Muhjah Falah Hassan1* , Hind Abdul-Kadim2, Ban Jaber Edan3
, Hind Abdul-Kadim2, Ban Jaber Edan3 , Sulagna Dutta4
, Sulagna Dutta4 , Pallav Sengupta5*
, Pallav Sengupta5*
1Department of Anatomy, Histology and Embryology, College of Medicine, University of Kerbala, Kerbala, Iraq
2Department of Uro-surgery and Infertility, College of Medicine, University of Kufa, Kufa, Iraq
3Depatment of Physiology and Medical Physics, College of Medicine, University of Babylon, Babylon, Iraq
4School of Medical Sciences, Bharath Institute of Higher Education and Research (BIHER), Tamil Nadu, India
5Physiology, Department of Biomedical Sciences, College of Medicine, Gulf Medical University, Ajman, UAE
Corresponding Author E-mail: allav_cu@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2668
Abstract
Background: Polycystic ovary syndrome (PCOS) is a major cause of ovulatory dysfunctions among reproductive-aged women. PCOS impairs folliculogenesis leading to suboptimal oocyte maturation, impaired embryonic development and pregnancy failure. Intracytoplasmic sperm injection (ICSI) is a popular option for PCOS patients to attain pregnancy. However, there is no specific determinant to ascertain successful pregnancy outcome in PCOS women undergoing ICSI. Objectives: The purpose of this study was to determine the influence of PCOS on embryo quality and subsequent pregnancy rate in Iraqi women who had undergone ICSI. Methods and materials: Over the course of three months, one hundred and three infertile couples who were referred to Al-Sadr Medical City, Kufa, Iraq between October 2017 and June 2018 were enrolled in this study. The couples were divided into two groups: those with PCOS, and those who did not have PCOS. The amounts of hormones were determined. The evaluation of embryo attributes with grading, as well as the determination of the fertilization rate, cleavage rate, and pregnancy rate, were carried out. Results: The difference in fertility and cleavage rates between the PCOS (P=0.40) and non-PCOS (P=0.59) groups was not statistically significant. When comparing the two groups, the mean number of good quality embryos in the PCOS group was higher (P=0.07), whereas the pregnancy rate in the former was considerably lower (P=0.02) than in the latter. Conclusion: According to our findings, PCOS had no negative impact on the quality of the embryos produced by Iraqi women who underwent ICSI treatment. Because PCOS is a complicated disorder characterized by a variety of endogenous physiological variables that may either directly or indirectly interfere with conception, the low likelihood of pregnancy in these patients suggests that good embryo quality is not the only predictor of successful pregnancy.
Keywords
Embryo Quality; Fertilization Rate; Hyperandrogenemia; ICSI Outcome; PCOS; Pregnancy Outcome
Download this article as:| Copy the following to cite this article: Hassan M. F, Kadim H. A, Edan B. J, Dutta S, Sengupta P. Embryo Quality and Intracytoplasmic Sperm Injection (ICSI) Outcome in Iraqi Women with Polycystic Ovary Syndrome (PCOS): A Cohort Prospective Study. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Hassan M. F, Kadim H. A, Edan B. J, Dutta S, Sengupta P. Embryo Quality and Intracytoplasmic Sperm Injection (ICSI) Outcome in Iraqi Women with Polycystic Ovary Syndrome (PCOS): A Cohort Prospective Study. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/42sQU2g |
Introduction
Polycystic ovarian syndrome (PCOS) is a disorder of chronic anovulation in women of reproductive age and usually occurs due to imbalance of reproductive hormones 1. It reflects heterogeneous collection of signs and symptoms, with wide spectrum of disorders. For some, there are mild symptoms while others experience severe disturbances in reproduction, endocrine and metabolic functions 2. Its prevalence varies between 2% and 26% among women across different populations 3,4, and constitutes of 80% to 90% of group II anovulatory sub-fertility, as per the World Health Organization (WHO) 1. PCOS is diagnosed as per the Rotterdam 2003 criteria: menstrual problems (oligo- and/or anovulation), elevated levels of male hormones (clinical and/or biochemical hyperandrogenism) and by trans-vaginal ultrasound of ovaries 5.
PCOS accompanies various associated factors that account for compromised fertility, which is not restricted only to anovulation 2, and these factors include increased body weight, inflammatory conditions, metabolic and endocrine defects with subsequent impairments of oocyte quality, embryo development and future fetal wellbeing 6,7. Increased luteinizing hormone (LH) in PCOS 8 also adversely affects the quality of embryo (early developmental delay and arrest) and increases the rate of miscarriages 9,10. The incidence of miscarriage in PCOS is three times higher than normal women and is believed to be a result of hypersecretion of LH, and insulin, as well as excess body weight 11. However, this tends to be diverse as PCOS women with normal androgen levels still have the ability to produce developmentally normal embryos9. Impairment of endometrial blood flow, growth factors, cytokines, and adhesive molecules also may contribute to fertility disruptions in PCOS patients 12. Moreover, high serum androgen level may also serve as causative factors owing to its adverse effect on the normal endometrial development by reducing expression of endometrial protein 13.
Infertile women with PCOS are usually successfully treated with first line ovulation inducing agents such as clomiphene citrate and insulin-sensitizing medications. Women who fail to conceive even first line treatment, are candidates for gonadotrophin treatment or laparoscopic ovarian drilling. Assisted reproductive technologies (ART) are offered to women with PCOS failing to ovulate with these protocols 14. In addition, ART may be considered when there is a severe accompanying infertility factor, such as severe male factor necessitating intracytoplasmic sperm injection (ICSI) 15. Excessive response to gonadotropins manifested by possibly life-threatening ovarian hyperstimulation syndrome (OHSS) is a potential complication of controlled ovarian hyperstimulation (COH) in these patients 16. The ART performance of patients with PCOS, employing either In vitro fertilization (IVF) or ICSI, has been reported to be comparable to control groups mainly consisting of tubal factor or male factor infertility 17,18. Further in-depth research is required to unveil the overall impact of PCOS on various aspects of the female reproductive potential, and the use of ART to bypass their fertility problems is a subject of scientific and ethical debate. It is essential to understand the exact effects of PCOS on various fertility parameters across different population. There are inadequate reports on Iraqi population pertaining to embryo quality in PCOS women. Thus, the present study, conducted on Iraqi women, aimed at evaluating the influence of PCOS on the embryonic quality, embryonic development, and major pregnancy-associated parameters, following ICSI.
Materials and methods
Ethical considerations and study population
This is a prospective cohort study that was conducted at IVF Center, Al-Sadr Medical City, Kufa, Iraq. The Institutional Medical Ethics Committee of University of Kufa has approved (FOM/8/10.10.17) the study proposal. One hundred three infertile couples were included in this study and all of them were involved in ICSI program throughout the period from October 15, 2017 to June 30, 2018. The age of female partners was ≤35 years old. The infertile couples were divided in two groups: Group 1 females with PCOS selected according to Rotterdam 2003 criteria [19] with male partners having mild-to-moderate semen quality impairment, and Group 2 females were without PCOS (normal ovulatory women who attended the fertility clinic for mild-to-moderate male factor infertility or women with tubal obstruction. Females with (a) endometriosis, (b) abnormal renal or hepatic functions, (b) individuals with hyperprolactinemia/hypothyroidism, (c) individuals with secondary causes of androgen excess, (d) individuals with gynecological age less than three years, (e) women who suffered from genetic disorders, such as Turner’s syndrome, primary hypopituitarism, primary premature ovarian failure, (f) primary insulin resistance (IR), (g) male partners with severe impairment of semen quality, or frozen sperms (from testis or epididymis), (i) couples with unexplained infertility (normal female and females with no identified cause of sub-fertility) had been excluded from this study.
Preparation of subjects, anthropometric and hormonal measurements
Male and female partners of both groups had been evaluated by urologists and gynecologists. Females of both groups had been subjected to pituitary downregulation using either gonadotropin releasing hormone (GnRH) antagonist; Cetrotide 0.25 mg (Serona) from Day-6 (fixed protocol) or agonist; Decapeptyle 0.1 mg (Serona) (depending upon the treatment protocol which was followed by the specialists in the center which is individualized according to each couples’ characteristics), i.e. antral follicle counts (AFC), body mass index (BMI), earlier response to the treatment in previous cycle and the male semen parameters, from the Day-2 of cycle (CD2) then controlled ovarian hyperstimulation by recombinant follicle stimulating hormone (r-FSH); Follitrope 75×2 IU(Merck) which was done under a close supervision by serial trans-vaginal ultrasound (TVUS) and hormonal assay for 10-14 days. Ovulation trigger was done either by human chorionic gonadotropin (HCG); Pregnyl 5000 IU×2 (Merck) injection or Decapeptyle 0.2 mg (depending upon the risk of OHSS; clinical symptoms of nausea, vomiting and abdominal discomfort, ultrasound; more than 20 follicles and some free fluid in the abdomen, hormonal; high serum estrogen >2500 pg/ml at the day of trigger and the preference of fresh embryo transfer) when the total number of the follicles and their size are adequate (7-12 follicles of more than 16 mm size). Oocyte pickup was done by the gynecologist under general anesthesia using trans-vaginal approach. The oocytes were denuded by hyaluronidase enzyme; 80 IU/l and mechanical way by repeated aspiration through a sequence of denuding pipettes. Then the oocytes were washed with the culture medium and the maturity of oocytes was assessed. ICSI was commenced in all cases as the center uses ICSI in nearly 99% of cases, fertilization was assessed 16-18 hrs. after injection, Subsequent evaluation of the embryo quality was done depending on blastomere number, their shape, equality, mono-nucleation, and the percentage of fragmentations. Embryos were classified as good quality (grade I and II) when they have 4 cells at 48 hrs. after injection or have 6-8 cells 72 hrs. with even sized blastomeres, little or no fragmentation (for good 10-20% and for bad more than 20%) and single, clearly visible nuclei per each blastomere. Anything else were classified as bad quality embryos (grade III and IV ) 20.
All participants had their body weight and height was measured in bare feet on a plane surface and with minimal garments. The body mass index (or Quetelet Index) was measured by using the following formula: BMI=weight (kg)/(Height in m)2 21. Hormonal parameters of the subjects were measured following the standard protocol.
Statistical analysis
Statistical analysis was done assuming a confidence level of 95%, and obtained data were arranged in Microsoft Office Excel spreadsheet 2007, analyzed by SPSS (v. 22.0, IBM, Chicago, IL, USA) and MedCalc (v. 19.05, Ostend, Belgium). Data of the continuous variables are expressed in means±standard deviation (SD). Independent sample Students’ t-test and Chi-square test were applied to analyze the obtained data. P-value <0.05 was set as statistically significant.
Results
Age, anthropometry and induction protocol
The descriptive information about age, BMI, durations of primary and secondary infertility of the study group and control subjects have been expressed in Table 1. It reflects that the age of female respondents of both groups (P=0.55) and the durations of infertility (P=0.40) are not statistically significant. The BMI, which is one of the key anthropometric predictors of PCOS was also reported to be non-significant between the two groups (P=0.37). The types of induction protocols that were used during COH was statistically significant between the two groups (P=0.001), whereas no significant difference was recorded between the total doses of gonadotropins (P=0.945) (Table 2).
Table 1: Comparison of patient information for PCOS and non-PCOS groups.
| Data | Non-PCOS (n=50) | PCOS (n=53) | P-value |
| Age (years) | 27.96±4.06 | 27.98±3.85 | 0.55 |
| BMI (kg/m2) | 27.83±4.87 | 28.92±4.51 | 0.69 |
| Duration (years) | 7.74±4.06 | 7.28±3.43 | 0.37 |
| Primary infertility | 34 | 40 | 0.40
|
| Secondary infertility | 16 | 13 | |
| Spermiogram | 0.45 | ||
| Normal | 19 | 24 | |
| Abnormal | 31 | 29 | |
values are expressed in mean±SD; P<0.05
Table 2: Comparison of stimulation protocols and total dose of gonadotropins in PCOS and non-PCOS females.
| Protocol | Non-PCOS
(n=50) |
PCOS
(n=53) |
Total | P-value |
| Antagonist | 17 | 41 | 58 | 0.001*
|
| Short agonist | 30 | 12 | 42 | |
| Long agonist | 3 | 0 | 3 | |
| Total | 50 | 53 | 103 | |
| Total dose of gonadotropin (IU) | 1925.2±968.7 | 1663.20±615.2 | 0.945 | |
values are expressed in mean±SD; P<0.05
Hormonal profiles, endometrial thickness and ICSI outcome
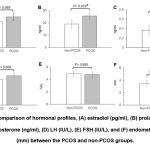
Comparison of hormonal profiles on cycle day-2 (CD2) did not show any significant difference for LH (P=0.608), FSH (P=0.895) and estradiol (E2) levels (P=0.089) between PCOS and non-PCOS groups. Contrarily, serum prolactin levels (P=0.01) and total testosterone (P=0.008) were reported to be significantly higher in PCOS women than control subjects. Endometrial thickness (ET) of PCOS women on CD2 was also not significantly different from non-PCOS subjects (Fig. 1).
The ICSI outcomes in form of fertilization rate (FR), cleavage rate (CR) and embryo quality and pregnancy rate have been expressed in Table 3. There was no statistically significant difference between the two groups regarding FR and CR despite being less in the PCOS group (P=0.40 and 0.59, respectively). While the mean total number of good quality embryos was higher in the PCOS group than the non-PCOS group with no significant statistical difference (P=0.074). Regarding pregnancy rate (PR) in both groups, it was higher in the non-PCOS group 52.17% compared to 27.5% in the PCOS counterparts (P=0.02). Four non-PCOS women and thirteen PCOS women were not included in PR calculation due to the development of OHSS and the cancellation of fresh embryo transfer.
Table 3: Comparison of fertilization rate, cleavage rate, mean total number of embryos, their quality and pregnancy rate between the studied groups.
| Parameters | Non-PCOS (n=46) | PCOS (n=40) | P-value |
| Fertilization rate | 73.38±24.32 | 71.49±21.84 | 0.402 |
| Cleavage rate | 95.77±14.81 | 93.86±20.55 | 0.597 |
| Total number of embryos | 5.12±3.89 | 6.46±4.49 | 0.113 |
| Good quality embryos, Total no. (%) | 4.65±3.43(90.8) | 6.08±4.39(94.04) | 0.074 |
| Bad quality embryos, Total no. (%) | 0.46±1.01(9.16) | 0.38±1.00(5.95) | 0.679 |
| Pregnancy rate, Total no. (%) | |||
| Pregnant | 24/46(52.17) | 11/40(27.5) | 0.02* |
| Not pregnant | 22/46(47.80) | 29/40(72.5) |
values are expressed in mean±SD; P<0.05
So, the total number of females included in the calculation of PR in PCOS and non-PCOS woman was 40 out of 53 and 46 out of 50 respectively. and the rate of developing OHSS was 24.5% and 8% respectively.
Discussion
PCOS represents a major cause of infertility, and its prevalence varies across various population 4,7. It is imperative to understand the influence of this disease on the reproductive system, particularly at oocyte and subsequent embryo development on specific population 14. Thus, the present study, conducted in Iraq, aimed to investigate impact of altered intrafollicular microenvironment in women with PCOS, on pregnancy rate, embryo quality, and embryonic development following ICSI as a fertility treatment measure.
The study revealed that 94.04% of embryos derived from PCOS women were of good quality in comparison with 90.8% in the non-PCOS group (P=0.074) (Table 3). This might be related to the ICSI procedure itself which enables the embryologist to select the best quality gametes to be injected so the probability of producing good quality embryos is augmented. A study by Hassan et al., is consistent with the current results 22 but, contrasting report is also available demonstrating significantly higher total number of embryos in PCOS women compared to non-PCOS control, but with significantly lower percentage of good quality embryos [23]. Some researchers believed that only lean PCOS women produced good quality embryos while obese women did not 24,25. A recent study had done morphokinetic analysis and showed that embryos from PCOS patients developed earlier to the nine-cell staged form as compared to the controls26. It is known that human embryo compaction is mediated via high degree integrated cell-to-cell signaling. Cells bind tightly to each other, having no distinct cell borders, with the synchronized actions of the desmosomes, gap junctions, tight junctions, and various other adhesion molecules, to form the embryo 27. Followed by these processes, cellular polarity develops and cell continues differentiating resulting in the formation of inner cell mass and trophectoderm 27. Not much is revealed regarding these mechanisms, but there are few key proteins, such as the connexins and cadherins, have been shown to play critical roles 27,28. In PCOS patients, nonetheless, connexins (Cx43) and E-cadherins expressions reportedly increase which may aid the robust embryo formation and differentiation 28,29.
Our results also depict that both FR and CR were lower in PCOS women (in comparison to non-PCOS ones (71.5% vs. 75.4% and 93.8% vs. 95.7%) (Table 3). Similar results were obtained from different studies that showed lower FR in PCOS patients 30-32. There are also reports showing significantly lower FR and higher CR in PCOS women compared to the non-PCOS control 33,34. Moreover, among the studies pertaining to PR in PCOS women, some demonstrated significant lower PR while few could find no significant difference in PR between PCOS and the control 30-32. In the present study, PR was observed to be significantly lower in PCOS women (27.5% vs. 52.17%) despite a high yield of good quality embryos. There must be other factors related directly or indirectly to PCOS, responsible for the failed conception and pregnancy loss. PCOS is associated with overproduction of ovarian androgens, aberrant hypothalamic-hypophyseal signals, environmental and genetic variables and others that are integral to the ultimate pathways similar to various metabolic disorders such as insulin resistance, glucose intolerance, and obesity 27-29. High LH and prolactin levels, low glycodelin level, infertility treatments and protocols of induction together with other intra-ovarian factors might also play role in the implantation failure and miscarriage in females with PCOS 11. Our result shows significantly higher total testosterone levels in PCOS patients (Fig. 1), which is reportedly linked with disrupted endometrial growth during the luteal phase leading to failed implantation and pregnancy loss 13. Although the mechanism underlying these observations is yet unknown, it might be linked to changes in aromatase activity and cumulus cell-oocyte interactions 35. Because of a recent link discovered between embryo morphokinetics and cumulus cell gene expression in women with PCOS, this might be an intriguing area to pursue 35.
In women with PCOS, prolactin levels during pregnancy may be relevant as a long-term risk marker for metabolic health. Currently, androgen status and obesity are proposed as predictors of individual risk of metabolic disorders in PCOS; however, metabolic disturbances are also enhanced in non-hyperandrogenic PCOS, and there are presently no ideal predictors for detecting those at higher risk of metabolic and cardiovascular complications 36. Prolactin promotes -cell proliferation in pancreatic islets as a physiological response to the development of insulin resistance during pregnancy 37. In a large population-based cohort, high prolactin within the normal range was linked to a reduced prevalence of diabetes and poor glucose tolerance 38. However, elevated prolactin level beyond physiological levels leads to pregnancy loss and lowering its level by medication before ICSI improves implantation 39. In the present study, PCOS women had a significantly higher prolactin level (Fig. 1) and the induction protocol of choice was the antagonist protocol which has negative impact on implantation40.
Conclusions
Overall, this study reveals that PCOS women undergoing IVF had good embryo quality and fast embryonic growth. However, embryo quality is not a sole predictor of successful pregnancy, as PCOS is a multifaceted disorder with variable phenotypes. Various factors, such as insulin intolerance, disrupted endocrine axes, hyperandrogenemia, intrinsic embryonic factors, etc. may directly or indirectly affect pregnancy rate in PCOS women. Further research into the mechanisms is needed to better understand and act in order to improve the oocyte health of PCOS women undergoing IVF and to enhance viability of the future offspring.
Competing Interest
The authors declare that they do not have any conflict of interest.
Funding Sources
There are no funding sources
References
- Gupta M, Singh D, Toppo M, Priya A, Sethia S, Gupta P. (2018) A cross sectional study of polycystic ovarian syndrome among young women in bhopal, central india. Int J Comm Med Pub Health 5: 95-100.
CrossRef - Brassard M, AinMelk Y, Baillargeon JP. (2008) Basic infertility including polycystic ovary syndrome. Med Clin North Am 92: 1163-1192.
CrossRef - Chanson P, Dominique M. (2019) The epidemiology, diagnosis and treatment of prolactinomas: the old and the new. Best Prac Res Clin Endocrinol Metab 33: 101290.
CrossRef - Deswal R, Narwal V, Dang A, Pundir CS (2020) The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci 13: 261.
CrossRef - Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41-47.
CrossRef - Susie J, Thomas H, Adam H. (2018) Polycystic ovary syndrome and assisted reproduction. Textbook of assisted reproductive techniques. David K. and Colin M.(eds.). 5th ed. CRC Press Taylor & Francis Group. 762-772.
- Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Mukhopadhyay LD, Syamal AK. (2021) Waist-to-height ratio and bmi as predictive markers for insulin resistance in women with pcos in kolkata, india. Endocrine 1-10.
CrossRef - Ambiger S, Patil S, Rekha M, Dhananjaya S. (2017) Role of leutinizing hormone lh and insulin resistance in polycystic ovarian syndrome. Int J Reprod Contracept Obs Gyncol 6: 3892-3896.
CrossRef - Le MT, Nguyen TV, Nguyen TT, Nguyen TTT, Nguyen TAT, Nguyen QHV et al. (2019) Does polycystic ovary syndrome affect morphokinetics or abnormalities in early embryonic development? Eur J Obs Gynecol Reprod Biol 3: 100045.
CrossRef - Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribiero S, Blockeel C, et al. (2019) Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod 34: 1595-1607.
CrossRef - Apostolos T. (2018) Polycystic ovary syndrome and early pregnancy loss: A review article. EC Gynecol 7: 35-42.
- Lu Y, Yan J, Liu J, Tan J, Hong Y, Wei D et al. (2020) Prednisone for patients with recurrent implantation failure: study protocol for a double-blind, multicenter, randomized, placebo-controlled trial. Trials 21: 1-7.
CrossRef - Pearson A, Tahir AM. (2020) Obesity and recurrent miscarriage. Obes Gynecol 2020:91-96.
CrossRef - Mumusoglu S, Mehmet S, Gurkan B. (2020) Polycystic Ovarian Syndrome and Medically Assisted Reproduction. Textbook of Assisted Reproduction. Springer, Singapore, 2020. 241-8.
CrossRef - Duan N, Wang H, Niu Z, Guo Y, Wang S, et al. (2018) A meta-analysis of oocyte quality and clinical outcome of intracytoplasmic sperm injection cycles in patients with polycystic ovary syndrome. J Med Imag Health Inform 8:1886-94.
CrossRef - Nelson SM. (2017) Prevention and management of ovarian hyperstimulation syndrome. Thromb Res 151: S61-S64.
CrossRef - Sourinejad H, Savabi Esfahani M, Tarrahi MJ, Adib Moghaddam E (2021) Outcomes and Challenges associated with In vitro Fertilization in Women with Polycystic Ovary Syndrome: A review study. J Sabzevar Univ Med Sci 28: 359-371.
- Kitaya K, Takaya Y, Nishiyama R, Yamaguchi K, Matsubayashi H, Takeuchi T, et al. (2019) Myoinositol supplementation on intracytoplasmic sperm injection outcome in Japanese infertile polycystic ovarian syndrome women with non-obese less-androgenic phenotype: a prospective controlled observational study. Clin Exp Obs Gynecol 46: 549-52.
CrossRef - Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. (2012) Consensus on women’s health aspects of polycystic ovary syndrome (pcos): The amsterdam eshre/asrm-sponsored 3rd pcos consensus workshop group. Fertil Steril 97: 28-38. e25.
CrossRef - Sakkas D, Gardner DK. (2012) Evaluation of embryo quality: Analysis of morphology and quantification of nutrient utilization and the metabolome. Textbook of Assisted Reproductive Techniques. London: Informa Healthcare. 240-253.
CrossRef - World Health Organization. (2006) Bmi classification. Global database on body mass index. World Health Organization.
- Hassan M, Hind A. (2018) Oocyte dysmorphisim in women with pcos and its’ effect on embryo development following icsi. World J Pharm Med Res. 4: 78-84.
- Zamaniyan M. et al. (2021) Epidemiologic aspects and risk factors associated with infertility in women undergoing assisted reproductive technology (ART) in north of Iran. Education 40:27-36.
- Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. (2006) Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obs Gynecol 108: 61-69.
CrossRef - Qiu M, Tao Y, Kuang Y, Wang Y (2019) Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertil Steril 112: 1172-9.
CrossRef - Chappell NR, Barsky M, Shah J, Peavey M, Yang L, Sangi-Haghpeykar H, Gibbons W, Blesson CS. (2020) Embryos from polycystic ovary syndrome patients with hyperandrogenemia reach morula stage faster than controls. Fert Steril Rep 1: 125-132.
CrossRef - Sozen B, Can A, Demir N. (2014) Cell fate regulation during preimplantation development: A view of adhesion-linked molecular interactions. Dev Biol 395: 73-83.
CrossRef - Yang M, Li J, An Y, Zhang S. (2015) Effects of androgen on immunohistochemical localization of androgen receptor and connexin 43 in mouse ovary. Tissue Cell 47: 526-532.
CrossRef - Lopes IMRS, Maganhin CC, Oliveira-Filho RM, Simões RS, Simões MJ, Iwata MC, Baracat EC, Soares Jr JM. (2014) Histomorphometric analysis and markers of endometrial receptivity embryonic implantation in women with polycystic ovary syndrome during the treatment with progesterone. Reprod Sci 21: 930-938.
CrossRef - Yahia W, Mahmoud S, Tamer F. (2013) Prediction of intracytoplasmic sperm injection outcome in patients with polycystic ovary syndrome using follicular antimullerian. Al-Azhar Assiut Med J 10.
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. (2011) A validated model of serum anti-müllerian hormone from conception to menopause. PloS One. 6: e22024.
CrossRef - Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, Brown K, Simpson ER, Mason HD. (2011) Anti-müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril 96: 1246-1251. e1241.
CrossRef - Okohue J, Onuh S, Ikimalo J, Onuh S. (2011) Challenges in running/establishing an assisted conception centre in the niger-delta region of nigeria. West Afr J Assist Reprod 1: 1-17.
- Almasi-Hashiani A, Mansournia MA, Sepidarkish M, Vesali S, Ghaheri A, Esmailzadeh A, Omani-Samani R. (2018) Comparison of in vitro fertilization/intracytoplasmic sperm injection cycle outcome in patients with and without polycystic ovary syndrome: A modified poisson regression model. Int J Fertil Steril 11: 309.
- Vuong LN, Le AH, Ho VN, Pham TD, Sanchez F, Romero S, et al. (2020) Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J Assist Reprod Genet 37: 347-57.
CrossRef - Pinola P, Puukka K, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, et al. (2017) Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril 107: 788-795.e782.
CrossRef - Baeyens L, Hindi S, Sorenson RL, German MS. (2016) Β-cell adaptation in pregnancy. Diab Obes Metab 18 Suppl 1: 63-70.
CrossRef - Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, et al. (2013) Circulating prolactin associates with diabetes and impaired glucose regulation: A population-based study. Diab Care 36: 1974-1980.
CrossRef - Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. (2012) Urinary bisphenol a concentrations and implantation failure among women undergoing in vitro fertilization. Env Health Perspect 120: 978-983.
CrossRef - Li W, Ma N, Laird S, Ledger W, Li T. (2013) The relationship between serum prolactin concentration and pregnancy outcome in women with unexplained recurrent miscarriage. J Obs Gynaecol 33: 285-288.
CrossRef
Abbreviations
PCOS Polycystic ovary syndrome
ICSI Intracytoplasmic sperm injection
LH Luteinizing hormone
ART Assisted reproductive technologies
OHSS Ovarian hyperstimulation syndrome
COH Controlled ovarian hyperstimulation
IVF In vitro fertilization
AFC Antral follicle counts
BMI Body mass index
r-FSH Recombinant follicle stimulating hormone
TVUS Trans-vaginal ultrasound
HCG Human chorionic gonadotropin
ET Endometrial thickness
FR Fertilization rate
CR Cleavage rate
PR Pregnancy rate