Amr Farhan1,2,3* , Aissam Lyazidi1,2
, Aissam Lyazidi1,2 , Badreddine Labakoum1,2
, Badreddine Labakoum1,2 , Mourad Rattal1,2
, Mourad Rattal1,2 and Azeddine Mouhsen1
and Azeddine Mouhsen1
1Hassan First University of Settat, Radiation-Matter Instrumentation Laboratory (RMI), Settat, Morocco.
2Hassan First University of Settat, Higher Institute of Health Sciences (ISSS), Laboratory of Health Sciences and Technologies, Settat, Morocco.
3Authority of Al-Thawra Hospital Taiz, Yemen.
Corresponding Author E-mail:amrfarhan70@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2681
Abstract
Chronic stress overactivates the sympathetic nervous system, which alters the body, mind, and behavior. Purpose Stress can indeed be employed to preserve homeostasis when there is a physical or mental imbalance brought on by damaging stimuli. Currently, there isn't a standard reference point for quantifying stress. The purpose of this meta-analysis is to assess studies that support the use of heart rate variability (HRV) as an indicator of stress. Methods and Materials: Studies concerning HRV that have been published in bibliographic database from 2013 to 2023 were firstly selected. A total of 181 articles were found, 69 in Elsevier, 51 in Google Scholar, 38 PubMed, and 23 in other databases. The criteria selected were human study, HRV reactivity and the HRV as an objective indicator of psychological stress. Only human study was restrained. Finally, ten publications that fit criteria were found. Results: Majority of research selected found that HRV components changed in response to stress brought on by diverse techniques. The most often cited component associated with variance in HRV variables was a drop in the high-frequency band and an increase in the low-frequency band, both of which are markers of diminished parasympathetic activity. HRV may be related to cortical areas that are engaged in evaluating stressful situations, according to neuroimaging research. Conclusion: The findings support the use of HRV for the purpose of evaluating stress and mental health objectively, and neurobiological data suggests that HRV is impacted by stress reactions.
Keywords
Autonomic nervous System; ECG; Heart Rate Variability; Stress
Download this article as:| Copy the following to cite this article: Farhan A, Lyazidi A, Labakoum B, Rattal M, Mouhsen A. Impact of Heart Rate Variability on Physiological Stress: Systematic Review. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Farhan A, Lyazidi A, Labakoum B, Rattal M, Mouhsen A. Impact of Heart Rate Variability on Physiological Stress: Systematic Review. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3NpfI5u |
Introduction
The heart generates an electrical signal called an electrocardiogram (ECG). The electrical impulse drives the human heart’s muscles to compress and decompress blood in a similar cardiac cycle. cardiac cycle1. The ECG signal is a useful tool for a variety of non-invasive biomedical applications, including heart rate estimation, heart rate monitoring, emotion recognition, biometric identification, and cardiac anomaly diagnosis 2,3. The electrodes can be used to identify electrical impulses coming from different parts of the heart4. The ECG measures heart rates and rhythms, including irregular or regular heartbeats5,6. Heart rate variability (HRV) is the term used to describe the time gap between subsequent heartbeats, and the standards were established by the Taskforce of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology two decades ago7. HRV analysis is a tool for assessing cardiovascular processes in close touch with the parasympathetic system, whose activity is observed non-invasively because it is connected to the body’s heart rate and muscular activity8. HRV analysis has utilized both linear and nonlinear techniques 9.By revealing the difference between consecutive heartbeats and the interval between them, this fluctuation can provide an evaluation of a person’s autonomic nervous system activity10,11. For determining autonomic alterations in a range of functional and clinical problems, it is one of the most straightforward, non-invasive, and accurate tests available12. HRV has been regarded as declining when sympathetic activity increases, parasympathetic activity decreases, or both13,14. Stress, according to Hans Selye, is “a response to changing intended to maintain the homology or stability that the body has managed to maintain despite the stimuli intended to undermine the body’s ability to maintain both mental and physical homeostasis and stabilization”15. In addition, stress was described as a maladaptive condition that results in acute or long-term psychological, behavioral impairment, and physical because sympathetic nervous system is overactive 16. Due to a number of challenges, finding stress biomarkers is still a difficult effort for academics and physicians. The absence of agreement on the concept of stress is one barrier. Furthermore, there is a lack of a thorough structure for comprehending how organisms interact with their environments and modify to changing conditions 17. There isn’t yet a single universal method for assessing stress. Numerous research has looked at biological markers (such as cortisol and amylase) and used established stress measuring techniques (such as body change position and psychological evaluations of stress). The sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis are the main pathways via which psychological stress affects the body 18. The SNS and parasympathetic nervous system, in conjunction with the ANS, quickly promotes physiological changes (PNS). The sympathetic response to stress, also known as the “fight-or-flight reaction,” is encouraged by the PNS by reducing the inhibitory effect19. The HPA axis, which is activated during the stress response, causes a number of endocrine changes to begin with the production of corticotropin-releasing hormone from the hypothalamus20. By blocking or suppressing the SNS and HPA axis, the PNS specifically plays a significant role in reducing the stress response in people. Stress is linked to changes in autonomic activity that interfere with homeostatic mechanisms19. An indicator of stress and stress susceptibility may be the parasympathetic tone measurement. Additionally, stasis, a sign of immediate physiological discomfort, is the absence of endogenous variability in peripheral neurally mediated systems, such as the heart rate. A growing number of studies are also being done on stress and cardiovascular variability (HRV). The variability of heartbeat periods is known as HRV21. HRV is a measure of how quickly the heart can react to various physiological and external cues22. Low HRV indicates a heart rate that is monotonously consistent. Furthermore, decreased HRV is associated with lowered regulatory and homeostatic autonomic nervous system (ANS) performance, which reduces the body’s ability to react to both stimuli both internal and external23. In various clinical settings, the HRV is a non-invasive ECG technique that can be utilized to quantify ANS (e.g., during psychological stress assessments)24. Assuming that HRV is a trustworthy indicator of stress, many researchers have conducted studies in which stress was measured using HRV. Few research have, however, confirmed whether HRV is a reliable stress indicator9.
The purpose of this meta-analysis is to assess studies that support the use of heart rate variability (HRV) as an indicator of stress.
Materials and Method
Study period and its type
Retrospective meta-analysis between January 2013 to January 2023, on psychological stress using HRV measurement.
Study selection criteria and research strategy
The research motors used were: PubMed, Elsevier, Google Scholar, and other motors, and Keywords were: (“Autonomic Nervous System”, And “Heart Rate Variability”, And “Electrocardiogram”, and “body postures”, and “stress”). Published research across all languages were included in the investigations. The requirements for each study’s inclusion were that it involved human subjects to employ HRV as an objective indicator of psychological stress and assess any HRV variables derived from frequency-time-based or frequency-based measurements in order to determine the degree of HRV reactivity. The study also included secondary literature and other articles that offered theoretical justification for the selection of HRV like a stress indication or the part played by the ANS in relation to heart rate and psychological stress. From database Scopus the search options were “keywords, abstract and title”. There was a total of 181 articles that we located in the databases (51, 69, 38, and 23 articles found in PubMed, Elsevier, Google Scholar, and other motors)25,26. 171 of the articles were eliminated from our analysis of the data (Figure 3) which includes the reasons for elimination. Finally, after analyzing the data, we chose 10 articles.
Exclusion Criteria
Following the search, we eliminated papers outside the chosen time period, studies involving the animal, and studies that were similar to our topic but went in a different direction. In accordance with the GraphPad Prism 9 paradigm, we conducted a meta-analysis. Only 10 articles were described using the removal criteria shown in the chart below (Figure 3).
Statistical analysis
For the statistical study, we used two different programs, each of which has its own benefits and features.GraphPad Prism 9: for repeatability and exclusion criteria, as well as for plotting publishing years and areas.SPSS for tabulation, data collecting, data comparison, and numerical entry.
Results
Studies on stress using HRV published between 2013 and 2023
We plotted the number of studies published during the selected period 2013-2023 (Figure1). The majority of studies occurred in 2016, followed by 2013, 2021, 2014, 2020 and 2023.
The number of Studies that were published in various nations within the same period that we found (Figure 2). Four investigations were conducted in India, two in Brazil, one each in Belgium, Mexico, Morocco, and the United States.
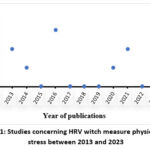 |
Figure 1: Studies concerning HRV witch measure physiological stress between 2013 and 2023 |
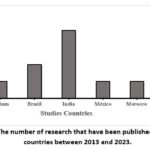 |
Figure 2: The number of research that have been published in various countries between 2013 and 2023. |
Characteristics of the studies incriminated
The diagram below provides an overview of the key traits of the ten papers that were part of this systematic evaluation (Figure 3). The foundation for each study was analytical surveys and investigations of physiological stress utilizing HRV measurements.
 |
Figure 3: Flow chart for studies selection using GraphPad Prism, demonstrates the number of articles included in physiological stress literature search, as well as how we processed the articles and the elimination criteria used. |
Psychological stressors Studies on the response of heart rate variability in human healthy individuals:
In each of ten publications selected, the findings and traits from the previous ten years that discussed heart rate variability and how it relates to stress were looked. We examined the following factors for each study: the topic, the country, the year of publication, the number of participants, their age, the stress assessment, the heart rate variability measurements, the most notable findings, the parameters that changed significantly, and the sources that served as the basis for this research. Less studies were undertaken in 2014, 2020, and 2023, while the majority were conducted in 2013, 2016, and 2021. Four of the research founded in India, two in Brazil, one each in the United States, México, Belgium, and Morocco (Table 1).
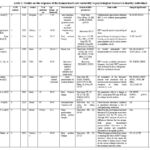 |
Table 1: Studies on the response of the human heart rate variability to psychological stressors in healthy individuals |
Tables 2 and 3 list the key HRV variables. The North American Society of Pacing and Electrophysiology (NASPE) and the Task Force of the European Society of Cardiology (ESC) outlined and set criteria for HRV measurement, physiological interpretation, and clinical usage in 1996.[36].
Table 2: Time-domain of Heart rate variability (HRV) [36]
|
Variable |
Units |
Description |
|
SDNN |
ms |
Standard deviation NN intervals |
|
SDANN |
ms |
Standard deviation of the Mean of NN intervals for all 5-minute |
|
RMSSD |
ms |
square root of mean of successive differences between NN heartbeats |
|
SDNN index |
ms |
the average of all the NN intervals’ standard deviations for every five minutes of a 24-hour HRV. |
|
NN50 count |
|
The average of times per hour that the difference between two consecutive normal sinus (NN) intervals is greater than 50 ms. |
|
pNN50 |
% |
The NN50 divided by total number NN intervals |
Table 3: Heart rate variability (HRV) frequency-domain measures[36]
|
|
Variable |
Units |
Description Frequency |
range |
|
Short time (5 min) |
Total power |
ms2 |
Variation of NN intervals across the temporal section |
≈≤0.4 Hz |
|
VLF |
ms2 |
VLF Power range |
≤0.04 Hz |
|
|
LF |
ms2 |
LF Power range |
0.04–0.15 Hz |
|
|
LF norm |
nu |
LF normalized units LF/(total power-VLF)×100 |
|
|
|
HF |
ms2 |
HF Power range |
0.15–0.4 Hz |
|
|
HF norm |
nu |
HF normalized units HF/(total power-VLF)×100 |
|
|
|
LF/HF |
|
Ratio of LF /HF |
|
Studies used HRV measurement in the time-frequency domain
A study published in Belgium in 2013, HRV Data were collected for five minutes with salivary cortisol at a rate of four samples per day for two days, by filling out a stress-related questionnaire [27]. Where the questionnaire contains the emotions, problems and negative events of a group of children with an average age of 10 [27].
By measuring (RMSSD, HF), it was shown that they are linked to anger, anxiety, and sadness, which means a decrease in parasympathetic activity. Additionally, a higher ratio of low frequency to high frequency is linked to feelings of concern, anger, and anxiety. Using multilevel modeling, it was found that HRV patterns with reduced parasympathetic activity were also related with higher cortisol levels, a larger cortisol stimulus-response, and a steeper daily drop.
A Study in India (2014), A study involving 50 male young adults was done. Young adult males who changed their posture from supine to sitting to standing revealed substantial variations in heart rate variability measures such the mean R-R interval, mean LF, and mean HF. With changes in posture from lying to sitting to standing, there is a correlation between a drop in parasympathetic tone and an uptick in sympathetic impact.
Another study conducted in India in 2016 used the HRV and Stroop test as a method to examine the relationship between mental stress and the cardiovascular autonomic nervous system response to mental stress. During the Stroop Color Word Test, they examined the mean RR interval, blood pressure, and HRV markers (SCWT). 50 healthy participants in all took part in this investigation.
When compared to the resting condition, a statistically significant change in heart rate, RR interval, and blood pressure was seen during the stressful condition. Each of the healthy person’s HRV parameters (SDNN, RMSSD, NN50, PNN50, LF, HF, and LF/HF) were sensitive to stress. When compared to women, men were more vulnerable to stress.
In the USA (2016), Data were gathered from 909 volunteers who ranged in age between 35 to 85. During telephone interviews conducted on 8 consecutive evenings, participants discussed unpleasant emotions and small stressful situations. On a different occasion, HRV was assessed using an electrocardiogram (ECG) signal during a laboratory-based psychophysiology routine while the subject was at repose. To assess the correlations of HRV parameters: HF, RMSSD, and SDRR and daily stress processes, regression models have been utilized. Stressor frequency was observed to be unrelated to HRV. Individuals with higher reported stressor severity, however, exhibited lower resting SDRR. All three HRV indicators were considerably lower in those with greater affective reactivity to stresses. Additionally, a lower RMSSD was associated with a cumulative daily negative effect.
Another study In India (2016), the Physiology Department researchers did a cross-sectional analytical investigation. First-year MBBS medical students who volunteered were 150 (78 female and 72 male). The stress questionnaire used by medical students was used to evaluate the stressors. Using an ECG, a short-term HRV recording was done. By employing an automated blood pressure monitor, the oscillometric approach was used to capture the basal heart rate (BHR), diastolic blood pressure (DBP), and systolic blood pressure (SBP).
According to the findings, more female pupils than male students fell into the group of high and severe stress. With the exception of LFnu, which dramatically rose, all of the frequency domain indexes (HF, HFnu, LF, and TP) decreased as stress intensity raised. The cumulative stress score significantly correlated with all of the HRV measures and coefficient of variation parameters as the level of stress increased.
Finally study In India (2020), Ten individuals’ data were collected in three different body positions. Data sets were collected when individuals were resting, seated, and standing. The R-peak obtained from the ECG is used to analyze the HRV, three body positions are used to evaluate linear HRV variables using various time- and frequency-domain indexes, including HFnu, LFnu, LF/HF, RMSSD, RR, HR, SDNN, pNN50, and NN50. All parameters refer to a change in HRV, which alters PSN and SNS due to a change in body posture.
Table 4: Studies that used HRV measurement in the time-frequency domain
|
Authors |
Year |
No.of patients |
Age Range or Mean±SD |
Stress assessment |
Measured HRV parameters |
Main results |
changed significantly |
P.V |
|
Michels et al. |
2013 |
334 |
5–10 |
auto symptoms of stressful situations (problems,events , and emotions) |
Short-term: |
Children exhibit signs of stress when their HRV is low (reduced parasympathetic activity). |
HF, LF/HF, RMSSD |
P<0.05 |
|
Sanhita Rajan Walawalkar |
2014 |
50 |
18- 25 |
Body positions Change |
Short-term: LF, HF, LFnu, HFnu, LF/HF, Mean RR, TP, RMSSD, Mean HR, SDNN,NN50, PNN50, |
postural changes from laying to sitting to standing are associated with a decrease in parasympathetic activity and an increase in sympathetic activity. |
LF, HF, Mean RR |
P<0.05 |
|
Chiranjeevi Kumar et al |
2016 |
50 |
28.5±0.71 |
Stroop Test |
Short-term : RMSSD, SDNN, HR, NN50 RR, PNN50, TP, HF HFnu, LF, LFnu, LF/HF |
All healthy participants’ HRV measurements were sensitive to stress. |
RR, HR, RMSS D, SDNN, HF, LF, LF/HF |
P<0.05 |
|
Nancy L. Sin et al |
2016 |
909 |
35-85 |
Interviews telephonic reported negative influence and minor stress |
Short-term: HF,RMSSD, SDNN, |
HRV was not correlated with stressor frequency. but, both 3 HRV measures were decreased in those with greater affective reactions to stresses. |
HF, RMSSD, SDNN |
P>0.05 |
|
Pushpanathan Punita et al |
2016 |
150 |
medical student volunteers |
stress questionnaire for medical students |
Short term: RR, SDNN, RMSSD, pNN50, NN50, HF, HFnu, LF, LFnu, TP, LF/HF,
|
all parameters of the frequency domain were decreased with an increase in the stress intensity with the exception of LFnu, which substantially raised. |
RR, RMSSD, SDNN, pNN50, NN50, HF ,HFnu , LF, LF/HF, TP
|
P<0.05 |
|
Prashant et al |
2020 |
10 |
20-25 |
Body Postures change |
Short-term: TP, LF, HF, LF/HF, HR, RMSSD, SDNN, pNN50 |
All parameters refer to a change in HRV, which alters PSN and SNS due to a change in body posture. |
HR, SDNN, RMSSD, pNN50, LF/HF |
– |
Studies used HRV measurement in the frequency domain
A study in México (2013), compared the outcomes of DFA and HRV using the scaling parameter alpha and the area beneath the low frequency spectrum. The evaluation included 57 healthy women aged between 40 and 60. Data was gathered using an ECG in the sitting position for ten minutes, followed by three minutes of a stress test and three minutes of an ECG in the sitting position.
assessing the psychological stress response to the Stroop test in short-term recordings. As opposed to DFA, which offers details concerning a more delayed response in the final stage of the paradigm, HRV is sensitive to different stages of the paradigm employed. The HRV provides a quick reply to psychological stress (resting state).
In Brazil (2021), After cardiopulmonary exercise testing, the study examined whether cardiorespiratory fitness had an impact on cardiovascular autonomic recovery. Where Sixty volunteers were split into three groups based on their level of cardiorespiratory fitness: high, moderate, and low.
Prior to and following a cardiopulmonary activity test, HRV parameters, BPV, and BRS were conducted. Lower baseline HR values and shorter HR recovery times were seen in the groups with increased cardiorespiratory fitness. The spectral analysis of HRV revealed that low-frequency (LF) oscillations in absolute units and high-frequency (HF) oscillations in both absolute and normalized units decreased when resting and recovering periods were compared. Additionally, it revealed a rise in LF oscillations of blood pressure. In light of the findings, it can be said that, in contrast to HR recovery, cardiorespiratory fitness has no impact on the cardiovascular autonomic modulations that occur following cardiopulmonary exercise testing.
Another investigation in Brazil (2021), Eight to twelve-year-old children were divided into two groups, according to their gender, age, peak oxygenation consumption, and their body mass index. Heart rate variability (HRV) assessments of spectral, symbolic, and complexity were performed on both groups while their postures changed in order to compare how much stress and anxiety they were experiencing.
According to the finding When stress/anxiety levels were assessed using data from the questionnaire, the p-DCD group displayed higher stress symptoms than the TD group, although HRV studies revealed no differences between the two groups. Both groups demonstrated parasympathetic dominance in the supine position and sympathetic dominance in the upright position.
In Morocco (2023), The recordings of fifteen student volunteers. For five minutes, data were gathered in both the supine and standing positions. The RR-peak, which is also necessary for HRV analysis, is evaluated using the R-peak acquired from an ECG. Two body locations are used to interpret linear HRV values using various time-domain indices and frequency-domain indices. The researchers found that the RR interval is longer in the supine position than in the standing position, and that the heart rate is higher in the standing position than in the more relaxed supine posture. This has an impact on the ANS and tiredness index readings, starting from the supine position where they are low before rising in the standing position.
Table 5: Studies used HRV measurement in the frequency domain.
|
Authors |
Year |
No. of patients |
Age Range or Mean±SD |
Stress assessment |
Measured HRV parameters |
Main results |
changed significantly |
P.V |
|
M. Vargas-Luna et al |
2013 |
57 |
40-60 |
3-minute stoop test |
Short-term: HF, LF |
HRV responds quickly to psychological stress. |
HF, LF
|
P<0.05 |
|
Facioli et al |
2021 |
60 |
Rang( 18-45) |
cardiopulmonary exercise test |
Short-term HR, LF, LFnu, HF, HFnu, LF/HF |
Cardiovascular autonomic regulation is unaffected following a test of cardiopulmonary endurance |
HR |
– |
|
Daniel T et al |
2021 |
30 boys |
Rang ( 8–12) |
Stress questionnaire during posture changes. |
Short-term HF, LF, LF/HF |
Data from the questionnaire indicated that the p-DCD group had more stress symptoms than the TD group did, although HRV analyses revealed no differences between the two groups. Both groups demonstrated parasympathetic dominance in the supine position and sympathetic dominance in the upright position. |
Non |
P<0.05 |
|
Amr Farhan et al |
2023 |
15 (10M,5F) |
Rang ( 19–40) |
Body position Change |
Short-term HF, LF, LF/HF |
When changing positions from supine to standing, the values of HRV change (increase), which in turn causes the stress index to change (raise), which in turn causes a change (value rise) in the autonomic nervous system. |
HF, LF, LF/HF |
P<0.05 |
Clinical uses for HRV
HRV can be utilized as an objective measure of stress and mental health in light of observations of HRV change related to stress and existing neurobiological research. Due to the enormous range of psychiatric disorders’ origins and symptoms, it is difficult to acquire consistent biological measurements in patients with mental illness. Thus, a patient’s psychosocial and medical histories Should have been considered equally when analyzing HRV results.
As opposed to specific mental diseases or disease states, Heart Rate Variability can be seen as a tool that represents the activity of heart and overall auto- nomic health. While researching stress using HRV in therapeutic practice, objective and physiological evaluations as well as self-reporting should be included because stress is a concept with both biological and psychological components. Many research have indicated a correlation between HRV and mental wellness. These findings are challenging to interpret, though, because HRV is linked to a variety of stress variables, stress duration, personal coping mechanisms, and lifestyle behaviors. Respiration , body posture, circadian rhythms, non-modifiable factors as sex, age, and genetics, factors of lifestyle are modifiable like metabolism disease, smoke, exercise, and obese, and alcohol use, and additional factors as medication (such as stimulants, betablockers, and anticholinergics) can all have an impact on HRV results[37] [38]. A three-stage stress response paradigm was proposed by Hans Seyle. The fight-or-flight response of the organism to a stressor and activation of the SNS constitute the stage one, known as the “alarm reaction stage.” During the second stage, referred to as the “resistance stage,” the body adapts to the stressor. The body focuses all of its energy on the stressor when The PNS assists several physiological processes in returning to normal throughout this phase. The organism appears normal on the outside, but the levels of blood sugar, cortisol, and adrenalin are still high[39] [40]. If a stressor persists longer than the body can handle it, the organism exhausts its resources and becomes vulnerable to illness event death. When the acquired resistance or adaptability is no longer present, the “exhaustion stage” is achieved. This three-stage procedure should be kept in mind when interpreting HRV results in a clinical environment to determine how severe a patient’s stress level is. Stress alters physiological function at each level, which is reflected in variations in HRV. While assessing the connection between HRV and stress, it is essential to comprehend the full autonomic context and take into account a patient’s medical and mental history due to the variety of probable stressors and distinctive stress reactions.
Discussion:
In this data analysis on stress and its relation with HRV, we selected 10 articles carried out over the last 10 years. HRV is responsive to modifications in autonomic nervous system (ANS) activity (i.e., alterations in the Parasympathetic and Sympathetic) brought on by stress.
In order to control human neurophysiological activities, the ANS is crucial. In latest years, HRV analysis has grown significantly in significance as a method for examining ANS activity and as a crucial early marker for detecting both physiological and pathological states. An effort was undertaken to investigate how stress affected heart rate variability as training adaptation and heart rate variability in elite endurance athletes.
The majority of studies found that HRV features changed in response to stress that was caused in a variety of methods. Reduced of PNS activity, which is shown by a decreasing in the Height Frequency and an increasing in the Low Frequency, was the reason most frequently cited as being responsible for changes in HRV features. HRV activities may also be influenced by a flexible network of brain regions that are dynamically structured in reaction to environmental cues.
According to neuroimaging research, brain areas associated with the assessment of stressors might act as a mediator between HRV and reduced threat perception. In clinical settings, the HRV can be a tool that serves to demonstrate cardiac activity and overall voluntary health as opposed to mental illness or specific pathological conditions. As a result, it is crucial to take into account the patient’s psychological and medical history as well as the general autonomic context when assessing the association between stress and HRV.
Conclusions
Due to the non-invasive nature,
simplicity, strong reproducibility, and prognostic information that heart rate
variability analysis provides regarding stress, it has grown in importance as a
technique to evaluate ANS. The sympathetic nervous system’s parasympathetic and
sympathetic functions have been studied using HRV, which has proven to be a
useful technique. In conclusion, the findings support the use of HRV for the
objective assessment of both stress and mental health, and according to
neurobiological evidence, stress responses have an effect on HRV.
Conflict of Interest
There is no conflict of interests in association with the material presented in this paper.
Funding Source
This study did not receive any funding.
References
- A. Alberdi, A. Aztiria, and A. Basarab, “Towards an automatic early stress recognition system for office environments based on multimodal measurements: A review,” Journal of Biomedical Informatics, vol. 59, pp. 49–75, 2016, doi: 10.1016/j.jbi.2015.11.007.
CrossRef - R. E. Gregg, R. Firoozabadi, and S. Babaeizadeh, “Can Peak-Picked ECG Be Used for Heart Rate Variability?,” Computing in Cardiology, vol. 2018-Septe, 2018, doi: 10.22489/CinC.2018.181.
CrossRef - P. Janbakhshi and M. B. Shamsollahi, “ECG-derived respiration estimation from single-lead ECG using gaussian process and phase space reconstruction methods,” Biomedical Signal Processing and Control, vol. 45, pp. 80–90, 2018, doi: 10.1016/j.bspc.2018.05.025.
CrossRef - J. Olkkonen, DISCRETE WAVELET TRANSFORMS – THEORY AND APPLICATIONS Edited by Juuso Olkkonen. 2011.
CrossRef - M. Hammad, A. Maher, K. Wang, F. Jiang, and M. Amrani, “Detection of abnormal heart conditions based on characteristics of ECG signals,” Measurement: Journal of the International Measurement Confederation, vol. 125, no. May, pp. 634–644, 2018, doi: 10.1016/j.measurement.2018. 05.033.
CrossRef - X. Xu and Y. Liu, “ECG QRS complex detection using Slope Vector Waveform (SVW) algorithm,” Annual International Conference of the IEEE Engineering in Medicine and Biology – Proceedings, vol. 26 V, pp. 3597–3600, 2004, doi: 10.1109/iembs.2004.1404011.
CrossRef - C. Kiran kumar, M. Manaswini, K. N. Maruthy, A. V. Siva Kumar, and K. Mahesh kumar, “Association of Heart rate variability measured by RR interval from ECG and pulse to pulse interval from Photoplethysmography,” Clinical Epidemiology and Global Health, vol. 10, no. December 2020, p. 100698, 2021, doi: 10.1016/j.cegh.2021.100698.
CrossRef - E. Mejía-Mejía, K. Budidha, T. Y. Abay, J. M. May, and P. A. Kyriacou, “Heart Rate Variability (HRV) and Pulse Rate Variability (PRV) for the Assessment of Autonomic Responses,” Frontiers in Physiology, vol. 11, no. July, pp. 1–17, 2020, doi: 10.3389/fphys.2020.00779.
CrossRef - B. Shah et al., “Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence,” Indian Pacing and Electrophysiology Journal, vol. 22, no. 2, pp. 70–76, 2022, doi: 10.1016/j.ipej.2022.01.004.
CrossRef - D. G. Weissman and W. B. Mendes, “Correlation of sympathetic and parasympathetic nervous system activity during rest and acute stress tasks,” International Journal of Psychophysiology, vol. 162, no. February, pp. 60–68, 2021, doi: 10.1016/j.ijpsycho.2021.01.015.
CrossRef - G. Panina, U. N. Khot, E. Nunziata, R. J. Cody, and P. F. Binkley, “Assessment of autonomic tone over a 24-hour period in patients with congestive heart failure: Relation betweeen mean heart rate and measures of heart rate variability,” American Heart Journal, vol. 129, no. 4, pp. 748–753, 1995, doi: 10.1016/0002-8703(95)90325-9.
CrossRef - A. A. Khan, G. Y. H. Lip, and A. Shantsila, “Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system,” European Journal of Clinical Investigation, vol. 49, no. 11, pp. 1–8, 2019, doi: 10.1111/eci.13174.
CrossRef - K. Maheshkumar, K. Dilara, K. N. Maruthy, and L. Sundareswaren, “Validation of PC–based sound card with biopac for digitalization of ECG recording in short–term HRV analysis,” North American Journal of Medical Sciences, vol. 8, no. 7, pp. 307–311, 2016, doi: 10.4103/1947-2714.187150.
CrossRef - C. K. Peng et al., “Non-equilibrium dynamics as an indispensable characteristic of a healthy biological system,” Integrative Physiological and Behavioral Science, vol. 29, no. 3, pp. 283–293, 1994, doi: 10.1007/BF02691332.
CrossRef - H.-G. Kim, E.-J. Cheon, D.-S. Bai, Y. H. Lee, and B.-H. Koo, “Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature,” Psychiatry Investig, vol. 15, no. 3, pp. 235–245, Mar. 2018, doi: 10.30773/pi.2017.08.17.
CrossRef - M. Campkin, “Stress management in primary care,” Family Practice, vol. 17, 2000.
CrossRef - J. F. Thayer, F. Åhs, M. Fredrikson, J. J. Sollers, and T. D. Wager, “A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health,” Neuroscience & Biobehavioral Reviews, vol. 36, no. 2, pp. 747–756, Feb. 2012, doi: 10.1016/j.neubiorev.2011.11.009.
CrossRef - A. H. Marques, M. N. Silverman, and E. M. Sternberg, “Evaluation of Stress Systems by Applying Noninvasive Methodologies: Measurements of Neuroimmune Biomarkers in the Sweat, Heart Rate Variability and Salivary Cortisol,” Neuroimmunomodulation, vol. 17, no. 3, pp. 205–208, 2010, doi: 10.1159/000258725.
CrossRef - S. W. Porges, “Cardiac vagal tone: A physiological index of stress,” Neuroscience & Biobehavioral Reviews, vol. 19, no. 2, pp. 225–233, Jun. 1995, doi: 10.1016/0149-7634(94)00066-A.
CrossRef - K.-A. Egliston, C. McMahon, and M.-P. Austin, “Stress in pregnancy and infant HPA axis function: Conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure,” Psychoneuroendocrinology, vol. 32, no. 1, pp. 1–13, Jan. 2007, doi: 10.1016/j.psyneuen.2006.10.003.
CrossRef - Guidelines, T. N. American, and Guidelines, “Guidelines Heart rate variability,” European Heart Journal, vol. 17, pp. 354–381, 1996, doi: 10.1161/01.CIR.93.5.1043.
CrossRef - U. Rajendra Acharya, K. Paul Joseph, N. Kannathal, C. M. Lim, and J. S. Suri, “Heart rate variability: a review,” Med Bio Eng Comput, vol. 44, no. 12, pp. 1031–1051, Dec. 2006, doi: 10.1007/s11517-006-0119-0.
CrossRef - A. Farhan, A. Lyazidi, S. Elkettani, B. Labakoum, M. Rattal, and A. Mouhsen, “Linear Analysis of ECG Data Variability to Assess The Autonomic Nervous System in Two Different Body Positions,” The Egyptian Journal of Hospital Medicine, vol. 90, no. 1, pp. 459–464, Jan. 2023, doi: 10.21608/ejhm.2023.279661.
- J. Sztajzel, “Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system,” Swiss medical weekly, vol. 134, no. 35–36, pp. 514–522, 2004.
- A. G. M. Al-Selwi and A. Barkat, “Antibiotic prescription in Morocco, national data: Meta-analysis,” Journal of Pharmaceutical Negative Results, vol. 13, no. 4, pp. 1074–1082, Nov. 2022, doi: 10.47750/pnr.2022.13.04.148.
CrossRef - A. G. M. Al-Selwi and A. Barkat, “Antibiotic Resistance of Streptococcus Pneumoniae, Neisseria Meningitidis, Haemophilus Influenzae and Staphylococcus Aureus in Morocco, National Data: Meta- Analysis.,” Biomedical and Pharmacology Journal, vol. 16, no. 1, pp. 251–263, Mar. 2023.
CrossRef - N. Michels et al., “Children’s heart rate variability as stress indicator: Association with reported stress and cortisol,” Biological Psychology, vol. 94, no. 2, pp. 433–440, Oct. 2013, doi: 10.1016/j.biopsycho.2013.08.005.
CrossRef - M. Vargas-Luna, M. R. Huerta-Franco, and J. B. Montes, “Evaluation of the Cardiac Response to Psychological Stress by Short-Term ECG Recordings: Heart Rate Variability and Detrended Fluctuation Analysis,” in World Congress on Medical Physics and Biomedical Engineering May 26-31, 2012, Beijing, China, M. Long, Ed., in IFMBE Proceedings, vol. 39. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013, pp. 333–335. doi: 10.1007/978-3-642-29305-4_89.
CrossRef - D. S. R. Walawalkar, “A Study Of Variation In Heart Rate Variability With Change In Posture In Young Adult Indian Males,” vol. 2, no. 3, 2014.
CrossRef - K. E. Chiranjeevi and T. Sonali, “Evaluation Of Cardiac Responses To Stress In Healthy Individuals A Non Invasive Evaluation By Heart Rate Variability And Stroop Test, IJSR – International Journal of Scientific Research(IJSR), IJSR | World Wide Journals.” https://www.worldwidejournals.com/international-journal-of-scientific-research-(IJSR)/article/evaluation-of-cardiac-responses-to-stress-in-healthy-individuals-a-non-invasive-evaluation-by-heart-rate-variability-and-stroop-test/ODQzOQ==/?is=1 (accessed Jan. 29, 2023).
- N. L. Sin, R. P. Sloan, P. S. McKinley, and D. M. Almeida, “Linking Daily Stress Processes and Laboratory-Based Heart Rate Variability in a National Sample of Midlife and Older Adults,” Psychosom Med, vol. 78, no. 5, pp. 573–582, Jun. 2016, doi: 10.1097/PSY.0000000000000306.
CrossRef - P. Punita, K. Saranya, and S. Kumar, “Gender difference in heart rate variability in medical students and association with the level of stress,” Natl J Physiol Pharm Pharmacol, vol. 6, no. 5, p. 431, 2016, doi: 10.5455/njppp.2016.6.0102325042016.
CrossRef - P. Kumar, A. K. Das, Prachita, and S. Halder, “Time-domain HRV Analysis of ECG Signal under Different Body Postures,” Procedia Computer Science, vol. 167, pp. 1705–1710, 2020, doi: 10.1016/j.procs.2020.03.435.
CrossRef - T. P. Facioli et al., “Study of heart rate recovery and cardiovascular autonomic modulation in healthy participants after submaximal exercise,” Sci Rep, vol. 11, no. 1, p. 3620, Feb. 2021, doi: 10.1038/s41598-021-83071-w.
CrossRef - D. T. Gama, M. C. Ferracioli-Gama, J. A. Barela, A. C. M. Takahashi, A. M. Pellegrini, and C. Y. Hiraga, “Autonomous nervous system modulation in supine and standing postures in children with probable developmental coordination disorder,” Heliyon, vol. 7, no. 1, p. e06111, Jan. 2021, doi: 10.1016/j.heliyon.2021.e06111.
CrossRef - M. Malik, “Heart rate variability: Standards of measurement, physiological interpretation, and clinical use,” Circulation, vol. 93, pp. 1043–1065, Mar. 1996.
CrossRef - M. E. Altuncu, O. Baspinar, and M. Keskin, “The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome,” Cardiology Journal, vol. 19, no. 5, Art. no. 5, 2012, doi: 10.5603/CJ.2012.0091.
CrossRef - M. Romanowicz, J. E. Schmidt, J. M. Bostwick, D. A. Mrazek, and V. M. Karpyak, “Changes in Heart Rate Variability Associated With Acute Alcohol Consumption: Current Knowledge and Implications for Practice and Research,” Alcohol: Clinical and Experimental Research, vol. 35, no. 6, pp. 1092–1105, 2011, doi: 10.1111/j.1530-0277.2011.01442.x.
CrossRef - “Stress in Health and Disease – 1st Edition.” https://www.elsevier.com/books/stress-in-health-and-disease/selye/978-0-407-98510-0 (accessed Apr. 10, 2023).
- “Effects of Work Stress on Ambulatory Blood Pressure, Heart Rate, and Heart Rate Variability | Hypertension.” https://www.ahajournals.org/doi/10.1161/01.hyp.35.4.880?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org& rfr_dat=cr_pub%20%200pubmed (accessed Apr. 10, 2023).








