Khandokar Nabila Sultana Amee1, Zahirul Islam2 , Ferdousi Jahan Sumi3, Mohammad Ibrahim Mondol1, Mahfuza Afroz Soma4
, Ferdousi Jahan Sumi3, Mohammad Ibrahim Mondol1, Mahfuza Afroz Soma4 , Md. Harun-Or-Rashid2
, Md. Harun-Or-Rashid2 , Md. Iqbal Shahid5
, Md. Iqbal Shahid5 , Md. Hassan Kawsar1
, Md. Hassan Kawsar1 , Md. Jamal Hossain1
, Md. Jamal Hossain1 , Raquibul Hasan6
, Raquibul Hasan6 , and Md. Moklesur Rahman Sarker1,5*
, and Md. Moklesur Rahman Sarker1,5*
1Department of Pharmacy, State University of Bangladesh, 77, Satmasjid Road, Dhanmondi, Dhaka 1205, Bangladesh.
2Department of Pharmacy, World University of Bangladesh, Dhaka 1205, Bangladesh.
3Department of Botany, Government BM College, Barishal 8200, Bangladesh.
4Department of Pharmacy, University of Asia Pacific, 74/A, Green Road, Dhaka 1205, Bangladesh.
5Pharmacology and Toxicology Research Division, Health Med Science Research Network, 3/1 Block F, Lalmatia, Dhaka 1207, Bangladesh
6Department of Pharmaceutical Sciences, College of Pharmacy, Mercer University, Atlanta, GA 30341, United States
Corresponding Author E-mail: moklesur2002@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2684
Abstract
The present study was undertaken for phytochemical screening as well as evaluating in vitro antioxidant, thrombolytic, and antimicrobial properties of crude methanolic extracts of Moringa oleifera Lam leaf and its diversified extractives. The antioxidant potential was investigated by ascertaining the capability of the samples to DPPH scavenging with phenol compositions estimated. Besides, thrombolytic and antibacterial properties were assessed following clot lysis and disc diffusion methods, respectively. All fractions were found to contain flavonoids, reducing sugars, tannins, gums, saponins, and quinines, while ME, PESF and CTSF comprise glycosides, steroids, and terpenoids. In the antioxidant activity assay, CTSF possessed the uppermost phenolic content (34.38 mg of GAE/gm) and the scavengers of DPPH free radicals potential with IC50 value of 2.96 µg/mL associated to the standard drug ascorbic acid (2.48µg/mL). AQSF displayed the highest percentage of clot lysis (25.00%), compared to the conventional drug streptokinase (63.74%). The methanol extract, PESF and CSF of M. oleifera displayed antimicrobial activity against all tested microorganisms. Therefore, the outcomes of the existing study were accompanied by the validation of the antioxidant, thrombolytic, and antimicrobial properties of M. oleifera leaves, which justified the plant's use in traditional medicine.
Keywords
Antioxidant; Antimicrobial activities; Extract; Moringa oleifera Lam.; Phytochemicals; Thrombolytic
Download this article as:| Copy the following to cite this article: Amee K. N. S, Islam Z, Sumi F. J, Mondol M. I, Soma M. A, Rashid M. H. O, Shahid M. I, Kawsar M. H, Hossain M. J, Hasan R, Sarker M. M. R. Phytochemical Profiling and Evaluation for Anti-oxidant, Thrombolytic, and Antimicrobial Activities of Moringa oleifera Lam Leaves Extracts. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Amee K. N. S, Islam Z, Sumi F. J, Mondol M. I, Soma M. A, Rashid M. H. O, Shahid M. I, Kawsar M. H, Hossain M. J, Hasan R, Sarker M. M. R. Phytochemical Profiling and Evaluation for Anti-oxidant, Thrombolytic, and Antimicrobial Activities of Moringa oleifera Lam Leaves Extracts. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/43dpvl1 |
Introduction
The Moringaceae is a monogeneric family, containing only one genus all over the world. Moringa is a small genus with 13 species distributed throughout the world1,2. Moringa oleifera is a renowned and extensively widespread plant in the genus Moringa. Bangladesh, India, Pakistan, Sri Lanka, Afghanistan, Thailand, Malaysia, Myanmar, Myanmar, Egypt, Indonesia, Philippines, Singapore, Nepal, Mexico, Nigeria, Jamaica, and Cuba are among the countries where this tall deciduous tree originates3,4. It is called “Sajna” in Bengali and Drumstick tree, Horseradish tree5, Benzolive tree, Ben oil tree, etc.in English6.It is a multipurpose tree. It is correspondingly recognized as the natural gift, the tree of life, and the never-die tree. The tree is generally cultivated to 10 or 12 m in height7.
M. oleifera has enormous nutritional importance. Moreover, various portions of the plant have been utilized to treat several ailments. M. oleifera has been widely used in folklore medicine in Bangladesh and has also been used in Ayurvedic medicine in India for a long time. Hence, the plant is called as a miracle tree8. According to herbal old-fashioned Chinese medicine, M. oleifera can prevent 300 types of ailments, and this plant has been utilized for both defensive and therapeutic applications9. This plant is considered as the best friend of a mother because its leaves are used to increase milk supply of a lactating mother10. The leaves are often used to treat fevers, dyspepsia, and infections of the eyes11. The seed plant is used as a vegetable, a spice and in the production of cosmetic oil9. M. oleifera seeds have a high-quality fatty acid composition and content ranging from 33 to 41%. The oil of M. oleifera, often referred to as “Ben oil” or “Behen oil,” contains 70% oleic acid12. The oil is used as a lotion and skin moisturizer in body and hair care. Since ancient Egyptian times, this oil has been utilized in skin preparations and ointments13. It is well-known as a plant enclosing an active coagulating compound14. Traditional uses of the plant include stimulant, diuretic, anthelmintic, antipyretic, asthma, fatty liver, diabetes, spleen, cardiac tonic, antitumor, antiepileptic, expectorant, and antispasmodic6.
Moringa oleifera is a great source of phytochemicals, mostly secondary metabolites. The phytocompounds are isolated from plants as bioactive compounds resembling vitamin A, vitamin C, carotenoids, polyphenols, phenolic acids, flavonoids, flavone glycosides, alkaloids, tannins, saponins, oxalates, amino acids, fatty acids, terpenes, sucrose, vanillin, carbohydrates, beta-carotene, methionine, cysteine, glucosinolates, isothiocyanates, and thiocarbonates9,15. For instance, the plant comprises a high amount of protein, vitamin C, calcium, potassium, carotene, quercetin, kaempferol, morphine, moriginine, B-sitosterol-3-O-β-D-glucopyranoside, oleic acid, glucomoringin and other nutrients16,17. Ascorbic acid, flavonoids, phenolics, and carotenoids, among other bioactive components contained in leaves, operate as natural antioxidants18. Multiple studies have already been conducted for the evaluation of biological activities of M. oleifera leaf, root and fruit extracts which resulted in significant antioxidant19, antimicrobial20,21, anticancer22, hepatoprotective, cardio-protective, gastroprotective, antiulcerant, neuropharmacological, hematological, antiasthmatic, antiobesity3, antidiarrheal23, antipyretic24, wound healing25, anti-inflammatory26, antifungal27, hyperglycemic28, hypolipidemic and antifungal activities29. The isolation of plant compounds and their pharmacological activities must be extensively explored to validate the traditional usages and establish their association with phytochemicals of herbal medicines in Bangladesh30, 31. In this light, the contemporary study seeks to examine the antioxidant, thrombolytic and antimicrobial properties of crude methanolic extractive of M. oleifera leaf and its miscellaneous liquefiable fractions. To the best of our knowledge, these soluble fractions have not been evaluated for exploring their antioxidant, thrombolytic and antimicrobial activities before19-21.
Methodology
Chemicals and Reagents
Methanol, chloroform, carbon tetrachloride, and petroleum ether of analytical quality were bought from local vendors (manufacturer Merck, Germany).
Collection and Identification of Plant
Moringa oleifera Lam. leaves were collected from Noakhali and acknowledged by authorities at BNH (Bangladesh National Herbarium), Dhaka, Bangladesh. The receipt specimen had been well-preserved for future usage (Accession No.: DACB-66750).
Preparation of Extract
Moringa oleifera leaves (5 kg) were collected and shade dried for 10 days. The dried leaves were then pulverized and kept in a tightly sealed container. The powered substance (250 gm) was immersed in 1.5 liters of methanol for about 15 days. Plant extracts used in pharmacological activities were extracted using methanol and ethanol. In comparison to ethanol, methanol demonstrates the highest bioactive constituent concentration and a higher extraction yield. The leaf extracts were strained first through a clean microfiber cloth pad and then through Whatman No. 1 filter papers. The subsequent filtrate had been condensed at a lower temperature (less than 400C) and pressure (337 mbar) to yield 16 gm crude extract using a rotary vacuum evaporator (Rotavapor, Butch, Switzerland). VanWagenen et al. (1993)33 partitioned it into petroleum ether (0.85 g), carbon tetrachloride (0.65 g), chloroform (0.30 g), and aqueous (2.65 g) liquefiable fractions using the upgraded Kupchan method32.
Phytochemical Analysis
Following conventional procedures,34 alkaloids, glycosides, flavonoids, steroids, resins, phenols, saponins were qualitatively analyzed in the leaf extract of M. oleifera.
Antioxidant activity assay
Analysis of Total Phenolic Content (TPC)
Folin-Ciocalteu technique was utilized to assess total phenolic content35. In this experiment, 0.5 ml of extract and 7.5% w/v Na2CO3 (2.0 ml) liquefaction were diluted along with 2.5 ml of FCR (Folin-Ciocalteu reagent) at 10 % v/v. For the next 20 minutes, the combination was kept at room temperature. A UV spectrophotometer was utilized to detect the absorbance at 760 nm after 20 minutes, and the TPC of the test samples were reckoned using a standard curve created from gallic acid solutions of various concentrations. While UV spectrophotometers are useful, HPLC methods have greater precision than UV spectrophotometers. The TPC of the extracts were determined in milligrams of GAE (gallic acid equivalent) per gram.
Free Radical Scavenging Activity using DPPH method
The scavenging radical property was exploited to evaluate the antioxidant potential of various test samples of M. oleifera leaf samples. The reagent 1,1-diphenyl-2-picrylhydrazyl (DPPH) had been utilized36. Two mL of extract methanol solution (4000 to 1.5625 g/mL) were assorted with 3.0 mL of (DPPH) methanol dissolution (20 g/mL). Following a response time of 30 minutes at room temperature in the opaque residence, the absorbance was recorded with a UV spectrophotometer at 517 nm beside a blank of methanol. The antioxidant potential of the plant extracts was measured using a UV spectrophotometer to compare the brightening of the purple-colored methanol dissolution of DPPH radicals through the plant extracts with that achieved by ascorbic acid (AA). Ascorbic acid was designated as a reference due to its availability in a variety of food sources and its use in reducing power assays. One of the most potent antioxidants, radical scavengers, and stabilizers of oxygen, nitrogen, and thyl radicals, ascorbic acid also serves as the body’s main line of defense against aqueous radicals in the blood. The inhibition proportion of the reactive oxygen species DPPH occurred as intended for the succeeding formula:
(I%) = (1 – Asample / Ablank). X 100 %
The results were compared to the standard given as the half-maximal inhibitory concentration (IC50).
Thrombolytic activity assay
In vitro thrombolytic potential of M. oleifera leaf extracts was assessed using the technique designated by Parsad et al 37 with streptokinase (SK) employed as a positive control and water as a negative control/blank. In order to increase the likelihood of a patient surviving a heart attack, streptokinase is given to break up blood clots that have developed in the blood vessels. Additionally, pulmonary embolism and deep vein thrombosis are conditions that this medication is used to treat. An intravenous blood sample was drawn from ten energetic human participants and located in sterilized petri dishes that had previously been heated at 37 °C for 45 minutes to allow coagulation. The liquid produced during incubation was removed. After that, the tubes were weighed again. As a standard and control, 100 liters of each of the SK and aqueous solutions were introduced into the clot-enclosing pipes. The jars were then heated at 37°C for 90 minutes to detect thrombus destruction. After culture, the unrestricted liquid was extracted, and the ampoules were weighed yet again to evaluate if the weightiness variance, and subsequently the clot breakdown, was significant. The proportion of clot lysis was premeditated by expending the succeeding expression:
% of clot lysis = (wt. of released clot /clot wt.) × 100 %
Antimicrobial activity assay
Test organisms
The Bangladesh Council of Scientific and Industrial Research (BCSIR), in Dhaka, Bangladesh, used purified culture to harvest nine different bacterial species (4 gram positive and 5 gram negative, as mentioned in Table 4).
Antibacterial assay
In order to build an antimicrobial assay of all extract fractions against nine bacteria, the disc diffusion method’s in vitro antibacterial activity was evaluated.38 A suitable amount of the test chemicals had been impregnated into filter paper discs (6 mm in diameter) that had been dried and sterilized. The test ingredients (400 g/disc) were distributed onto discs, which were then evenly seeded with the pathogenic test microorganisms. In separate dishes, four gram-positive and five gram-negative bacterial strains were raised on nutritious agar media. The conventional antibiotic Ciprofloxacin (5 g/disc) acted as a positive control, and blank discs acted as a negative control (impregnated with solvents). Conventional Ciprofloxacin (5 g/disc) discs served as a positive control to demonstrate that the typical antibiotics had been effective alongside the test microorganisms and to compare the repercussions elicited by the recognized antimicrobial mediator with those elicited by the experimental samples. Ciprofloxacin, a fluoroquinolone antibiotic, was active alongside together gram-positive and gram-negative microbes and outperformed preceding medications in terms of antibacterial activity. The culture plates were then hatched for 24 hours at 37 °C to promote microbial evolution. Microorganism growth was hindered by the test materials’ antibacterial potential, and a distinctive zone of inhibition was seen to circle the disc. The thickness of the zone of inhibition in millimeters was used to calculate the test drugs’ antibacterial efficacy. A calculation of the average zone of inhibition followed the acceptance of the experiment in triplicate.
Results
Phytochemical screening
The presence of different phytochemicals, namely alkaloids, flavonoids, tannins, carbohydrates, saponins, resins, gums, glycosides, steroids, terpenoids, and quinines, was screened into the crude methanolic extractive of leaves of M. oleifera (ME) and its altered partitionates; (PESF, CTSF, CSF and AQSF). All fractions appeared to contain flavonoids, reducing sugars, tannins, gums, saponins and quinines, with the exception of alkaloids and resins (Table 1). Glycosides, steroids and terpenoids were also found to be present in ME, PESF and CTSF.
Table 1: Results of phytochemical screening of several fractions of M. oleifera
|
Test for |
ME |
PESF |
CTSF |
CSF |
AQSF |
|
Alkaloids |
– |
– |
– |
– |
– |
|
Flavonoids |
+ |
+ |
+ |
+ |
+ |
|
Reducing sugar |
+ |
+ |
+ |
+ |
+ |
|
Saponins |
+ |
+ |
+ |
+ |
+ |
|
Resins |
– |
– |
– |
– |
– |
|
Tannins |
+ |
+ |
+ |
+ |
+ |
|
Gums |
+ |
+ |
+ |
+ |
+ |
|
Glycosides |
+ |
+ |
+ |
– |
+ |
|
Steroids |
+ |
+ |
+ |
+ |
– |
|
Terpenoids |
+ |
+ |
+ |
+ |
– |
|
Quinines |
+ |
+ |
+ |
+ |
+ |
|
+ Indicates presence, – Indicates absence |
|||||
Determination of (TPC) total phenolic content
According to TPC, extraction reports ranged from 3.91 mg of GAE/gm to 34.38 mg of GAE/gm of Moringa oleifera extractions (Table 2 and Figure 1). CTSF seemed to have the highest phenolic concentration (34.38 mg of GAE/gm), considered by PESF (18.75 mg of GAE/gm) and ME (10.16 mg of GAE/gm).
 |
Figure 1: Total phenolic contents (mg of GAE/gm of extractives) of different extracts of M. oleifera. Here, ME, Methanol extract; PESF, Petroleum ether soluble fraction; CTSF, Carbon tetrachloride soluble fraction; CSF, Chloroform soluble fraction; AQSF, Aqueous soluble fraction. |
DPPH free radical scavenging activity
The scavengers of DPPH radical potential of crude methanol extract of M. oleifera leaf and its various liquefiable subdivisions were tested using DPPH. The IC50 values of different fractions were found to be ME (5.78 µg/mL), PESF (3.70 µg/mL), CTSF (2.96 µg/mL), CSF (7.55 µg/mL) and AQSF (6.38 µg/mL) (Table 2 and Figure 2). Ascorbic acid (AA) was employed as a standard reference in this experiment, and the IC50 value of the AA was 2.48 µg/mL.
Table 2: Total Phenol Contents (TPC) and scavenging of DPPH free radical activity of leaves of M. oleifera.
|
Sample / Standard |
TPC (mg of GAE/gm of extracts) |
IC50 (µg /mL) |
|
ME |
10.16 |
5.78 |
|
PESF |
18.75 |
3.70 |
|
CTSF |
34.38 |
2.96 |
|
CSF |
3.91 |
7.55 |
|
AQSF |
7.81 |
6.38 |
|
AA (Std) |
|
2.48 |
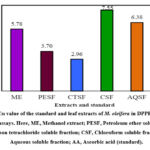 |
Figure 2: IC50 value of the standard and leaf extracts of M. oleifera in DPPH free radical scavenging assays. Here, ME, Methanol extract; PESF, Petroleum ether soluble fraction; |
Thrombolytic Property
In vitro clot lysis activity study, compared to the conventional drug streptokinase 63.74% lysis of RBC, the aqueous solvent (AQSF) fractionate revealed a maximal potentiality of 25.00% lysis, monitored by the petroleum ether soluble (PESF) fraction at 18.75% and the methanol extracts of the leaves of M. oleifera at 18.46% lysis of RBC. (Table 3 and Figure 3)
Table 3: Thrombolytic Activity of methanol extract and its various fractions of M. oleifera.
|
Fractions |
Weight of empty vial (W1)gm |
Weight of vial with clot (W2) gm |
Weight of clot (W3= W2-W1)gm |
Weight of vial after clot lysis (W4)gm |
Weight of lysis clot (W5=W2-W4)gm |
% of clot lysis |
|
ME |
4.810 |
5.460 |
0.65 |
5.34 |
0.12 |
18.46 |
|
PESF |
4.820 |
5.300 |
0.48 |
5.21 |
0.09 |
18.75 |
|
CTSF |
4.820 |
5.540 |
0.72 |
5.41 |
0.13 |
18.06 |
|
CSF |
4.740 |
5.110 |
0.37 |
5.05 |
0.06 |
16.22 |
|
AQSF |
4.680 |
5.160 |
0.48 |
5.04 |
0.12 |
25.00 |
|
Blank |
4.714 |
4.956 |
0.24 |
4.95 |
0.01 |
4.04 |
|
SK |
5.340 |
6.250 |
0.91 |
5.67 |
0.58 |
63.74 |
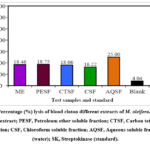 |
Figure 3: Percentage (%) lysis of blood cloton different extracts of M. oleifera. Here, ME, Methanol extract; PESF, Petroleum ether soluble fraction; CTSF, Carbon tetrachloride soluble fraction; |
Antibacterial activity
The disc diffusion process is comprehensively performed to explore the antibacterial activity of natural substances and plant extractices. M. oleifera leaves had been investigated against renowned bacteria and the growth inhibition was compared with the standard drug, ciprofloxacin (Table 4). The methanol extract, PESF and CSF of M. oleifera displayed antimicrobial activity against all tested organisms. The ME exhibited the highest zone of inhibition alongside Gram-positive Bacillus subtilis (40 mm) and Gram-negative Salmonella typhi (36 mm), whereas the lowest inhibitions were found against gram-positive Sarcina lutea (29 mm) and gram-negative Vibrio mimicus (29 mm). The uppermost zones of inhibition by PESF were also shown to be beside gram-positive B. subtilis (37 mm) and gram-negative S. typhi (33 mm). On the other hand, CTSF and CSF displayed highest activity beside gram-negative S. typhi (31 mm) and gram-positive Bacillus cereus (31 mm), respectively. However, no inhibitory effect was detected by AQSF alongside both microorganisms.
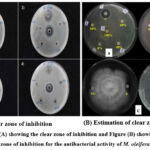 |
Figure 4: Figure (A) showing the clear zone of inhibition and Figure (B) showing the estimation of the clear zone of inhibition for the antibacterial activity of M. oleifera leaf extract |
Table 4: Antimicrobial property of methanol crude extract and various soluble fractions of M. oleifera
|
Test organisms |
Diameter of a zone of inhibition (mm) |
|||||
|
ME |
PESF |
CTSF |
CSF |
AQSF |
Ciprofloxacin |
|
|
|
Gram-positive Bacteria |
|||||
|
Bacillus cereus |
35 |
32 |
29 |
31 |
– |
43 |
|
Bacillus subtilis |
40 |
37 |
24 |
27 |
– |
45 |
|
Staphylococcus aureus |
33 |
31 |
– |
23 |
– |
47 |
|
Sarcinalutea |
29 |
30 |
21 |
20 |
– |
45 |
|
|
Gram-negative Bacteria |
|||||
|
Salmonella typhi |
36 |
33 |
31 |
30 |
– |
47 |
|
Shigelladysenteriae |
34 |
29 |
25 |
27 |
– |
49 |
|
Vibrio parahaemolyticus |
31 |
32 |
– |
23 |
– |
44 |
|
Escherichia coli |
30 |
30 |
23 |
18 |
– |
41 |
|
Vibrio mimicus |
29 |
27 |
20 |
21 |
– |
42 |
Discussion
The medicinal significance of the plants is associated with the presence of bioactive phytochemicals, which have a specific physiological action on humans and can be used in treating numerous diseases39. The study was done to establish the scientific validity of traditional uses of the plant for safe and effective treatment. In this study, phytochemical tests verified the existence of flavonoids, reducing sugars, tannins, gums, saponins, and quinines in all extracts of M. oleifera leaves in variable quantities.
Plant extracts have been recognized to significantly contain polyphenolic compounds (like flavonoids, terpenoids, etc.) that can operate as free radical scavengers. These groups can absorb free radicals and reactive oxygen species (ROS), which can induce a variety of diseases, including cancer.40 When compared to different soluble fractions and a crude methanol extract of M. oleifera leaves, CTSF seemed to have the highest phenolic concentration. Since the leaf extracts are confirmed to contain antioxidant phytoconstituents (flavonoids, tannins, terpenoids, etc.), it justifies the free radical neutralizing properties of the plant.
The DPPH scavenging assay is a fast and dependable method for estimating the antioxidant property of plant quiddity. In the ongoing study, CTSF and PESF of a crude methanol extract of M. oleifera leaves showed promising scavenging effects on the DPPH free radical compared to standard ascorbic acid. All the other fractions indicated DPPH free radical potential to a moderate extent. This scavenging activity might protect reactive radical species from harmful biomolecules in susceptible natural and food systems.
As a part of exploring cardio-protective medicines that come from natural sources, the methanol crude extracts and its altered organic soluble portions of M. oleifera leaves were screened to reveal their thrombolytic activity. The extracts of the plant exhibited mild thrombolytic activity when compared to the conventional thrombolytic agent streptokinase. Among the extracts, the soluble fraction AQSF showed the highest activity, followed by PESF and ME from M. oleifera leaf.
Infectious disorders are becoming increasingly challenging to treat because of the antibiotic resistance of bacteria, especially Gram-positive microorganisms. The disc diffusion methodology is comprehensively used to explore the antimicrobial property of natural substances and plant extracts. The leaves of M. oleifera were studied alongside renowned bacteria, and the growth inhibition was compared with the standard drug, ciprofloxacin. The methanol extract, PESF and CSF of M. oleifera demonstrated potential antimicrobial potentiality beside all tested organisms (gram-positive and gram-negative bacteria).
Our research clearly demonstrates the value of M. oleifera extracts as potent antioxidants, moderate thrombolytics, and antimicrobials. To find drugs from M. oleifera, however, requires more research.
Conclusion
In this investigation, the phytochemical screening of the methanolic crude extracts of the leaves of Moringa oleifera and its miscellaneous soluble fractionates revealed the presence of certain bioactive molecules, for instance, flavonoids, reducing sugars, tannins, gums, saponins, quinines, glycosides, steroids, and terpenoids. Moreover, the plant extracts demonstrated significant antioxidant and moderate antibacterial activities, along with mild thrombolytic activity. Therefore, the ongoing study rationalizes the uses of M. oleifera in folk medicine for various diseases caused by microbes. Further research should be undertaken to isolate the active chemical constituents responsible for the pharmacological properties.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Sources
There are no funding source
References
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007; 21(1):17-25.
CrossRef - Arora DS, Onsare JG, Kaur H. Bioprospecting of Moringa (Moringaceae).: Microbiological perspective. J Pharmacogn Phytochem. 2013;1(6):193-215.
CrossRef - Bhattacharya A, Tiwari P, Sahu PK, Kumar S. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J Pharm Bioallied Sci. 2018; 10(4):181-191.
CrossRef - Sandeep G, Anitha T, Vijayalatha KR, Sadasakthi A. Moringa for nutritional security (Moringa oleifera Lam.). Int J Botany Stud. 2019; 4(1):21-24.
CrossRef - Ramachandran C, Peter KV, Gopalakrishnan PK. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ Bot. 1980; 34(3):276-283.
- Dixit S, Tripathi A, Kumar P. Medicinal properties of Moringa oleifera: A review. Int J Edu Sci Res Rev. 2016; 3(2):173-185.
- Parrotta JA. Moringa oleifera. Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie. 2004; 13:1-8.
- Das PK, Siddika MA, Asha SY, Aktar S, Islam F, Khanam JA, Rakib MA. Investigation of phytochemicals and antioxidant activities in the leaves methanolic extract from Moringa oleifera plants grown in Bangladesh. J Pharmacogn Phytochem. 2019; 8(4):2502-2508.
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int JMol Sci. 2015;16(6):12791-12835
CrossRef - Chukwuebuka E. Moringa oleifera “the mother’s best friend”. Int J Nutr Food Sci. 2015; 4(6):624-630.
CrossRef - Sánchez-Machado DI, Núñez-Gastélum JA, Reyes-Moreno C, Ramírez-Wong B, López-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods. 2010; 3(3):175-180.
CrossRef - Rashid U, Anwar F, Moser BR, Knothe G. Moringa oleifera oil: A possible source of biodiesel. Bioresour Technol. 2008; 99(17):8175-8179.
CrossRef - Mishra SP, Singh P, Singh S. Processing of Moringa oleifera leaves for human consumption. Bull Env Pharmacol Life Sci. 2012; 2(1):28-31.
- Okuda T, Baes AU, Nishijima W, Okada M. Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 1999; 33(15):3373-3378.
CrossRef - Mehta K, Balaraman R, Amin AH, Bafna PA, Gulati O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol. 2003; 86(2-3):191-195.
CrossRef - Kumar PS, Mishra D, Ghosh G, Panda CS. Medicinal uses and pharmacological properties of Moringa oleifera. IntJ Phytomedicine. 2010;2(3): 210-216.
- Oleg P, Ilona G, Alexey G, Irina S, Ivan S, Vladimir K. Biological activities of derived bioactive components from Moringa species: An overview. Entomol Appl Sci Lett. 2018; 5(1):82-87.
- Razis AA, Ibrahim MD, Kntayya SB. Health benefits of Moringa oleifera. Asian Pac JCancer Prev. 2014; 15(20):8571-8576.
CrossRef - Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.). leaves. J Agric Food Chem. 2003; 51(8):2144-2155.
CrossRef - Bukar A, Uba A, Oyeyi T. Antimicrobial profile of Moringa oleifera Lam. extracts against some food–borne microorganisms. Bayero J Pure Appl Sci. 2010; 3(1):43-48.
CrossRef - Sayeed MA, Hossain MS, Chowdhury ME, Haque M. In vitro antimicrobial activity of methanolic extract of Moringa oleifera Lam. fruits. J Pharmacogn Phytochem. 2012; 1(4):94-98.
- Jafarain A, Asghari G, Ghassami E. Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv Biomed Res. 2014; 3:194-198.
- Misra A, Srivastava S, Srivastava M. Evaluation of antidiarrheal potential of Moringa oleifera (Lam.). leaves. J Pharmacogn Phytochem. 2014; 2(5):43-46.
- Bhattacharya A, Behera R, Agrawal D, Sahu PK, Kumar S, Mishra SS. Antipyretic effect of ethanolic extract of Moringa oleifera leaves on albino rats. Tanta Med J. 2014; 42(2):74-78.
CrossRef - Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int. 2013; 2013: 974580.
CrossRef - Ezeamuzie IC, Ambakederemo AW, Shode FO, Ekwebelem SC. Antiinflammatory effects of Moringa oleifera root extract. Int J Pharmacogn. 1996; 34(3):207-212.
CrossRef - Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol. 2007; 98(1):232-236.
CrossRef - Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009; 123(3):392-396.
CrossRef - Jain PG, Patil SD, Haswani NG, Girase MV, Surana SJ. Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Rev Bras Farmacogn. 2010; 20:969-973.
CrossRef - Soma MA, Hasan MM, Jannat T, Sufian MA. In vivo analgesic, anti-hyperglycaemic and CNS depressant studies of Commelina paludosa Blume. Bangladesh Pharm J. 2020; 23(2):103-108.
CrossRef - Pinkey AA, Khan ZI, Taher MA, Soma MA. Elaeocarpus serratus L. exhibits potential analgesic and antidiarrheal activities in mice model. Int J Med Med Res. 2020; 6(2):44-51.
CrossRef - Kupchan chemistry of terpenoid tumor inhibitors. Pure Appl Chem. 1970; 21(2):227-246.
CrossRef - VanWagenen BC, Larsen R, Cardellina JH, Randazzo D, Lidert ZC, Swithenbank C. Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J Org Chem. 1993; 58(2):335-337.
CrossRef - Patel P, Patel N, Patel D, Desai S, SM. Recent advances in the Meshram D. Phytochemical analysis and antifungal activity of Moringa oleifera. Int J Pharm Pharm Sci. 2014; 6(5):144-147.
- Harbertson, J.F. and Spayd, S., 2006. Measuring phenolics in the winery. Am J Enol Vitic. 2006; 57(3): 280-288.
CrossRef - Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995; 1; 28(1):25-30.
CrossRef - Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J. 2006; 4(1):1-4.
CrossRef - Bauer AW. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966; 45: 149-158.
CrossRef - Farnsworth NR, Soejarto DD. 1991. Global importance of medicinal plants. In: The conservation of medicinal plants. Cambridge, UK: Cambridge University Press 25-51.
CrossRef - Kabir F, Jaman AU, Rumpa RA, Jannat T, Alam S, Saha T, Islam MA, Soma MA. In vitro and in vivo investigations provide new insights into bioactivities of Blumea clarkei Hook. f. leaves. Bangladesh Pharm J. 2021; 24(2):149-158.
CrossRef








