer Activity
Out of 4 crude fractions of C. auriculata i.e., methanol, ethyl acetate, chloroform and hexane, methanol fraction exhibited high capability of antioxidant potential shown by FRAP test, Nitric oxide antioxidant scavenging activity and DPPH test. Subsequently methanolic crude fraction was chosen for assessment of anticancer action on A549 human cancer cell lines of the lungs by MTTassay. MTT test is a well known in vitro strategy for surveying cytotoxicity against cancer cell lines. The percentage of growth inhibition was measured with the help of MTT assay. With crude fraction of methanol at all concentrations varying from (12.5–200 𝜇g/mL) for 24 hour decreased the viability of cancer cells was observed. The dead cells were expanded by expanding the concentration of the methanol fraction. The peak level of inhibition percentage against A549 cell (78.64%) was noted by methanol crude fraction at a concentration of 200𝜇g/mL. IC50 value was calculated with the help of graph constructed against percentage of inhibition and concentration on A549 cell lines. The half inhibitory concentration of methanol fraction (IC50) was calculated from the standard graph as indicated by Geran et al; and National Center Institute of United States51, the levels of activity named as given: IC50 > 500 µg/mL = inactive, IC50 201-500 µg/mL = weakly active, IC50 21-200 µg/mL = reasonably active and IC50 ≤ 20 µg/mL = extremely active. Methanol fraction indicated intense cytotoxic impacts with the IC50 estimations of115.14 𝜇g/mL in A549 cell line which falls in the range of (21-200 µg/mL = reasonably active). The IC50 esteems demonstrated that the crude fraction methanol is considered as promising anticancer potential Fig.6 (a, b, &c).
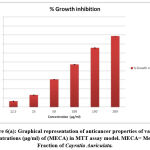 |
Figure 6(a): Graphical representation of anticancer properties of various Concentrations (μg/ml) of (MECA) in MTT assay model. MECA= Methanol Fraction of Cayratia Auriculata. |
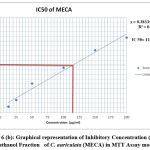 |
Figure 6 (b): Graphical representation of Inhibitory Concentration (IC50) of Methanol Fraction of C. auriculata (MECA) in MTT Assay model. |
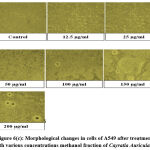 |
Figure 6(c): Morphological changes in cells of A549 after treatment with various concentrations methanol fraction of Cayratia Auriculata. |
Conclusion
Free radicals are responsible for damage of cellular components such as cellular membranes, proteins and DNA leading to onset of many diseases including cancer apart from possessing vital role in cellular functions52. The antioxidant activity of phytochemicals is responsible for the significant decrease in mortality rates and occurrence of numerous human disorders including cancer53-54.Based on the strong interactions of phenols with lipid peroxy radical (LOO.), hydroxyl radical (HO.), superoxide anion radical (O2–) which are highly reactive oxygen free radicals, it is indicated that antioxidant potentials of plant fraction directly proportional to their phenolics content55-57. Hence, several antioxidant-rich plants have anticancer activity58-59. Current study, methanol fraction exhibited significantly potent antioxidant ability, due to this reason methanol fraction may be consider as a plant derived natural source of potent promising antioxidant compound for treating disorders that are mainly due to oxidative stress. Methanol fraction in the present study exhibited potent anticancer capability; this provides strong evidence that this plant could be a natural source of new drug development for the treatment of cancer. Because of negligible side effects when compared to modern drugs used in the treatment of cancer therapy. Tremendous increasing interest towards plant derived phytoconstituents in present era. Being a natural Medicinal plant, C. auriculata may be significantly useful in the treatment of malignancies60. Furthermore, in- vitro as well as in vivo investigations are required for the isolation as well as characterization of the particular phytoconstituents underlying for such properties.
Acknowledgment
We (Authors) are expressing our gratitude to our Honorable Chairman, Dean and Principal of GSL Medical College and General hospital, Rajahmundry, Andhra Pradesh, for supporting and providing infrastructures which is necessary to carry out our research.
Conflict of Interest
We (Authors) are announcing that we have no conflict of interest.
Funding Source
There is no funding sources.
References
- World Health Organization. (2014).Global status report on noncommunicable diseases2014. World Health Organization. https://apps.who.int/ iris/handle/ 10665/148114.
- Harun-ur-Fiashid M, Gafur M, Sadik M.G and Rahman A. Biological activities of a new acrylamide derivative from Ipomoea turpethum. J. Biol. Sci., 2002; 5:968–69.
CrossRef - David J.N and Gordon M.C. Natural products as sources of new drugs from 1981 to 2014. Nat. Prod., 2016; 79:629–661.
CrossRef - Xu D.P, Li Y, Meng X, et al. Natural Antioxidants in Foods and Medicinal Plants: Fractionion, Assessment and Resources. Int. J. Mol. Sci., 2017; 18: 96.
CrossRef - Fouche G, Cragg, G. M, Pillay P, Kolesnikova N, Maharaj V. J and Senabe J. In vitro anticancer screening of South African plants. Ethnopharmacol., 2008; 119:455–461.
CrossRef - Kamatou G.P.P, Van Zyl R.L, Davids H, Van Heerden F.R, Lourens A.C.U, and Viljoen A.M. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. Afr. J. Bot., 2008; 74:238–243.
CrossRef - Shoeb M. Anti-cancer agents from medicinal plants. Bangladesh J. Pharmacol., 2006; 1:35–41.
CrossRef - Joyce DA. Oxygen radicals in disease. Adverse Drug React. Bull., 1987; 127:476‑9.
CrossRef - Braca A, Tommasi N.D, Bari L.D. et al. Antioxidant Principles from Bauhinia Terapotensis. Nat. Prod., 2001; 64: 892-895.
CrossRef - Namiki M. Antioxidants/Antimutagens in Food. Crit Rev Food Sci Nutr., 1990; 29: 273-300.
CrossRef - Prasad N.K, Xie H, Hao J. et al. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem., 2010; 118:62–66.
CrossRef - Sadangi N, Sahu RK.Traditional vetenary herbal practice of Kalahandi district, Orissa, India. Nat. Remedies., 2004; 4:131-136.
- Jain A, Katewa S.S, Galac P, Nag A. Some therapeutic uses of biodiversity among the tribals of Rajasthan. Indian J. Tradit. Knowl. 2008; 7:256-262.
- Srivastava A. Patil S.P, Mishra R.K, Vashistha RK, Singh A,and Puskar A.K. Ethnomedicinal importance of the plants of Amarkantak region Madhya Pradesh, India: j. med. aromat. Plants. 2010; 2:53-59.
- Ahirrao YA, Patil DA. Ethnomedicinal investigation in Nandurbar district of Maharasthtra. Sci. Life; 2007; 27:50-56.
- Saheb TS. A Study on Medicinal Climbers of Nallamalais, Andhra Pradesh. j. multidiscip. res. Dev., 2014; 1: 172-176.
- Naidu M.T, Kumar O.A and Venkaiah M.Taxonomic Diversity of Lianas in Tropical Forests of Northern Eastern Ghats of Andhra Pradesh, India. Sci. Biol., 2014;6:59-65.
CrossRef - Matthew, K M. The Flora of the Tamilnadu Carnatic. Tiruchirapalli, India: Rapinat Herbarium, St. Joseph’s College, 1983; 1-3.
- Reddy K.N, Trimurthulu G and Reddy C.S. Plants use by ethenic people Krishna district, Andhra Pradesh. Indian J. Tradit. Knowl. 2009; 9:313-317.
- Wath M and Sangeeta J.S. Ethnoveterinary survey of herbal therapy treating livestocks of for Melghat region (Maharashtra). j. plant animal env. sci., 2014; 4:42-48.
- Patil U.S and Deshmukh O.S. Traditional ethno-veterinary practices in Betul district Madhya Pradesh India. J. Curr. Res., 2015; 4:423-428.
- Anuj S.K, Srivastava P, Tiwari B.N, Mishra J.N, Behera B.R and Shrivastava A.K.A. Plant (Cissus quadrangularis) with various ethnopharmacologicalaction: A review. Pharm. Res., 2011; 4:1887-1890.
- ensen W.B. The origin of the Soxhlet extraction. Chem. Educ., 2007; 84:1913.
CrossRef - E. Trease and W. C. Evans, “Phenols and phenolic glycosides,” in Textbook of Pharmacognosy., 1989; 12: 343–383.
- Singleton V.L and Rossi J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am J Enol Vitic., 1965; 16:144–158.
CrossRef - Oyaizu M. Studies on products of browning reaction. The Japanese Journal of Nutrition and Dietetic.,, 1986; 44:307-15.
- Chakraborthy GS. Free radical scavenging activity of Costus speciosus Indian J Pharm Educ Res., 2009; 43:96–8.
- Kim D.O, Jeong S.W, and Lee C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem., 2003; 8: 321–326.
CrossRef - Prieto P, Pineda M and Anguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdaenum complex: specific application to the determination of vitamin E. Biochem. 1999; 269:337-341.
CrossRef - Denizot F and Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Immunol. Methods. 1986; 89: 271–277.
CrossRef - Vijayalakshmi A, Kumar P.R, Sakthi Priyadarsini S, Meenaxshi C.In Vitro Antioxidant and Anticancer Activity of Flavonoid Fraction from the Aerial Parts of Cissus quadrangularis Linn. against Human Breast Carcinoma Cell Lines. Chem., 2013.
CrossRef - Shoibe M, Chy M.N.U, Alam M, Adnan M, Islam M.Z, Nihar S.W, Rahman N and , Suez E. Vitro and In Vivo BiologicalActivitiesof Cissus adnata(Roxb.). Biomedicines., 2017; 5:63.
CrossRef - Prashith K.T.R, Vinayaka K.S, Soumya K.V, Ashwini S.K and Kiran R. Antibacterial andAntifungal activity of methanolic fraction of Abruspulchellus wall and Abrusprecatorius Linn- A comparative study. J. Toxicol. Pharmacol; 2010; 2:26-29.
- Sahu NP, Mahato SB. Anti-inflammatory triterpene saponins of Pithecellobium dulce: characterization of an echinocystic acid bisdesmoside. Phytochemistry. 1994; 37:1425-27.
CrossRef - Luziatelli, G., Sørensen, M., Theilade, I. et al.Asháninka medicinal plants: a case study from the native community of Bajo Quimiriki, Junín, Peru. Ethnobiol. Ethnomedi., 2010; 6: 21-27.
CrossRef - Shah B.N, Nayak B.S, Seth A.K, Jalalpure S.S, Patel K.N, Patel M.A and Mishra A.D. Review Article Search for medicinal plants as a source of anti-inflammatory and anti- arthritic agents.Pharmacogn Mag., 2006; 2:77-86.
- Falodun A and Agbakwuru E.O.P. Phytochemical analysis and laxative activity of the leaf fractions of Euphobiaheterophylla Linn. (Euphorbiaceace). J. Sci. Ind. Res., 2004; 47:345-348.
- Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y,Halici M et al Comparison of antioxidant activity and phenolic content of three lichen species. Res., 2004; 18: 938–941.
CrossRef - Wang M, Shao Y, Li J, Zhu N, Rangarajan M, LaVoie EJ et al Antioxidative phenolic glycosides from sage (Salvia officinalis). Nat. Prod., 1999; 62:454-456 .
CrossRef - Jain S, Dwivedi J, Jain P.K, Satpathy S, and Patra A. Medicinal Plants for Treatment of Cancer: A Brief Review. J., 2016; 8:87-102.
CrossRef - Govindaraju K, Darukeshwara J and Srivastava A.K. Studies on protein characteristic and toxic constituents of Simarouba glauca oilseed meal. Food Chem. Toxicol., 2009; 47:1327-1332.
CrossRef - Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks Food Chem. Toxicol., 2008; 46:2326-31.
CrossRef - Kähkönen M.P, Hopia A.I, Vuorela H.J, Rauha J.P, Pihlaja K, Kujala T.S and Heinonen M. Antioxidant activity of plant fractions containing phenolic compounds. Agric. Food Chem., 1999; 47:3954-3962.
CrossRef - Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci., 2008; 4:89-96.
- Ialenti A, Moncada S and Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. J. Pharmacol., 1993; 110: 701–706.
CrossRef - Jagetia G.C, Rao S.K, Baliga M.S and Babu K. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: a preliminary study Res., 2004; 18:561-565.
CrossRef - Madson H.L, Andersen C.M, Jorgensen L.V and Skibsted L.H. Radical scavenging by dietary flavonoids. A kinetic study of antioxidant efficiencies. Food Res. Technol., 2000; 211: 240-246.
CrossRef - Kim H.K, Choen B.S, Kim Y.H, Kim S.Y and Kim H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure activity relationship. Pharmacol. 1999; 58: 759‐765.
CrossRef - Yamamoto Y and Gaynor R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. Clin. Investig. 2001; 107:135-42.
CrossRef - Aderogba M.A, Okoh E.K and Idowu T. O. Evaluation of antioxidant activity of secondary metabolites from piliostigma reticulatum (DC.) hochst.Biol. Sci., 2005; 5:239-245.
CrossRef - Geran R.I. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep., 1972; 13:1-87.
- Diplock AT, Charuleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, et al. Functional food science and defence against reactive oxidative species. Br J Nutr., 1998; 80:77–112.
CrossRef - Anderson K.J, Teuber S.S, Gobeille A, Cremin P,Waterhouse A.L and Steinberg F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. Journal of nutrition; 131(11):2837-2842(2001).
CrossRef - Shigenaga MK, Ames BN. Oxidants and mitogenesis as causes of mutation and cancer: the influence of diet. Basic Life Sci. 1993; 61:419–36.
CrossRef - Torel J, Cillard J, and Cillard P. Antioxidant activity of flavonoids and reactivity with peroxy radical. 1986; 25: 383-385.
CrossRef - Hussain S.R. Cillard J. Cillard P. Hydroxyl radical scavenging activity of flavonoids. 1987; 2: 2489-2491.
CrossRef - Afanas’ev I.B, Dorozhko A.I, Brodskii A.V, Kostyuk V.A and Potapovitch A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Pharmacol. 1989; 38:1763-1769.
CrossRef - Greenwell M, Rahman P. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res. 2015; 6:4103.
- Alam AHMK, Hossain ASMS, Khan MA, Kabir SR, Reza MA, Rahman MM, et al. The Antioxidative Fraction of White Mulberry Induces Apoptosis through Regulation of p53 and NFκB in EAC Cells. PLoS ONE., 2016;11:1-18.
CrossRef - Kumar D, Kumar S, Gupta J, Arya R, Gupta A. A review on chemical and biological properties of Cayratia trifolia Linn.(Vitaceae).Pharmacognosy Rev.,2011;5 :184-188
CrossRef