Manuscript accepted on :27-01-2023
Published online on: 01-07-2023
Plagiarism Check: Yes
Reviewed by: Dr. Amani Eyad
Second Review by: Dr. Shamma Aboobacker
Final Approval by: Dr. Ayush Dogra
Prashant Tripathi1 and Arti Malviya2*
and Arti Malviya2*
1LNCT University Bhopal-462042, Madhya Pradesh, India.
2Lakshmi Narain College of Technology, Bhopal-462021, Madhya Pradesh, India.
Corresponding Author E-mail:art_7920@yahoo.co.in
DOI : https://dx.doi.org/10.13005/bpj/2696
Abstract
Imidazole heterocycles possess a very special place in biological chemistry making their derivatives receive considerable attention among researchers. Several natural products including nucleic acids, histamine, and histidine consist of the imidazole nucleus. It is an ionizable compound that renders good pharmacokinetic properties to the compounds contained in it. The nucleus presents some interesting pharmacological properties like antibacterial, antitubercular, anticancer, larvicidal, and antifungal. The present paper attempts to review the significant pharmacological actions of imidazole derivatives over the past few years. The paper summarizes the preparation methods like condensation method, microwave-assisted method, ultrasonic method and heating process employed for synthesis of imadazoles. The paper summarizes the current improvements of imidazole-based mixtures in the entire range of restorative science. The significant analysis of the published research infers that optimization of the microwave method for synthesis of the imidazole nucleus could be an effective method in the preparation of the motif.
Keywords
Anticancer; Antibacterial; Heterocycle; Histamine; Imidazole; Larvicidal
Download this article as:| Copy the following to cite this article: Tripathi P, Malviya A. Imidazole Scaffold: A Review of Synthetic Strategies and Therapeutic Action. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Tripathi P, Malviya A. Imidazole Scaffold: A Review of Synthetic Strategies and Therapeutic Action. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3XyhMN6 |
Introduction
The design of novel and newer potent molecules has been the thirst of researchers worldwide. An important approach towards this incorporates the modification of the existing molecules with established biological actions utilizing the various approaches for drug designing. Heterocyclic compounds either of synthetic or natural origins have found applicability in several physiological neurotransmitters as well as in the modulators of certain neurotransmission processes 1.
Heterocyclic compounds containing two N atoms at 1-3 positions are gifted with a broad spectrum of microbial actions. A plethora of sulphur and nitrogen-possessing compounds are found in the biological and non-biological systems 2. Amongst the heterocyclic compounds containing sulphur and nitrogen, the six and five-membered heterocycles have gathered the maximum interest, owing to their biological and industrial applications 3.
Since the time of its disclosure during the 1840s, the examination and advancements of imidazole based mixtures have been a significant quickly creating, and progressively dynamic region inferable from their wide expected applications as restorative medications, agrochemicals, synthetic materials, unnatural acceptors, supramolecular ligands, biomimetic impetuses, and so on 4.
Imidazole ring is a 5-membered heterocycle structure having two nitrogen iotas and amphoteric nature with exceptionally polar characteristics 5. It occurs in two identical tautomeric structures, in which the hydrogen (H) particle can be situated on both of the two nitrogen (N) molecules. It is a sweet-smelling compound. Besides, the electron-rich nitrogen heterocycle couldn’t promptly acknowledge or give proton, yet in addition, effectively structure different powerless cooperations. These exceptional primary qualities of the imidazole ring are helpful for its subsidiaries to promptly tie with an assortment of chemicals and receptors in natural frameworks employing hydrogen bonding, coordination linkages, particle dipole interaction, cation-pi, pi-pi stacking, hydrophobic impacts, van der Waals powers, etc, in this way displaying wide bioactivities 6.
Compounds containing the imidazole ring are vital in living frameworks, like nutrient B12 and a few pilocarpine alkaloids. Imidazole framework happens in the fundamental amino corrosive histidine; histidines inside catalysts are personally associated with catalysis requiring protein transfer 7. The existence of imidazole moiety in concerned compounds may be good for further developing water dissolvability somewhat because of its two nitrogen iotas effectively prompting the arrangement of hydrogen bonds. In addition, imidazole ring with different restricting destinations is fit for planning with a selection of inorganic metal particles or interfacing with natural atoms through noncovalent securities to form supramolecular drugs, which might have microbial activities of imidazoles themselves, however also the benefits of various supramolecular drugs, potentially applying twofold activity mechanisms that are useful to defeat drug resistance.
The imidazole ring is present in several natural and synthetic biologically active compounds, such as biotin, histidine, histamine, and pilocarpine alkaloids. This has led to several compounds based on the imidazole motif being optimized for commercial success as medicinal agents. Some of the most prominent marketed products containing imidazole are presented in Table 1.
Table 1: Marketed products with imidazole scaffold 8-20
|
Name |
Therapeutic category |
Mechanism of Action |
|
Zolpidem |
CNS depressant |
GABAA inhibitor |
|
Dacarbazine |
Antineoplastic |
DNA alkylator |
|
Mercaptopurine |
Antineoplastic/ Antimetabolite |
Cytochrome P450 3A4 inhibitor |
|
Nilotinib |
Antineoplastic |
Cytochrome P450 3A4 inhibitor |
|
Temozolomide |
Antineoplastic |
DNA Alkylation |
|
Ketoconazole |
Antifungal |
Impairs ergosterol synthesis |
|
Oxiconazole |
Antifungal |
Impairs ergosterol synthesis |
|
Butaconazole |
Antifungal |
Inhibition of steroid synthesis |
|
Clotrimazole |
Antifungal |
Impairs ergosterol synthesis |
|
Metronidazole |
Antibacterial |
Inhibits protein synthesis leading to loss of helical DNA structure |
|
Tinidazole |
Antibacterial |
Reduction of the NO2 (nitro) by susceptible bacteria |
|
Ornidazole |
Antibacterial |
Reduction of the NO2 (nitro) group by susceptible bacteria |
|
Clonidine |
Antihypertensive |
Alpha adrenoceptor antagonist |
Novel synthetic strategies for imidazole derivatives
It was seen from the literature that most of the imidazole and derivatives has been prepared through conventional synthesis method like Debus synthesis, Radiszewski synthesis, dehydrogenation, Wallach synthesis, etc., and takes several hours to the completion of the reaction. The classical methods also suffer from the disadvantages of poor yield, side reactions, slow rate, and inappropriate conditions. Researchers are constantly working to develop clean, high-yielding reactions with reusable catalysts using environmentally friendly reaction systems with an emphasis on their therapeutic advantages.
This review paper mentions the most relevant reaction strategies for the synthesis of imidazoles. The earlier reviews have not covered the research work done on imidazoles in the last 5 years, which have been included in our paper.
The present work highlights the important findings on the pharmacological actions of imidazole and its derivatives, especially during the last decade.
Substituted imidazoles were prepared by the multicomponent condensation method or microwave-assisted or heating method.
The synthesis of five optically active compounds based on the imidazole scaffold via a four-step reaction (Scheme 1) was reported by a team of researchers 21. The microwave-assisted method for synthesis could not yield the desired products while the condensation reaction of α-bromo ketones and formamidine acetate in presence of liquid ammonia was found to be a successful approach for the synthesis.
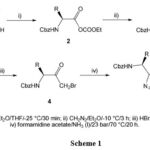 |
Scheme 1 |
Table 2 summarizes a few of the catalyst-based synthesis of imidazoles and derivatives.
Table 2: Catalyst-Based synthesis of Imidazoles 22
|
Catalyst type |
Category |
Operating conditions |
Yield |
|
TiCl3-SiO2 |
Silica-based |
Heat at 90 0C |
92% |
|
Activated fullers earth |
Clay-based |
Heat at 1000C |
95% |
|
Zeolite |
Mineral based |
Heat at 1100C |
90% |
|
Silica gel |
Heteropolyacids |
Microwave at 130 0C |
78.9% |
|
HPA |
Microwave |
95% |
|
|
PPA-SiO2 |
PPA |
Microwave |
96% |
|
ZrO2-Al2O3 |
Alumina |
Heat at 1200C |
99.2% |
|
Fe3O4 |
Magnetic nanoparticles
|
Heat |
99% |
|
Cu2O/Fe3O4 |
Ultrasonic at room temperature |
97% |
|
|
HMS-SA |
99% |
||
|
BNPs-SiO2 |
Silica based nanocatalyst |
Heat at 140 0C |
97% |
|
SiSaln |
Heat at 800C |
80% |
|
|
AgNP-CS |
Biohybrid nanocatalyst |
Heat at 1000C |
90% |
|
Au-RGO |
Heat at 5 0C |
90% |
A novel one-pot three-component scheme for the production of imidazole using ultrasonic irradiation has been reported 23. They used nickel catalyst in ethanol to obtain 2-phenyl-1H-phenanthro [9,10-d] imidazoles and reported a short reaction time.
Three imidazole derivatives from oxazolone and dinitrophenyl hydrazine using pyridine as a catalystwere synthesized 24. The prepared compounds were confirmed by FTIR and NMR spectra.
The synthesis of N-Arylimidazole derivatives using aluminium oxy-hydroxide-supported palladium nanoparticles has been reported 25. The finest results were reported by employing water-isopropyl solvent mixture. Ultrasonic conditions facilitated high yields of N-aryl imidazole derivatives. The catalyst employed was purified economically from the reaction medium and was reused without losing its efficacy, thereby presenting an environment-friendly and economical method. ICP-MS result of 1% validated the fact that the method is eco-friendly in approach.
A novel synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation as a novel neutral ionic liquid-catalyzed solvent-free method 26.
To achieve superior selectivity and output a wide range of catalysts have been explored. Researchers have employed titanium-based silica for one-pot synthesis of imidazole compounds 27. In another attempt, copper oxide over silica was employed for the synthesis of solvent-aided imidazole derivative and offered the advantage of simple and cost-effective preparation 28.
Similarly, SbCl3-silica catalyst was used to prepare microwave-assisted trisubstituted imidazole. This method proved advantageous over previously explored ones due to appreciable results, short reaction time, diminished expenses, and eco-friendliness. The catalyst was renewable with no significant reduction in activity 29.
Microwave irradiation was also used for solvent-free preparation of imidazole derivatives 30. The excellent yield with good reaction time was observed by the researchers. Easy and quick and high yield synthesis of trisubstituted imidazole was done by microwave-assisted one-pot cyclo condensation. Sodium hydrogen sulphate supported by silica was employed as a renewable and thermally stable catalyst.
Through this review, we could figure out that optimizing a microwave-assisted method for the synthesis of imidazoles could be beneficial in a significant reduction of reaction time as well as improving the yield of the product formed.
Therapeutic Efficacy of Imidazole Motif
The enormous utility of imidazole-based moieties in curative chemistry has led to a lot of work being directed toward the feasible prolific applications of imidazole derivatives in diverse areas. The current area is expected to summarize the current improvements of imidazole-based mixtures in the entire range of restorative science including anticancer, anti-neuropathic, fungal, antibacterial, antitubercular, antiparasitic, antihistaminic, anti-hypertensive, antiinflammatory, viral defending, so on.
Antimicrobial action
In a recent study, 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-4-yl)-1H-imidazole derivatives were synthesized (Figure 1) and examined for fungal and mycobacterial activity against Candida albicans strain and Mycobacterium tuberculosis H(37)Rv strain, respectively31.
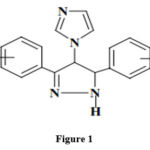 |
Figure 1 |
One of the research team performed the production of a run of novel imidazole-based compounds with broad chemotherapeutic properties. The compounds showed comparable degree of activity to metronidazole but lower activity than efavirenz. They screened the compounds for anti-HIV and antibacterial actions and suggested that the series of compounds synthesized could prove to be efficient starting moieties for broad-spectrum action32.
The synthesis of an innovative and new series of imidazole subordinates was achieved by refluxing 9, 10-phenanthraquinone with aryl aldehyde, essential amines, and ammonium acetic acid using a catalytic amount of glacial acetic acid. The combined mixtures were evaluated for antimicrobial exercises against Candida albicans. They reported 200µg/ml dose of every synthesized derivative had better activity than the dose of 100µg/ml 33.
Antimicrobial action of newly synthesized mono, di, and tri-substituted imidazole derivatives against bacteria and fungi was reported by researchers34. Streptomycin and Amphotericin B were employed as standards during the screening action.
Another investigator performed the synthesis of imidazoleby cyclo condensation of hippuric acid and m-methyl benzaldehyde. The synthesized compounds were evaluated for antimicrobial action. The antibacterial behavior of the compounds was studied against species like Staphylococcus aureus, Bacillus subtilis, E.coli, and klebsiella promioe at a concentration of 50microg/mL by agar cup plate method while the fungicidal action was studied at 103 ppm concentration in vitro 35.
Novel imidazole derivativeswere prepared by using a four-step reaction strategy involving the reaction of ethanone with selenium dioxide in dioxane followed by cyclization using aromatic aldehyde in the presence of acetic acid or ammonium acetate 36. The antibacterial effect of the synthesized compounds was also studied.
In another report, the synthesis of 2-aminoimidazole derivatives from conventional dicarbonyl compounds in the presence of ammonium acetate and acetic acid 37. The screening of the imidazoles for antimicrobial activity highlighted the significance of the amine group at the 2-position of the imidazole in enhancing the antimicrobial potential.
One-pot synthesis of imidazole compounds based on a quinoline scaffold has been reported 38 (Figure 2). The synthesis was carried out from benzil, ammonium acetate, and 2-phenoxyquinoline3-carbaldehyde employing ceric ammonium nitrate as a catalyst. The ready compounds were investigated for antimicrobial and antitubercular activities. Glucosamine-6-phosphate was considered a new target for antimicrobial action. They inferred that the compounds with the least binding energy act as a more active antimicrobial agent than standard drugs.
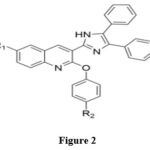 |
Figure 2 |
The scheme and synthesis of several 2-(substituted dithiocarbamoyl)-N-[4-((1H-imidazol-1-yl)methyl)phenyl]acetamide derivatives was reported by a team of researchers. They tested the compounds for antifungal effects on four fungal strains and also attempted to investigate the mode of action using molecular docking studies 39.
The synthesis of tetra-substituted imidazole derivatives using one-pot condensation reaction was also performed. All compounds were analyzed for their melting point, Carbon Hydrogen Nitrogen (CHN) analysis, Fourier transform Infrared Spectroscopy (FT-IR) , and UV-Visible spectra40. The biological activity was verified against Staphylococcus aureus, Bacillus subtilis, and Escherichia, and significant action against bacterial strains was reported.
Anticancer action
In an investigation, a series of 1,2-heteroannulated anthraquinones and anthra [1,2-d] imidiazole-6,11-dione homologues was synthesized, and reported the cell damage and human telomerase inhibition activities 41. A range of side-chain inclusion was done using synthetic routes including acylation, cyclization, condensation, and heterocyclization. Screening tests revealed varying levels of differential cytotoxicity.
A few derivatives of 2-(4-substituted piperazine-1-yl)-N-[4-(1-methyl-4,5-diphenyl-1H-imidazole-2-yl)phenyl] acetamide was synthesized by researchers 42. The Infra Red (IR) spectra, Nuclear Magnetic Resonance (NMR) images, and Electron Ionization Mass Spectrum (EI-MS) data confirmed the structures of the compounds. These combined mixtures were evaluated for anticancer action against colon carcinoma cell lines. The prepared derivatives effectively exhibited cytotoxicity against the cell lines and were supported by DNA fragmentation.
The production of a series of novel imidazolium derivatives and studied the cytotoxicity against 5 human tumor cell lines using an MTS assay was carried out. They suggested the presence of 5,6-dimethyl-benzimidazole ring, naphthylacyl group as a substituent, and the existence of alkyl chain length between aromatic rings was significant for the antitumor action. The prepared compounds amazingly induced cell cycle seize 43.
A report summarized the synthetic procedures of novel imidazole along with fused imidazole derivate. The key intermediate for the synthesis was 5-arylidene-2-hydrazino-3-phenyl imidazolin-4-one. Some of the prepared imidazoles were checked for cytotoxic action against carcinoma of the breast and colon cell lines and also for action against microbes using the cup plate diffusion method. A broad-spectrum activity was reported by the research team 44.
The synthesis of novel N-(6-substituted-benzothiazol-2-yl)-2-[[4,5-dimethyl-1-((p-tolyl/4-nitrophenyl)amino)-1H-imidazol-2-yl]thio]acetamide derivatives was reported by researchers 45. The synthesized compounds were winnowed for cytotoxic effect against C6 and HepG2 tumor cell lines. They reported an IC50 value of about 15.67 µg/mL via C6 tumor cell lines suggesting effective antiproliferative activity of the derivatives.
In a novel work, the synthesis of tetra aryl imidazole derivatives and evaluation of the compounds for cytotoxicity and anthelmintic actions was reported. They prepared a suitable Schiff’s base, which was reacted with ammonium acetic acid and isatin to yield the corresponding tetra aryl derivative. Noteworthy action against HEp2 cell lines was observed by the team 46.
Some phenylindole-linked imidazole compounds were blended for preparation 47. Plenty of these prepared compounds were investigated for their cytotoxic effects against 4 cancer cell lines.
Other actions
Researchers reported the biological activity of some (2E)-substituted-2- ethylidene-5,6-diphenylimidazo[2,1-b][1,3]thiazol-3-(2H)-ones 48 (Figure 3).
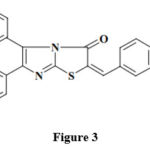 |
Figure 3 |
Anti-tubercular action of some newly synthesized imidazole derivatives was performed. Tetrabutylammonium bromide was utilized as a catalyst during the synthesis process. The compound was screened against Mycobacterium tuberculosis and excellent invitro antitubercular activity was observed 49.
The preparation of N-1-phenyl-3-substituted phenyl indole (2,3) imidazole derivatives by the condensation reaction between aryl aldehydes and N-1-phenyl isatin in an environment of ammonium acetate (NH4CH3CO2) and glacial acetic acid (C2H4O2) was performed and synthesized compounds were screened for anticonvulsant action and neurotoxicity. The anticonvulsant activity was checked by the maximal electroshock seizure (MES) method 50.
One-pot synthesis of imidazole derivatives using Mannich base method in the presence of copper catalyst was reported by an investigation team. Prepared compounds were analyzed by IR spectra, Proton NMR, Carbon NMR, and mass spectral data including elemental analysis. The synthesized imidazoles were evaluated for larvicidal actions using a standard bioassay protocol. The high larvicidal activity was confirmed based on their half-maximal lethal dose value 51.
Anticonvulsant action of a few novel tetra-substituted imidazoles against Pentylenetetrazole (PTZ-induced) convulsions and MES methods was performed by researchers 52. The maximum activity of 12.5 s of the Hind Limb Tonic Extensor Phase and convulsion time of 285.5 s supported the results. Also, imidazole-based mixtures have shown remarkable improvement in the entire range of restorative science 53-56.
Conclusions
Over the years, the quest for obtaining newer and better drug substances has been constantly increasing. The imidazole scaffold shares a special mention in the pharmaceutical field as it can be found in endogenous neurotransmitters.
The critical analysis of the published research infers that optimization of the microwave method for synthesis of the imidazole nucleus and the derivatives thereof would help reduce the overall time required for the synthesis with a much lesser workup. It would also help in increasing the yield of the product obtained to up to 97%. This would help in overcoming the research gap in drug development related to imidazole scaffold.
The presented review reflects excellent pharmacological activities of the imidazoles and derivatives, including antibacterial, anticancer, antitubercular, anticonvulsant, and anti-larvicidal activities supported by maximal lethal dose values, HLTE phase and convulsions time, etc. Researchers have succeeded in fabricating compounds with promising biological output by modifying the imidazole nucleus. The current review will help the scientific community to add new pharmacological profiles for safer and more effective therapeutic compounds.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Sources
There is no funding sources
References
- Belagali SL, Harish K, Boja P, Himaja M. Synthesis and biological studies of 5(p-chlorophenyl)-furan-2-carbony peptides and 4-[2′-(5′-formyl)-furyl]benzoyl peptides. Indian J Chem 1998; 37B: 370.
- Bansal RK. Heterocyclic chemistry. Synthesis, reactions and mechanism, New Delhi; Wiley Eastern Ltd. 1990.
- Sammes PG. Compressive organic chemistry of heterocyclic compounds. Oxford; Pergammon press; part 20-1, 1971.
- Zhang L, Pend XM, Damu GLV, Geng RX, Zhou CH. Comprehensive review in current developments of imidazole based medicinal chemistry. Med Res Reviews 2014; 34(2): 340-437. 10.1002/med.21290.
- Molina P, Tarraga A, Oton F. Imidazole derivatives: A comprehensive survey of their recognition properties. Org Biomole Chem 2012;10:1711–1724. doi.org/10.1039/C2OB06808G
- Shrivastava TP, Patil UK, Garg S, Singh MA. Divers pharmacological significance of imidazole derivatives- A review. Res. J. Pharm. and Tech 2013; 6 (1): 44-50 https://www.rjptonline.org/AbstractView.aspx?PID=2013-6-1-18
- Aleksandrova EV, Kravchenko AN, Kochergin PM. Properties of haloimidazoles (review). Chem Heterocyclic Comp 2011; 47: 261–289. doi.org/10.1007/s10593-011-0754-8
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5732, Zolpidem; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Zolpidem
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 2942, Dacarbazine; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Dacarbazine
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 667490, Mercaptopurine; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Mercaptopurine
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 644241, Nilotinib; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Nilotinib
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5394, Temozolomide; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Temozolomide
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 3823, Ketoconazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Ketoconazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5353853, Oxiconazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Oxiconazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 47472, Butoconazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Butoconazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 2812, Clotrimazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Clotrimazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 4173, Metronidazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Metronidazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5479, Tinidazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tinidazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 28061, Ornidazole; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Ornidazole
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 2803, Clonidine; [cited 2022 Apr. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Clonidine.
- Marek A, Kulhanek J, Ludwig M, Bures F. Facile synthesis of optically active imidazole derivatives. Molecules 2007; 12: 1183-1190. doi: 10.3390/12051183.
- Patel SR, Patil RV, Chavan JU, Beldar AG. Polymer based advanced recipes for imidazoles: a review. Turkish J Chem. 2022; 46 (3): 624-664. doi.org/10.55730/1300-0527.3357
- Dianati HR, Nazif AR, Salimi S, Teymori V. Novel methodology for the synthesis of 1H-phenanthro[d] Imidazole derivatives via Ni (II) Schiff base catalysts. Res J Chem Environ Sci 2014; 2(2): 45-48.
- Idriss IKH, Saeed AEM. Synthesis and characterization of some imidazole derivatives. Inter J Innovative Sci and Eng Tech 2016; 3(8): 34-42.
- Goksu H, Demir E, Zengin N. Synthesis of N-arylimidazole derivatives from imidazole and arylhalides in the presence of recoverable Pd/AlO(OH) NPs. Biomedical J Sci Technical Res 2020; 27(4): 20948-20954. doi.org/10.26717/BJSTR.2020.27.004537
- Xia M, Lu YD. A novel neutral ionic liquid-catalyzed solvent-free synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation. J of Molecular Catalysis A 2007; 265: 205-08. doi.org/10.1016/j.molcata.2006.10.004
- Subba R, Dasgupta HR, Saha B, Pariyar GC, Tamang A, Ghosh P. TiCl3-silica: A recyclable solid support for efficient synthesis of substituted imidazoles. Asian J Nanoscience and Materials 2021; 4 (1): 31-45. 10.26655/AJNANOMAT.2021.1.3
- Gupta M, Gupta M. Doping of copper (i) oxide onto a solid support as a recyclable catalyst for acetylation of amines/alcohols/phenols and synthesis of trisubstituted imidazole. J Iran Chem Soc 2016; 13 (2): 231-241. doi.org/10.1007/s13738-015-0730-9
- Safari J, Naseh S, Zarnegar Z, Akbari Z. Applications of microwave technology to rapid synthesis of substituted imidazoles on silica-supported SbCl3 as an efficient heterogeneous catalyst. J Taibah Uni Sci 2014; 8 (4): 323-330. doi.org/10.1016/j.jtusci.2014.01.007
- Safari J, Khalili SD, Banitaba SH. Three-component, one-pot synthesis of 2,4,5- trisubstituted imidazoles catalyzed by TiCl4-SiO2 under conventional heating conditions or microwave irradiation. Syn Communications 2011; 41 (16): 2359- 2373. doi.org/10.1080/00397911.2010.502994
- Zampieri D, Mamolo MG, Laurini E, Scialino G, Banfi E, Vio L. Antifungal and antimycobacterial activity of 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-4yl)-1H–imidazole derivatives. Bioorganic Medicinal Chem 2008; 16: 4516-22. doi.org/10.1016/j.bmc.2008.02.055
- Ganguly S, Vithlani VV, Kesharwani AK, Kuhu R, Baskar L, Mitramazumder P, Sharon A, Dev A. Synthesis, antibacterial and potential anti-HIV activity of some novel imidazole analogs. Acta Pharmaceutica 2011; 61: 187-201. doi: 10.2478/v10007-011-0018-2
- Narwal S, Singh G, Saini D. Synthesis of novel imidazole compounds and evaluation of their antimicrobial activity. Indo Global J Pharmaceutical Sci 2012; 2(2): 147-153. doi: 10.35652/igjps.2012.18
- Mahalakshmi CM, Karthick M, Shanmugam M, Chidambaranathan V. Synthesis, spectral characterization and antimicrobial studies of novel imidazole derivatives. Der Pharma Chemica 2015; 7(1): 14-19. http://derpharmachemica.com/archive.html
- Shah PJ. Preparation, characterization and antimicrobial studies of novel imidazole combined molecule. Organic Chemistry- An Indian J 2015; 11(2): 77-81.
- Singh RK, Bhatt A, Ravi K. Design and synthesis of some novel imidazole derivatives as potent antimicrobial & antimalarial agents. Der Pharmacia Lettre 2016; 8(7): 188-194. http://scholarsresearchlibrary.com/archive.html
- Gupta S, Verma P, Singh V. Synthesis and antimicrobial study of 2-amino-imidazole derivatives. Indian J Chem 2018; 57B: 679-686.
- Shobhashana PG, Prasad P, Kalola AG, Patel MP. Synthesis of imidazole derivatives bearing quinoline nucleus catalysed by can and their antimicrobial, antitubercular and molecular docking studies Res J Life Sci, Bioinformatics, Pharma Chemical Sci 2018; 4(3): 175-186. doi: 10.26479/2018.0403.15
- Altindag FD, Saglik BN, Cevik UA, Isikdag I, Ozkay Y, Gencer HK. Novel imidazole derivatives as antifungal agents: Synthesis, biological evaluation, ADME prediction and molecular docking studies. Phosphorus, Sulfur, and Silicon and the Related Elements 2019; 194 (9): 887-894. doi.org/10.1080/10426507.2019.1565761
- Shahzad K, Abbas F, Pandey D, Ajmal S, Khadim M, Tahir MU. Synthesis, characterization and biological evaluation of novel tetrasubsituted imidazole compounds. Chem Rxiv 2020; doi.org/10.26434/chemrxiv.12480605.v1
- Huang HS, Chen JC, Chen RH, Huang CF, Huang FC, Jhan JR, Chen CL, Lee CC, Lo Y, Lin JJ. Synthesis, cytotoxic and human telomerase inhibition activities of a series of 1,2-heteroannulated anthraquinones and anthra[1,2-d]imidazole-6,11-dione homologues. Bioorg Med Chem 2009; 17: 7418-7428. doi: 10.1016/j.bmc.2009.09.033
- Ozkay Y, Isikdag I, Incesu Z, Akalin G. Synthesis of 2-substituted-N-[4-(1-methyl-4,5-diphenyl-1H-imidazole-2yl)phenyl]acetamide derivatives and evaluation of their anticancer activity. European J Med Chem 2010; 45: 3320-28. doi.org/10.1016/j.ejmech.2010.04.015
- Liu LX, Wang XQ, Zhou B, Yang LY, Zhang H-B, Yang XD. Synthesis and antitumor activity of novel N-substituted carbazole imidazolium salt derivatives. Scientific Reports 2015; 5:13101. 10.1038/srep13101
- Eatedal H Abd El-Aal, Fattah HAA, Osman NA, Seliem IAA. Synthesis of novel imidazole and fused imidazole derivatives as cytotoxic and antimicrobial agents: molecular docking and biological evaluation. Inter J Pharmacy and Pharma Sci 2015; 7(10): 36-45.
- Yurttas L, Ertas M, Ciftc GA, Temel HE, Demirayak S. Novel benzothiazole based imidazole derivatives as new cytotoxic agents against Glioma (C6) and Liver (HepG2) Cancer cell lines. Acta Pharmaceutica Sciencia 2017; 55(1): 39-47. doi: 10.23893/1307-2080.APS.055.
- Kumar R, Saxena V, Saxena AK, Hoque M. Synthesis of Some Novel Tetra Aryl Imidazoles Compounds: Evaluation of in-vitro cytotoxicity and anthelmintic activity. Internat.. J Medical Research and Health Sci 2019; 8(8): 62-69.
- Yousif MNM, Hussein HAR, Yousif NM, El-Manawaty MA, El-Sayed WA. Synthesis and Anticancer Activity of Novel 2-Phenylindole Linked Imidazolothiazole, Thiazolo-s-triazine and Imidazolyl-Sugar Systems. J Applied Pharma Sci 2019; 9(1): 6-14. 10.7324/JAPS.2019.90102
- Bhaskar VH, Kumar M, Sangameswaran B, Balakrishnan BR. Biological activity of some (2E)- substituted -2- ethylidene-5,6-diphenylimidazo[2,1-b][1,3] thiazol-3-(2H)-ones. Asian J Res Chem 2008; 2: 218-23.
- Pandey J, Tiwari VK, Verma SS, Chaturvedi V, Bhatnagar S, Sinha S, Gaikwad AN, Tripathi RP. Synthesis and anti-tubercular screening of imidazole derivatives. European J of Medicinal Chem 2009; 44: 3350-55. https://doi.org/10.1016/j.ejmech.2009.02.013.
- Bhragual DD, Kumar N, Drabu S. Synthesis and pharmacological evaluation of some substituted imidazoles. J Chem Pharma Res 2010; 2: 345-49.
- Alaklab A, Kumar RS, Ahamed A, Arif IA, Manilal A, Idhayadhulla A. Synthesis of novel three compound imidazole derivatives via Cu(II) catalysis and their larvicidal and antimicrobial activities. Monatsh Chem 2017; 148:275-290. 10.1007/s00706-016-1746-2.
- Joseph L, George M, Murali A. Synthesis, characterization and screening of anti convulsant activity of novel tetra substituted imidazole derivatives. Inter J Pharmacy and Analytical Res 2018; 7(3): 490-493. 10.22159/ajpcr.2019.v12i10.34738.
- Kaushik K, Bhardwaj H, Sharma GK. Synthesis and biological evaluations of some novel Benzimidazole Derivatives. Res J. Pharm. and Tech. 2020; 13(1): 368-372. doi: 10.5958/0974-360X.2020.00073.6.
- Anand, K. and Wakode, S. Synthesis, characterization and biological evaluation of benzimidazole derivatives. Inter J of pharmaceutical sci and res, 2018, 9(2), 617-624. 10.13040/IJPSR.0975-8232.9(2).617-24.
- Imran M, Shah FA, Nadeem H, Zeb A, Faheem M, Naz S, Bukhari A, Ali T, Li S. Synthesis and Biological Evaluation of Benzimidazole Derivatives as Potential Neuroprotective Agents in an Ethanol-Induced Rodent Model. ACS Chem Neurosci. 2021 Feb 3;12(3):489-505. doi: 10.1021/acschemneuro.0c00659. Epub 2021 Jan 12. PMID: 33430586.
- Kavya M., Priya D. Green Chemistry approach for the synthesis of some Benzimidazole derivatives and their Biological Evaluation. Res J. Pharm. and Tech. 2019; 12(3): 1023-1030. doi: 10.5958/0974-360X.2019.00169.0







