Jeneth Berlin Raj1 , Parthasarathy S2*
, Parthasarathy S2* , Manimekalai K3, Srinivasan AR4
, Manimekalai K3, Srinivasan AR4
1Department of Physiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth.puducherry, India.
2Department of Anesthesiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth. Puducherry, India.
3Department of Pharmacology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth. Puducherry, India.
4Department of Biochemistry, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth. Puducherry, India
Corresponding Author E-mail: painfreepartha@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2673
Abstract
With the emerging trend of preferring naturopathy over modern medicine, common people are in more danger when consuming natural plant products without the knowledge of the toxic agents present in them. Aim and Objective: To evaluate the safety of methanolic leaf extract of Costus pictus D Don on albino Wistar rats. Methodology: The current study was designed strictly based on the organization for economic cooperation and development (OECD) guideline 423 for acute toxicity study to determine LD50 and guideline 407 for sub-acute toxicity study for hazard identification and risk assessment of the test solution. Methanolic leaf extract was prepared by soxhlation. 12 animals (each 12-week-old nulliparous, non-pregnant female Wistar rats with a mean weight of 142 ± 2 g), 3 per step were used for the acute toxicity study. The test was initiated with a single test dose of 300 mg/kg BW on three animals and continued till 2000 mg/kg BW. After ingesting the test dose each animal was observed individually for the first 4 hours and later every day for 2 weeks for signs of toxicity. For the sub-acute toxicity study, 30 adult Wistar rats (each 16-week-old rat weighing 250±12g) were randomized into 3 groups (1 control and 2 study groups) of 10 each consisting of five males and 5 females. Animals in the control group received 1% Carboxymethyl cellulose (CMC) at a dose of 10 ml/kg BW whereas the animals in the study group received 500 and 1000 mg/kg body weight (BW) of the extract respectively for 28 days. Later, all the animals were sacrificed and blood samples were studied for hematological and biochemical changes. Results: The lethal dose of Costus pictus D Don methanolic leaf extract was fixed as more than 2000 mg/kg Body weight. No obvious change was observed in feeding habits, weight, hematology, biochemical parameters, and histopathology. Conclusion: Methanolic leaf extract of Costus pictus D Don was observed to be absolutely safe when given orally in albino Wistar rats.
Keywords
Costus pictus D Don; Methanolic extract; safety; toxicity; viscera
Download this article as:| Copy the following to cite this article: Raj J. B, Parthasarathy S, Manimekalai K, Srinivasan A. R. Evaluation of Safety Profile of Costus Pictus D Don Methanolic Leaf Extract on Albino Wistar Rats. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Raj J. B, Parthasarathy S, Manimekalai K, Srinivasan A. R. Evaluation of Safety Profile of Costus Pictus D Don Methanolic Leaf Extract on Albino Wistar Rats. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/437v9FH |
Introduction
Plants and plant-derived products, which were once only used in folk medicine, are now widely consumed by the general public without the advice of experts. Researchers are more interested in the efficacy of plant extracts than in their toxicity. To protect themselves from microorganisms, insects, and animals, plants produce toxic substances. Some phytotoxins with bacterial-like properties may cause adverse effects in humans ranging from a mild itch to food poisoning 1. As a result, it is critical to investigate plant products in order to assess their toxic effect. With the rising cost of pharmacological diabetes treatment, the general public is turning to plants and plant-derived products for assistance. India, the medicinal plant botanical garden, has approximately 20,000 effective plant-based formulations used in traditional and folk medicine. Economic considerations and lower side effects are driving the expansion of Complementary and Alternative Medicine (CAM) as adjuncts or alternatives to Western medical approaches 2. According to WHO, India has the most diabetic patients (31.7 million) in the world. Even in populations that consume a lot of calories, micronutrient-rich foods are consumed in small amounts. Micronutrient deficiencies are prevalent in both urban and rural areas 3. Nutraceuticals are products that are extracted from natural sources (nature-like) or commercially produced synthetically (man-made) to replenish the diet and help in the treatment and prevention of disease and nutrient disorders 4. In India, approximately 2500 species of medicinal plants are used in the treatment of diabetes. Costus pictus D Don is one such plant recently studied by researchers for its diverse effects especially as antidiabetic drug owing to the presence of many phytochemicals like Bixin, Geraniol, abscisic acid etc. 5-7.
Materials and Methods
Plant materials
D Costus Pictus Don leaves were collected from a one-year-old plant in a Pondicherry Garden during the summer. The Department of Botany at Annamalai University in Chidambaram authenticated the plant material (No. 326). A specimen of the plant is kept at the Sri Balaji Vidyapeeth in Pondicherry
Preparation of plant extract
The leaves were air-dried in shade for 7-10 days. The dried leaves were then powdered and subjected to soxhlation with methanol. The final extract obtained was dried with a rotary evaporator and refrigerated in a brown airtight bottle.
Experimental animal
After obtaining institute ethical clearance (O3/IAEC/MG/2016), Healthy adult Wistar rats (12-week-old female rats for acute toxicity study and 16 weeks old male and female rats for sub-acute toxicity study) weighing > 160 g were used for the study. The animal was procured from Kings Institute, Chennai, and maintained in a standard rat cage under controlled temperature (25+2 °C), relative humidity of ~ 60 %, and light (12:12 light-dark cycle) in MGMC & RI central animal house. The animals will be fed with standard rat pellet and hygienic water ad libitum.
Oral acute toxicity study
An acute toxicity study to determine LD50 was performed as per the guidelines (OECD guideline 423) set by the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA). [8]. A total of 12 animals (each 12-week-old nulliparous, non-pregnant female Wistar rats with a mean weight of 142 ± 2 g) were used to determine the LD50 dose of Costus pictus D Don methanolic leaf extract (CPDDMLE). 3 animals per step were used for the acute toxicity study. After an overnight fast (no food but water was given), the animals were weighed and a single dose of test solution was given orally using a gavage tube. Food was withheld for the next 4 hours. The animals were observed for signs of toxicity like tremors, convulsions, increased salivation, diarrhea, lethargy, excess sleep, death, etc every 30 minutes on the first day and thereafter daily for the next 2 weeks. If no toxic signs or mortality were present, the test was repeated with the same dose for reconfirmation and then proceeded with higher strength. We initiated the test with a single test dose of 300 mg/kg BW on three animals and continued till 2000 mg/kg BW.
Each rat’s body weight was measured before the experiment began and every week thereafter with a digital weighing machine. Food and water intake were calculated as follows: Feed intake= daily intake (g or ml)/average body weight of rats in each cage (g).Following the conclusion of the experiment, all animals were sacrificed via intraperitoneal injection of 150mg/kg sodium pentobarbitone. The organs were dissected out, washed with saline, blotted dry, and weighed, including the heart, liver, kidney, pancreas, brain, eyes, and ovaries. A small amount of these organs were quickly fixed in 10% formalin.Following that, the tissues were processed using standard histopathological techniques (i.e. dehydration through graded isopropyl alcohol, clearing through xylene, and impregnated in paraffin wax for 2 hr). Wax blocks were created. Five sections were cut with a rotary microtome, stained with hematoxylin and eosin, and photographed.
Sub-acute toxicity study
According to OECD guideline 407 for hazard identification and risk assessment of the test solution [9], a subacute toxicity study was carried out on 30 adult Wistar rats (each 16-week-old rat weighing 250±12g). The animals were randomized into 3 groups (1 control and 2 study groups) of 10 each consisting of five males and 5 females. Animals in the control group received 1% CMC at a dose of 10 ml/kg BW whereas the animals in the study group received 500 and 1000 mg/kg BW of the extract respectively for 28 days. Feed intake and weight gain were measured before and after the test procedure on a regular basis. Every day following the administration of test doses, the animals were observed for signs of toxicity. After an overnight fast, all of the animals were sacrificed via intraperitoneal injection of 150mg/kg sodium pentobarbitone. Blood samples were taken from an intra-cardiac puncture to determine haematological parameters (total RBC, WBC, and platelet count) as well as biochemical parameters (total cholesterol, triglycerides, urea, creatinine, total protein, albumin, globulin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). To assess the toxic effect, organs were dissected and histopathological examinations were performed.
Result
Acute toxicity study
No significant toxic changes were observed in any of the animals for 14 days after ingestion of plant extract. Since one mortality was recorded at a repeat dose of 2000 mg/kg BW of CPDDMLE, we fixed the upper limit of the test dose at 2000 mg/kg. On studying the feed and weight gain, no significant change was seen (Fig 1) between the groups tested with 300 mg/kg BW and 2000 mg/kg BW of CPDDMLE. Histopathology of the organs studied also did not show any notable histological changes (Fig 2) in any of the groups and the dead animal tested with 2000 mg/kg BW. No toxic signs were observed on oral administration of C. pictus D Don methanolic leaf extract at the dosage of 300 & 2000mg/kg BW (Table 1). These results indicate that the medium lethal dose (LD50) is higher than 2000 mg/kg.
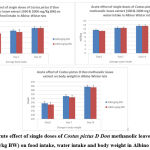 |
Figure 1: Acute effect of single doses of Costus pictus D Don methanolic leave extract (300 & 2000 mg/kg BW) on food intake, water intake and body weight in Albino Wistar rats |
Table 1: Signs of toxicity in Albino Wistar rats after acute exposure to Costus pictus D Don methanolic leave extract
| Signs of toxicity | Animals treated with 300 mg/kg BW | Animals treated with 2000 mg/kg BW |
| Changes in skin & fur | Normal | Normal |
| Lacrimation | Normal | Normal |
| Salivation | Normal | Normal |
| Diarrhoea | Normal rat dropping | Normal rat dropping |
| Tremors | No tremors | No tremors |
| Motor activity | Normal | Normal |
| Loss of righting reflex | Nil observed | Nil observed |
| Feed intake | Normal | Normal |
| Lethargy | Normally active | Normally active |
| Death/ mortality | Nil recorded | Nil recorded |
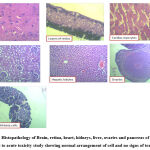 |
Figure 2: Histopathology of Brain, retina, heart, kidneys, liver, ovaries and pancreas of animals subjected to acute toxicity study showing normal arrangement of cell and no signs of toxic effect |
Sub-Acute toxicity study
An increase in feed intake after the 7th day was observed in rats tested with 500 mg/kg BW and 1000 mg/kg BW of CPDDMLE. Weight gain increased initially in response to an increase in feed intake, but by the 14th day, weight gain was under control (Fig 3). There were no significant hematological changes observed (Table 2). There were no changes in renal or liver parameters. Significant changes in glucose, total cholesterol, and triglyceride levels were observed in rats given CPDDMLE at doses of 500 mg/kg BW and 1000 mg/kg BW (Table 3). There was no difference in organ weight between the study and control groups of animals (Table 4) Histopathology of the heart, liver, kidneys, and brain revealed no toxic changes. (Fig.4).
 |
Figure 3: Effect on ingesting Costus pictus D Don methanolic leave extract (500 & 1000 mg/kg BW) for 28 days on food intake, water intake and body weight in Albino Wistar rats |
Table 2: Hematological changes after ingesting Costus pictus D Don methanolic leave extract (500 & 1000 mg/kg BW) for 28 days in Albino Wistar rats
| Hematological parameters | Control animals | Rats treated with 500 mg/kg of extract | Rats treated with 1000 mg/kg of extract | |||
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| Hemoglobin (g/dl) | 14.08 ± 0.17 | 14.12 ± 0.19 | 14.24 ± 0.16 | 14.31 ± 0.11 | 14.26 ± 0.27 | 14.24 ± 0.20 |
| RBC (10^6/µL) | 7.26 ± 0.2 | 7.26 ± 0.1 | 7.24 ± 0.11 | 7.32 ± 0.06 | 7.28 ± 0.06 | 7.32 ± 0.06 |
| WBC (10^3/µL) | 6.45 ± 0.25 | 6.49 ± 0.18 | 6.53 ± 0.15 | 6.72 ± 0.33 | 6.60 ± 0.12 | 6.63 ± 0.11 |
| Platelets (10^3/µL) | 926.3 ± 22.14 | 935 ± 23.19 | 925.3 ± 22.14 | 43.1 ± 1.66 | 921 ± 26.77 | 934 ± 20.57 |
| Hematocrit (%) | 41.4 ± 2.17 | 41.4 ± 1.71 | 43.1 ± 1.66 | 43.5 ± 0.97 | 42.9 ± 1.73 | 43.8 ± 1.32 |
| MCV (fL) | 57.04 ± 3.10 | 57.02 ± 2.37 | 59.56 ± 2.19 | 59.44 ± 13 | 58.95 ± 2.33 | 59.85 ± 1.67 |
| MCH (pg) | 19.40 ± 0.43 | 19.45 ± 0.33 | 19.68 ± 0.33 | 19.56 ± 0.25 | 19.60 ± 0.45 | 19.46 ± 0.35 |
| MCHC (g//dL) | 34.08 ± 1.60 | 34.16 ± 1.46 | 33.09 ± 1.39 | 32.91 ± 0.92 | 33.29 ± 1.55 | 32.54 ± 1.01 |
Table 3: Effect of ingesting Costus pictus D Don methanolic leave extract (500 & 1000 mg/kg BW) for 28 days on liver function parameter, kidney function parameters, lipid profile and glucose homeostasis in Albino Wistar rats
| Control animals | Rats treated with 500 mg/kg of extract | Rats treated with 1000 mg/kg of extract | ||||
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| Total protein (g/dl) | 6.69 ± 0.26 | 6.72 ± 0.27 | 6.74 ± 0.23 | 6.65 ± 0.30 | 6.63 ± 0.23 | 6.74 ± 0.23 |
| Albumin (g/dl) | 3.89 ± 0.31 | 4.00 ± 0.26 | 3.98 ± 0.29 | 3.88 ± 0.21 | 3.91 ± 0.22 | 3.96 ± 0.13 |
| Globulin (g/dl) | 1.71 ± 0.12 | 1.72 ± 0.14 | 1.72 ± 0.14 | 1.80 ± 0.09 | 1.73 ± 0.14 | 1.72 ± 0.12 |
| AST (U/L) | 80.60 ± 4.86 | 81.20 ± 3.91 | 81.50 ± 5.50 | 80.70 ± 4.50 | 80.90 ± 6.26 | 80.70 ± 4.90 |
| ALT (U/L) | 41.00 ± 2.00 | 42.00 ± 2.05 | 42.30 ± 2.87 | 42.20 ± 3.68 | 41.30 ± 3.37 | 41.50 ± 3.10 |
| Creatinine (mg/dl) | 0.87 ± 0.21 | 0.86 ± 0.22 | 0.90 ± 0.21 | 0.86 ± 0.16 | 0.86 ± 0.16 | 0.87 ± 0.21 |
| Blood Urea Nitrogen (mg/dl) | 17.69 ± 0.44 | 17.57 ± 0.30 | 17.91 ± 0.28 | 17.75 ± 0.27 | 17.75 ± 0.27 | 17.69 ± 0.44 |
| Triglycerides (mg/dl) | 72.10 ± 3.87 | 74.10 ± 3.81 | 73.50 ± 4.45 | 65.30 ± 3.53# | 73.90 ± 3.70 | 65.50 ± 4.40# |
| Total cholesterol (mg/dl) | 88.30 ± 6.00 | 88.50 ± 4.95 | 88.00 ± 6.38 | 72.70 ± 4.16* | 88.50 ± 4.95 | 72.80 ± 7.89* |
| Fasting glucose level (mg/dl) | 77.80 ± 7.51 | 79.70 ± 7.35 | 77.10 ± 7.48 | 62.70 ± 3.13** | 77.20 ± 6.56 | 61.80 ± 2.04** |
Data expressed in Mean+SD.#, *, ** à P<0.001, Tukey multiple comparison test
Table 4: Effect of ingesting Costus pictus D Don methanolic leave extract (500 & 1000 mg/kg BW) for 28 days on organ weight in Albino Wistar rats
| Group (n=10) | Avg. organ weight (g) | |||
| Heart | Liver | Kidney | Brain | |
| Control group animals – males | 0.97 + 0.07 | 7.25 + 0.68 | 1.82 + 0.09 | 1.76 + 0.09 |
| Animals treated with 500 mg/kg BW | 0.93 + 0.12 | 7.33 + 0.46 | 1.85 + 0.10 | 1.77 + 0.08 |
| Animals treated with 1000 mg/kg BW | 0.90 + 0.13 | 7.27 + 0.65 | 1.92 + 0.14 | 1.79 + 0.10 |
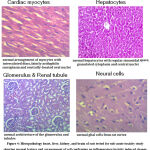 |
Figure 4: Histopathology heart, liver, kidney, and brain of rats tested for sub-acute toxicity study showing normal texture and arrangement of cell confirming no inflammatory/toxicity induced change |
Discussion
Acute toxicity Study
Because methanolic leaf extract preserved the greatest number of phytochemicals [10], it was chosen for our study as well. No abnormal behavioral changes or acute toxic effects were observed in animals treated for 14 days with a single dose of methanolic leaf extract of C.pictus D Don at doses ranging from 300 mg/kg to 2000 mg/kg BW. The limit dose was set at > 2000 – 5000 mg/kg body weight (category 5) after one death was observed on a repeat dose of the extract at 2000 mg/kg. There were no histopathological changes observed in any of the animals, including the diseased. As a result, the extract can be claimed to be non-toxic when taken orally 11.
Sub-acute toxicity study
After the 7th day, there was an increase in feed intake. This may be due to the hypoglycemic effect as observed by decreased fasting blood glucose levels 12. The initial increase in weight gain in accordance with the increase in feed intake was seen. After the 14th day, weight gain was under control which may be due to the suppression of fat accumulation as both. Decreased TC and TG levels in our study may be related to the same 13. The hematopoietic system is one of the most sensitive targets of toxic compounds and is an important index of physiological and pathological status in men and animals14. No hematological changes were observed in our study. This indicates that the extract is neither non-toxic to circulating blood cells nor interferes with their production. Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Total Protein, Albumin, and Globulin were the same in the control and study groups proving the extract non-toxic and safe for the liver. Normal values of kidney parameters such as blood urea nitrogen (BUN) and creatinine suggest that sub-acute administration of extract did not cause any damage to the kidney. The presence of hypolipidemic agents in the extract is indicated by a significant reduction in the lipid profile (total cholesterol and triglycerides). Plant sterols, like polyphenols and tannins, lower serum cholesterol by inhibiting absorption 15. The presence of hypoglycemic components in the extract is indicated by a significant reduction in fasting glucose levels in the study 16. This demonstrates that the extract can be used effectively as an anti-diabetic agent. There was no discernible difference in gross necropsy or microscopy in any of the animals. These findings back up the biochemical test that was performed. According to the findings, the extract’s No Observed Adverse Effect Level (NOAEL) is greater than 1000 mg/kg/day.
Conclusion
The lethal dose of methanolic leaf extract of C.pictus D Don is estimated to be more than 2000 mg/ kg BW. The extract has a promising effect in significantly reducing blood glucose and lipid profile. They are proven to be non-toxic and safe when consumed in oral form.
Conflict of Interest
There is no conflict of interest
References
- Patel S, Nag MK, Daharwal SJ, Singh MR, Singh D. Plant Toxins: An Overview. Research J Pharmacology and Pharmacodynamics. 2013;5(5):283-288.
- Pandey MM, Rastogi S, Rawat AKS. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med. 2013;2013:376327. doi:10.1155/2013/376327
CrossRef - int. Accessed April 3, 2023. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_part2.pdf
- Cunningham JJ. Micronutrients as nutriceutical interventions in diabetes mellitus. J Am Coll Nutr. 1998;17(1):7-10. doi:10.1080/07315724.1998.10718729
CrossRef - Raj J, Kalaivani R. Comparative in-vitro evaluation of anthelmintic property of leaves and rhizome of Costus pictus D. Don against albendazole. Natl J Physiol Pharm Pharmacol. 2016;6(5):438. doi:10.5455/njppp.2016.6.0205423032016.
CrossRef - Raj J, Kalaivani R. In-vitro evaluation of antimicrobial activity of Costus pictus D. Don aqueous leaf extract. Natl J Physiol Pharm Pharmacol. Published online 2018:1. doi:10.5455/njppp.2018.8.0310502042018.
CrossRef - Raj JB, Parthasarathy S, Manimekalai K, Srinivasan AR. Ameliorating Effect Of Costus pictus D Don Methanolic Leaf Extract On Free Radical-Induced Hepatic Damage In Prediabetic Rats. Committee for the Purpose of Control and Supervision of Experimental Animals. Published online 2022:2205-2211.
CrossRef - Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), OECD/OCDE, OECD Guidelines for the Testing of Chemicals, Revised Draft Guidelines 423: Acute Oral Toxicity- Acute Toxic Class Method, Revised Document.; 2000.
- OECD GUIDELINES FOR THE TESTING OF CHEMICALS repeated dose 28-day oral toxicity study in rodents. Nih.gov. Published 2008. Accessed April 3, 2023. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg407-2008.pdf
- Jothivel N, Ponnusamy SP, Appachi M, et al. Anti-diabetic activity of methanol leaf extract of Costus pictus D. don in alloxan-induced diabetic rats. J HEALTH Sci. 2007;53(6):655-663. doi:10.1248/jhs.53.655.
CrossRef - Acute toxicity study of aqueous and ethenolic extracts of Costus pictus D. Don and Enicostema littorale Blum in rats. The Journal of Bombay Veterinary College. 2013;20:27-33.
- Suganya S, Narmatha R, Gopalakrishnan VK, Devaki K. Hypoglycaemic effect of Costus pictus D. Don on alloxan induced type 2 diabetes mellitus in albino rats. Asian Pacific Journal of Tropical Disease. Published online 2012:117-123.
CrossRef - Ashwini S, Bobby Z, Joseph M, Jacob SE, Padmapriya R. Insulin plant (Costus pictus) extract improves insulin sensitivity and ameliorates atherogenic dyslipidaemia in fructose induced insulin resistant rats: Molecular mechanism. J Funct Foods. 2015;17:749-760. doi:10.1016/j.jff.2015.06.024.
CrossRef - Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musangacecropioides in rats. J Ethnopharmacol. 2006;105:374-379.
CrossRef - Ogbonnia S Adekunle AA, Bosa MK. Evaluation of acute an subacute toxicity of Alstoniacongensis Engler (Apocynaceae) bark and Xylopia aethiopica (Dunal) A. Rich (Annonaceae) fruits mixtures used in the treatment of diabetes. African journal of Biotechnology. 2008;7(6):701-705.
- Remya R, Daniel M. Phytochemical and pharmacognostic investigation of antidiabetic Costus pictus D Don. Int J Pharm Biomed Res. 2012;3(1):30-39.








