Nisrat Jahan1, 2 , Khozirah Shaari3
, Khozirah Shaari3 , Sk Nazrul Islam4
, Sk Nazrul Islam4 , ATM Zafrul Azam1 Fatema-Tuz-Zohora5*
, ATM Zafrul Azam1 Fatema-Tuz-Zohora5* and Monira Ahsan1
and Monira Ahsan1
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Dhaka 1000, Bangladesh.
2Department of Pharmacy, southeast University, 251/A and 252, Tejgaon Industrial Area, Dhaka - 1208, Bangladesh.
3Laboratory of Natural Products, Institute of Bioscience, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia.
4Institute of Nutrition and Food Science, University of Dhaka, Dhaka 1000, Bangladesh.
5Department of Pharmacy, University of Asia Pacific, 74/A, Green Road, Dhaka 1205, Bangladesh.
Corresponding Author E-mail: fatema.zohora41@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2664
Abstract
The study's objectives include phytochemical profiling and biological (antioxidant, thrombolytic and cytotoxic) analysis of pure chemicals from Jatropha pandurifolia stem bark ethyl acetate extract. Five different compounds including octacosanyl cis ferulate (1), hexacosyl (E)-ferulate (2) triacontyl ferulate (3), β-sitosterol (4) and stigmasterol (5) are elucidated. Their structures determine through 1HNMR analysis and comparison to published data, while three ferulic acid alkyl esters (1-3) were isolated for the first time from J. pandurifolia. Compounds 1, 2, and 3 all have significant thrombolytic potential with respective values of 68.92% ±1.17 (**P<0.01), 66.56% ±2.35 (**P<0.01) and 70.81%±0.98 (**P<0.01) with comparison to standard streptokinase (73.6%±0.76). When compared to BHT (6.82± 0.99 μg/ml) the IC50 (DPPH assay) values were 16.26±1.07 (**P<0.01), 14.12±1.23 (**P<0.01), and 13.16±1.70 μg/ml (**P<0.01). Comparing the three compounds to the reference vincristine sulphate (LC50: 0.52±0.18 μg/ml), of compound 1 (1.56±0.35 μg/ml) (**P<0.01), compound 2 (1.3±0.78 μg/ml) (**P<0.01) and compound 3 (1.29±0.33 μg/ml) (**P<0.01). The results can therefore be interpreted as a concept of isolated molecules having potential for application in additional pharmaceutical research.
Keywords
Antioxidant; Cytotoxic Activity; Hexacosyl (E)-Ferulate; Jatropha Pandurifolia; Octacosanyl Cis Ferulate; Triacontyl Ferulate; Thrombolytic
Download this article as:| Copy the following to cite this article: Jahan N, Shaari K, Islam S. K. N, Azam A. T. M. Z, Zohora F. T, Ahsan M. Chemical and Biological Profiling of Three Ferulic Acids Alkyl Esters Isolated from Jatropha pandurifolia (Family: Euphorbiaceae) Stem Bark. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Jahan N, Shaari K, Islam S. K. N, Azam A. T. M. Z, Zohora F. T, Ahsan M. Chemical and Biological Profiling of Three Ferulic Acids Alkyl Esters Isolated from Jatropha pandurifolia (Family: Euphorbiaceae) Stem Bark. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3pjLnxj |
Introduction
The class of organic molecules known as phytochemicals, which includes tannins, sugars, triterpenoids, alkaloids, flavonoids, steroids, and others, is rife in medicinal plants (plant chemicals). These phytochemicals are fantastic for offering health advantages and acting as a protection, avoiding a number of diseases 1.
Excessive generation of reactive oxygen species (ROS) leads to oxidative stress 2 that in turn trigger several diseases such in inflammation, hypertension, atherosclerosis, AIDS, DM and cancer etc. Antioxidative enzymes such as peroxidase, superoxide dismutase (SOD) is concealed by the over production of free radicals that consequences cellular mutation and damage 3. The brine shrimp bioassay is linked to cytotoxic act in living tissue and represents pesticidal activity 4,5. The mechanism of a thrombolytic drug is to liquefy blood clot (thrombus) in acutely occluded coronary arteries, pulmonary embolism, atrial fibrillation, thrombosis and prosthetic heart valves 6 that ensures sufficient blood supply to myocardium and to develop prospects with blood loss reduction. Market available thrombolytic agents like urokinase, alteplase, streptokinase, anistreplase, tissue-type plasminogen activator (tPA) offer some restrictions like side effects with associated bleeding history 7,8. Due to the presence of phytochemical elements, medicinal plants are essential in the prevention of various diseases, and their use and promotion fit into all current prevention efforts.
Jatropha (Family: Euphorbiaceae, a castor family) having 175 species of succulent, caudiciform, herbaceous perennials and woody plants including Jatropha pandurifolia (Jatropha integerrima) that is a tiny, evergreen tree or shrub with lustrous leaves with star-shaped red, pink, or vermilion blooms and also known as peregrine or spicy jatropha 9. In Asia, Latin America, and Africa, several plant parts including the leaves, stem, bark, roots, and oil, have long been used for a variety of ailments as styptic agent, emetic agent, antidote 10,11, purgative 12, in the treatment of warts, toothaches, ringworm, gastric emptying, gum bleeding, rheumatic pain and skin diseases 13,14. Numerous phytochemicals holding potential medicinal norms were reported from this plant including alkaloid (jatrophine, jatropham and curcin) 15, diterpene (jatrophone, jatrophatrione, jatropholone A–B) 16,17, sitosterol, glycoside, lignin, tannin, saponin 18, fatty acids (palmitic, oleic, and linoleic acids), carotenoid, flavonoid etc 19,20. This plant has been linked to antiinflammatory, antituberculosis, antiplasmodial, cytotoxicity 21,22, anticancer 23,24, antimicrobial 25-27, antifungal antioxidant potentials 28 according to previous studies. Following several chromatographic methods structure elucidations was carried out by HNMR spectroscopy. The chemical investigation revealed very first isolation of three ferulic acid alkyl esters from this species. β- sitosterol and stigmasterol were also isolated concurrently. In the current study the obtained pure three ferulic acid alkyl esters were subjected to antioxidant, thrombolytic and cytotoxic investigation.
Materials and Methods
Overall experimental measures
Whilst performing VLC (vacuum liquid chromatography) fine VLC-grade silica like Kiesel gel 60H was used in the column. With the aid of dichloromethane, ethyl acetate, and methanol, the elution was carried out while gradually increasing polarity. An appropriate solvent system was used to elute TLC plates that are pre coated with Silica gel 60, F254. The TLC plates were sprayed with vanillin-H2SO4, heated for 5-10 minutes at 110oC, and UV light at short wavelength (254) and long wavelength (366) nm were used to visualize the spots. NMR spectra were transcribed in CDCl3 on a 400 MHz Ultra shield NMR Spectrophotometer.
In August 2019, J. pandurifolia stem barks were collected from premises of University of Dhaka, Bangladesh. The plant was identified in National Herbarium, Dhaka and an identification no. was preserved (DACB Accession No. 65545). The air dried and powdered stem-barks of J. pandurifolia (3.0 kg) in ethyl acetate over the period of 15 days and filtered through a cotton pad then by Whatman filter paper. The extract was then evaporated using a Buchii rotary evaporator to be concentrated under reduced pressure.
Extraction and Isolation
An aliquot part of the crude ethyl acetate extract (40g) was partitioned by VLC technique in increasing order of polarity to provoke adsorption and seperation like a normal-phase column chromatography. Used solvents were petroleum ether, ethyl acetate and ethanol in sequence.VLC fractions were numbered from 1 to 41. White crystals were appeared in fraction VLC 10, 13 and 25. The crystals were washed with n-hexane, collected separately and were purified by PTLC (preparative TLC) with a convenient solvent system (1% ethyl acetate in toluene). By recrystallization technique JP-10 (Compound 1, colorless crystals, 5.0 mg, Rf value 0.65), JP-13 (Compound 2, colorless crystals, 10.0 mg, Rf value 0.45) and JP-25 (Compound 3, colorless crystals, 4.0 mg, Rf value 0.50), JP-5 (Compound 4, clear liquid, 3 mg, Rf value 0.60), JP-6 (Compound 5, clear liquid, 2 mg, Rf value 0.50) were yielded.
Spectral data of the compounds (C1-3)
Compound 01 (JP-10):
1HNMR (400 MHz, CDCl3); δ 5.82 d (J = 12 Hz, doublet,) (H-2), 6.79 d (J = 12 Hz, doublet) (H-3), 7.77 s (H-5), 6.88 (J = 8 Hz, doublet) (H-8), 7.10 (J = 8 Hz, doublet) (H-9), 4.11 (J = Hz, triplet, 2H) (H-1’), 1.27 (multiplet, 50H) (H 2’-26’), 1.64 (multiplet, 2H) (H-27’), 0.88 (6.5) (triplet, 3H) (H-28’), 3.93 (singlet, 3H) and 5.83 (doublet) for OCH3 and OH-7 groups.
Compound 02 (JP-13):
1HNMR (400 MHz, CDCl3); δ 6.29 (J = 16 Hz, doublet,) (H-2), 7.61 d (J = 16 Hz, doublet) (H-3), 7.03 (singlet, H-5), 6.91 (J = 8 Hz, doublet) (H-8), 7.07 (J = 8.1 Hz, doublet of doublets) (H-9), 4.18(triplet, 2H) (H1’), 1.28 (multiplet, 46H) (H 2’-24’), 1.64 (multiplet, 2H) (H-25’), 0.88 (6.5) (triplet, 3H) (H26’), 3.92 (singlet, 3H) and 5.93 (singlet with broad signal) for OCH3 and OH-7 groups.
Compound 03 (JP-25):
1HNMR (400 MHz, CDCl3); δ 6.29 (J = 16 Hz, doublet,) (H-2), 7.61 d (J = 16 Hz, doublet) (H-3), 7.03 (singlet, H-5), 6.91 (J = 8 Hz, doublet) (H-8), 7.07 (J = 8.1 Hz, doublet of doublets) (H-9), 4.18 (triplet, 2H) (H-1’), 1.28 (multiplet, 46H) (H 2’-28’), 1.68 (multiplet, 2H) (H-29’), 0.88 (6.5) (triplet, 3H) (H-30’), 3.93 (singlet, 3H) and 5.85 (singlet with broad signal) for OCH3 and OH-7 groups.
Structure elucidation of C-4 and C-5 were also performed by 1HNMR analysis and compared spectral data were compared to the reference data.
Protocol for biological investigation
Isolated pure compounds were taken for in vitro investigations according to established methods.
Thrombolytic activity
The percentage of human blood clot lysis was estimated 29 in comparison to Streptokinase (100μl equivalent to 30,000 I.U) as positive control and distilled water (100 μl) as negative control. The calculation as follows:
% of thrombolytic activity = (wt. of clot after treatment / wt. of clot before treatment) × 100
Antioxidant activity (DPPH free radical scavenging assay)
According to Brand-Williams et al. 30 DPPH assay was carried out in presence of different concentration by serial dilution technique. Butylated hydroxyl toluene (BHT) and methanolic DPPH solution were used as positive control and negative control accordingly. IC50 values were calculated from the regression equation by plotting concentrations vs percentage of scavenging the free radicals.
I % = 1- {A1 / A0} × 100
A0: absorbance of the control and A1: absorbance of test samples/standard.
The experiment was repeated in triplicate at each concentration.
Brine shrimp lethality bioassay
The cytotoxic test was anticipated by brine shrimp lethality bioassay 31 of pure compounds at different concentrations. The percentage of mortality was calculated and plotted against LogC Then lethal concentrations (LC50) were calculated to measure cytotoxic activity. Saline water and vincristine sulphate (VS) were employed as negative and positive controls, respectively.
% mortality = (no. of dead nauplii/initial total no. of live nauplii) × 100
Statistical Analysis
The results of every experiment were shown as mean SEM. Using SPSS 20 software, one-way analysis of variance (ANOVA) was used to compare the study groups to one another. A p value of 0.05 or less was considered statistically significant.
Results and Discussion
Structure Elucidation
Modern chromatographic purification and separation of the ethyl acetate-stem bark extract of J. pandurifolia provided a total of three compounds (1-3), the structures were elucidated by 1H NMR analysis.
 |
Figure 1: Structural comparison between 1H NMR spectrums of three compounds (C 1-3). |
Compound 01 (JP-10)
Colorless crystal. Black and bluish spots were observed under the both 254 nm and 356 nm UV light accordingly on TLC and deep purple spot with vanillin-H2SO4 and heating for 1 min at 1100C. Three aromatic protons were seen in the 1H NMR spectrum at δ 7.77 s, 6.88 d (J = 8 Hz) and 7.10 d (J = 8 Hz) assignable to H-5, H-8 and H-9 of phenyl ring respectively. Protons at position 2 and 3 having doublets at 5.82 (J = 12 Hz) and 6.79 (J = 12 Hz) respectively are close to the carboxylic group. A triplet was appeared at δ 4.11 representing a methylene group. The spectrum also revealed two multiplets at δ1.27 (50H) and 1.64 (-CH2 group) and a triplet at δ 0.88 for a -CH3 group. A broad singlet (δ3.93) and doublet (δ5.83) indicate methoxy and hydroxyl groups consecutively on the phenyl ring. Compared NMR data with those of compound-1 was determined to be octacosanyl cis ferulate 32.
Compound 02 (JP-13)
Colourless crystal. Black and bluish spots were observed under the both 254 nm and 356 nm UV light accordingly on TLC and deep purple spot with vanillin-H2SO4 and heating for 1 min at 1100C. Three aromatic protons were seen in the 1H NMR spectrum at δ 7.03 s, 6.91 d (J = 8 Hz) and 7.07 dd (J = 8.1 Hz) assignable to H-5, H-8 and H-9 of phenyl ring respectively. Protons at position 2 and 3 having doublets at 6.29 (J = 16 Hz) and 7.61 (J = 16 Hz) respectively are close to the carboxylic group. A triplet was appeared at δ 4.18 representing a methylene group. The spectrum also revealed two multiplets at δ1.28 (46H) and 1.64 (-CH2 group) and a triplet at δ 0.88 for a-CH3 group. A singlet (δ3.92) and broad singlet (δ 5.93) indicate methoxy and hydroxyl groups consecutively on the phenyl ring. Compared NMR data with those of compound-2 was determined to be hexacosyl (E)-ferulate 33.
Compound 03 (JP-25)
Colourless crystal. Black and bluish spots were observed under the both 254 nm and 356 nm UV light accordingly on TLC and deep purple spot with vanillin-H2SO4 and heating for 1 min at 1100C. The 1H NMR spectrum displayed three aromatic protons at δ 7.03 s, 6.91 d (J = 8 Hz) and 7.07 dd (J = 8.1 Hz) assignable to H-5, H-8 and H-9 of phenyl ring respectively. Protons at position 2 and 3 having doublets at 6.29 (J = 16 Hz) and 7.61 (J = 16 Hz) respectively are close to the carboxylic group. A triplet was appeared at δ 4.18 representing a methylene group. The spectrum also revealed two multiplets at δ1.28 (46H) and 1.68 (-CH2 group) and a triplet at δ 0.88 for a-CH3 group. A singlet (δ 3.93) and broad singlet (δ 5.85) indicate methoxy and hydroxyl groups consecutively on the phenyl ring. Compared NMR data with those of compound-3 was determined to be triacontyl ferulate 34.
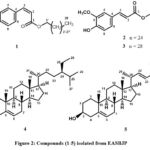 |
Figure 2: Compounds (1-5) isolated from EASBJP. |
Table 1: 1H NMR data for compounds (1-3) (at 400 MHz in CDCl3, δ in ppm, J in Hz)
|
Position (H) |
Compound 1 |
Compound 2 |
Compound 3 |
|
1 |
—- |
—- |
— |
|
2 |
5.82 (d, J = 12 Hz) |
6.29 (d, J = 16 Hz) |
6.29 (d, J = 16 Hz) |
|
3 |
6.79 (d, J = 12 Hz) |
7.61 (d, J = 16 Hz) |
7.61 (d, J = 16 Hz) |
|
4 |
— |
— |
— |
|
5 |
7.77 s |
7.03 s |
7.03 s |
|
6 |
— |
— |
— |
|
7 |
— |
— |
— |
|
8 |
6.88 (d, J = 8 Hz) |
6.91 (d, J = 8 Hz) |
6.91 d (J = 8 Hz) |
|
9 |
7.10 (d, J = 8 Hz) |
7.07 (dd, J = 8,1 Hz) |
7.07 (dd, J = 8,1 Hz) |
|
1’ |
4.11 (2H, t, J = Hz) |
4.18 (2H, t, J = Hz) |
4.18 (2H, t, J= 8 Hz) |
|
2’-26’ |
1.27 (50H, m) |
1.28 (46H, m) |
1.28 (46H, m) |
|
27’ |
1.64 (2H, m) |
1.64 (2H, m) |
1.68 (2H, m) |
|
28’ |
0.88 (3H, t, 6.5) |
0.88 (3H, tm, 6.5) |
0.88 (3H, t 6.5) |
|
OCH3 |
3.93 (3H, s) |
3.92 (3H, s) |
3.93 (3H, s) |
|
OH-7 |
5.83 |
5.93 (br s) |
5.85 |
The ferulic acid alkyl esters are derivatives of hydoxy cinnamic acid with Carbon-28, Carbon-26 and Carbon-30 attached to phenyl ring.
Biological investigations of J. pandurifolia
The isolated pure crystals confirmed various degrees of secondary metabolites when subjected to thrombolytic, antioxidant and brine shrimp cytotoxic activities.
Thrombolytic activity
Tissue plasminogen activator (t-PA), streptokinase, alteplase, reteplase, tenecteplase and urokinase etc. are commonly prescribed thrombolytic medication for the treatment of various CVD, myocardial infarction, cerebral thrombosis etc. Several secondary metabolites such as alkaloids, tannins, flavonoids, saponins exert thrombolytic properties as a natural supply 35.
Table 2: Thrombolytic activity of pure compounds (1-3) isolated from EASBJP.
|
Sample ID |
% of clot lysis |
|
|
Compound 1 |
68.92** ± 1.17 |
|
|
Compound 2 |
66.56**± 2.35 |
|
|
Compound 3 |
70.81**± 0.98 |
|
|
Streptokinase (STK) |
73.6 ± 0.76 |
|
|
Negative control (water) |
5.03± 0.70 |
|
Values are expressed as mean ± SD (n = 3); Significance level among different groups at P ≤0.05 (*P<0.05; **P<0.01, ***P<0.001); Test groups were compared to standard group; EASBJP represents ethyl acetate stem bark of J. pandurifolia
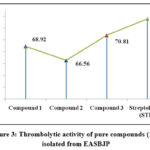 |
Figure 3: Thrombolytic activity of pure compounds (1-3) isolated from EASBJP. |
Compound 1, compound 2 and compound 3 showed significant clot rupture activity valued as 68.92% ± 1.17 (P<0.01), 66.56% ± 2.35 (P<0.01), and 70.81% ± 0.98 (**P<0.01) accordingly in comparison to Streptokinase (73.6% ± 0.76) (Tab: 02, Fig: 02).
Antioxidant activity
Other than superoxide dismutase, catalase enzymes or glutathione transferase diverse phytochemicals like polyphenolic compounds such as, flavonoids, phenolics, tocopherols, tannins etc. are to blame for inhibiting oxidation that may cause cell damage in living organism 36. The present study depicted IC50 value 6.82± 0.99 µg/ml for butylated hydroxy toluene (BHT).
Table 3: Antioxidant activity of pure compounds (1-3) isolated from EASBJP.
|
Sample ID |
DPPH scavenging activity (IC50, μg/ml) |
Regression equation |
|
Compound 1 |
16.26** ± 1.07 |
y = 0.019x + 0.191, R² = 0.853 |
|
Compound 2 |
14.12** ± 1.23 |
y = 0.025x + 0.147, R² = 0.928 |
|
Compound 3 |
13.16** ± 1.7 |
y = 0.031x + 0.092, R² = 0.800 |
Values are expressed as mean ± SD (n = 3); Significance level among different groups at P ≤0.05 (*P<0.05; **P<0.01, ***P<0.001); Test groups were compared to standard group; EASBJP represents ethyl acetate stem bark of J. pandurifolia
 |
Figure 04: Comparative Antioxidant activity of pure |
Compound 1, 2 & 3 exerted DPPH scavenging property with IC50 valued 16.26 ± 1.07 (P<0.01), 14.12 ± 1.23 (P<0.01) & 13.16 ± 1.70 µg/ml (**P<0.01) accordingly that projects on mild to moderate antioxidative potential of the isolated compounds that could be an innate reserve of polyphenolic compounds. Turkmen et al. (2006) hypothesized that solvent polarity may be a factor in the effectiveness of various extracts at scavenging free radicals 37.
Brine shrimp lethality bioassay
Table 4: Cytotoxic activity of pure compounds (1-3) isolated from EASBJP
|
Compound 1 |
1.56** ± 0.35 |
y = 29.90x + 3.478, R² = 0.951 |
|
Compound 2 |
1.3** ±0.78 |
y = 22.35x + 21.13, R² = 0.944 |
|
Compound 3 |
1.29** ±0.33 |
y = 26.27x + 16.16, R² = 0.972 |
Values are expressed as mean ± SD (n = 3); Significance level among different groups at P ≤0.05 (*P<0.05; **P<0.01, ***P<0.001); Test groups were compared to standard group; EASBJP represents ethyl acetate stem bark of J. pandurifolia
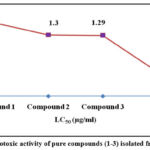 |
Figure 5: Cytotoxic activity of pure compounds (1-3) isolated from EASBJP |
Brine shrimp lethality bioassay is a quick and dynamic technique to compute the cytotoxicity of any compounds that might be noxious to health 38. The pure compounds showed significant brine shrimp larvicidal activity in comparison to positive control (Vincristine sulphate, LC50: 0.52 ± 0.18). LC50 were measured for compound 1, 2 and 3 were 1.56 ± 0.35 (P<0.01), 1.3 ±0.78 (P<0.01), 1.29 ± 0.33 (**P<0.01) (µg/ml) indication less toxicity compared to the positive control. The results point toward the presence of relevant influential phytotoxic secondary metabolites that may be deleterious to living organisms.
Earlier research has reported the cytotoxic and antimicrobial activity from the n- hexane stem bark extract of this plant 22. Antioxidant activity was reported from different extracts of root and leaf of J. gaumeri 39, J.Unicostata 40, J. macrantha 41. Investigations of J. curcas, J. integerrima, J. multifida, J. chevalieri, J. gossypiifolia, J. podagrica and J. pohliana have exerted cytotoxic activity towards toward melanoma cells, human cancer of the nasopharynx, artemia and other cell line analysis 42,43 whereas aqueous and ethanolic leaf extract J. macrantha showed toxicity on brine shrimp (Artemia sp.) model 44. Latex and leaf of J. curcas 45 and J. gossypiifolia 46 were turned up as blood thinner with probable estimation in clotting time reduction which could be correlated to thrombolytic property of the current plant. Biological investigations of the pure compounds from this plant strongly prove the abundance of ethno pharmacological activity. Further investigations of the isolated compounds are required specially, biological and molecular docking studies for unveiling probable activities.
Conclusion
The biological investigation found in this research justifies the pharmacological activity of this plant for curing miscellaneous malady. Different chain length of the ester increases the antioxidant potential of ferulic acids. Mild to strong thrombolytic, antioxidant and cytotoxic activities were unveiled by the pure compounds of ethyl acetate fraction that would be remarkable investigation of unrevealed ethno-pharmacological potency. The present study suggests exploring extensive research on this plant to discover many potent bioactive molecules.
Acknowledgement
The authors are thankful to Khozirah Shaari, University Putra Malaysia for her support for taking NMR spectra of the isolated compounds. Also expressing gratitude to Faculty of Pharmacy, University of Dhaka, Bangladesh for providing all the research amenities to bring out this work successfully.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Funding Source
There are no funding sources
References
- Edogo HO, Okwu DE, Mbaebie BO. Phytochemicals constituents of some Nigerian medical plants. Afr J Biotechnol, 2005; 4 (7): 685–688.
- Manik MIN, Ali MH, Islam MM, Zobayed A, Khan A. In vitro antioxidant, cytotoxic, thrombolytic activities and phytochemical evaluation of methanol extract of the Ampelocissus Barbata (Wall.) Leaves. Biomedical and Pharmacology Journal, 2022; 15(2): 911-923.
- Kohen R, Nyska A. Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions and methods for their quantification. Toxicologic pathology, 2002; 30(6): 620-650 .
- McLaughlin JL, Rogers LL, Anderson, JE. The use of biological assays to evaluate botanicals. Drug information journal, 1998; 32(2): 513-524.
- Apu AS, Muhit MA, Tareq SM Pathan AH, et al. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica Linn. J Young Pharm, 2010; 2 (1): 50–53.
- Bristol-Myers Squibb Company. Princeton; Coumadin (warfarin sodium) tablets crystalline and coumadin (warfarin sodium) for injection prescribing information. 2010.
- Wu DH, Shi GY, Chuang WJ, et al. Coiled\coil region of streptokinase gamma-domain is essential for plasminogen activation. J Biol Chem, 2001; 276:15025–15033.
- Ali R. Hossain M, Runa J, et al. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J Phytomed, 2014; 4 (6): 430–436.
- Duke JA. Medicinal plants. Science, 1985; 229:1036.
- Shetty S, Udupa SL, Udupa AL, Vollala VR. Wound healing activities of Bark extract of Jatropha curcas Linn in albino rats. Saudi Med, 2006; 27: 1473-6.
- Kirtikar KR, Basu BD. Ind. Medi. Plants. Popular Prakashan, Dehradun. 2002.
- Nayak BS, Patel KN. Pharmacognostic studies of the Jatropha curcas leaves. Int J Pharm Tech Res, 2010; 2 (1): 140–143.
- Pertino M, Schmeda-Hirschmann G, Rodriguez JA, Theoduloz C. Gastroprotective effect and cytotoxicity of semisynthetic jatropholone derivatives. Planta Med, 2007; 73: 1095–1100.
- Mujumdar AM, Misar AV. Anti-inflammatory activity of Jatropha curcas roots in mice and rats. Ethnopharmacol, 2004; 90 (1): 11-5.
- Luo MJ, Yang XY, Liu WX, et al. Expression, purification and anti-tumor activity of curcin. Acta Biochim et Biophy Sinica, 2006; 38 (9): 663– 668.
- Zohora FT, Sarkar P, Tareq FS, et al. Isolation of terpenes and coumarin from the stem bark of Jatropha pandurifolia (Andr.). Asian Journal of Chemistry, 2013; 25 (5): 2853.
- Yang J, Long YO, Paquette LA. Concise total syntheses of the bioactive mesotricyclic diterpenoids jatrophatrione and citlalitrione. J Am Chem Soc, 2003; 125: 1567– 1574.
- Kolawole OS, Rahaman AAA, Oladele FA. A numerical approach to the taxonomy of the genus Jatropha Linn. Using quantitative phytochemical constitutents. Europ. J of Exp Biol, 2014; 4 (6): 71-76.
- Kuspradini H, Rosiarto AM, Putri AS, Kusuma IW. Antioxidant and toxicity properties of anthocyanin extracted from red flower of four tropical shrubs. Nusantara bios, 2016; 8 (2): 135-140.
- Dietrich H, Rechner A. Bioactive compounds in fruit and juice. Fruit Process, 2004; 1: 50-55.
- Sutthivaiyakit S, Mongkolvisut W, Prabpai S, Kongsaeree P. Diterpenes, sesquiterpenes, and a sesquiterpene-coumarin conjugate from Jatropha integerrima. J Nat Prod, 2009; 72: 2024–2027.
- Akhter A, Rahman MS, Ahsan M. Preliminary antimicrobial and cytotoxic activities of n- hexane extract of jatropha pandurifolia. Lat Am J Pharm, 2008; 27 (6): 918-21.
- Mbwambo ZH, Moshi MJ, Masimba PJ, Kapingu MC, Nondo RSO. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Compl Altern Med, 2007; 7: 9.
- Moshi MJ, Innocent E, Magadula JJ, et al. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzania J. Health Res, 2010; 12 (1): 63-67.
- Gaikwad RS, Kakde RB, Kulkarni AU, et al. In vitro antimicrobial activity of crude extracts of Jatropha species. Current Botany, 2012; 3 (3): 09-15.
- Pertino M, Schmeda-Hirschmann G, Rodríguez JA, Theoduloz C. J Ethnopharmacol, 2007; 111: 553-9.
- Rampadarath S, Puchooa D, Sanmukhiya MR. Antimicrobial, phytochemical and insecticidal properties of jatropha species and wild ricinus communis L. Found in Mauritius. Int J of Pharmacog and Phyto Res, 2014; 6 (4): 831-840.
- Kuspradini H, Rosiarto AM, Putri AS, Kusuma IW. Antioxidant and toxicity properties of anthocyanin extracted from red flower of four tropical shrubs. Nusantara bios, 2016; 8 (2): 135-140.
- Prasad S, Kashyap RS, Deopujari JY, et al. Effect of fagonia arabica (dhamasa) on in vitro thrombolysis. BMC Complement Altern Med, 2007; 7: 36.
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Science and Tec, 1995; 28: 25-30. .
- Meyer BN, Ferrigni NR, Putnam JE, et al. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica, 1982; 45: 31– 4.
- Tlahque JG, Mancilla CLA, Palestina CL, et al. Constituents, antioxidant and antifungal properties of jatropha dioica var. dioica. Nat Pro Com, 2019; 14(5): 1–5.
- Fraga BM, Coloma AG, Gomez SA, et al. Bioactive constituents from transformed root cultures of Nepeta teydea. Phytochem, 2017; 133: 59-68.
- Ochieng CO, Shrivastava A, Chaturvedi U, et al. Antioxidant activities of triacontanyl-e-cinnamoatesmetabolites from caesalpinia volkensii root bark extracts. J of the Kenya Che Soc, 2017;10 (1): 3-9.
- Ali MR, Kuri S, Das A, Islam MA. Preliminary phytochemical screening and in vitro thrombolytic potential of the methanolic extract of Enhydra fluctuans Lour (leaves). Int J Pharmamed Ind, 2013; 1: 270- 280.
- Meena H, Pandey HK, Pandey P, Arya MCm, Ahmed Z. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Baccopa monnieri and Centella asiatica. Ind J Pharmacol, 2012; 44: 114-117.
- Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chemistry, 2006; 99 (4): 835-84.
- Mirzaei M, Mirzaei A. Comparison of the Artemia salina and Artemia uramiana bioassays for toxicity of four Iranian medicinal plants. Int Res J Biol Sci, 2013; 2: 49- 54.
- Sãnchez-Medina A, Garcia-Sosa K, May-Pat F, et al. Evaluation of biological activity of crude extracts from plants used in Yucatecan traditional medicine part L. Antioxidant, Antimicrobial and beta-glucosidase inhibition activities. Phytomed, 2001; 8 (2): 144–151.
- Mothana RAA. Anti-inflammatory, antinociceptive and antioxidant activities of the endemic Soqotraen Boswellia elongata Balf. and Jatropha unicostata Balf in different experimental models. Food and Chem. Toxicol, 2011; 49, 2594–2599.
- Desmarchelier C, Repetto M, Coussio J, et al. Total reactive antioxidant potential (trap) and total antioxidant reactivity of medicinal plants used in Southwest Amazona (Bolivia and Peru). Int J of Pharmacog, 1997; 35 (4): 288–296.
- Ankli A, Heinrich M, Bork P, et al. Yucatec Mayan medicinal plants: evaluation based on indigenous uses. J of Ethnopharmacol, 2002; 79: 43–52.
- Kupchan SM, Sigel CW, Matz M.J. Jatrophone, a novel macrocyclic diterpenoid tumor inhibitor from Jatropha gossypiifolia. J of American Chem. Society, 1970; 92 (14): 4476.
- Bussmann RW, Malca G, Glenn A, et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J of Ethnopharmacol, 2011; 137:121–140.
- Osoniyi O, Onajobi F. Coagulant and anticoagulant activities in Jatropha curca latex. J of Ethnopharmacol, 2003; 89: 101–105.
- Oduola T, Avwioro OG, Ayanniyi TB, et al. Suitability of the leaf extract of Jatropha gossypiifolia as an anticoagulant for biochemical and haematological analyses. Afr J of Biotech, 2005; 4 (7): 679–681.








