Afifah Afifah1* , Khusnul Muflikhah2
, Khusnul Muflikhah2 , Viva Ratih Bening Ati1
, Viva Ratih Bening Ati1 , Eman Sutrisna1
, Eman Sutrisna1 , Fajar Wahyu Pribadi1
, Fajar Wahyu Pribadi1 , Lantip Rujito3
, Lantip Rujito3 , Tzania el Izz Avinda1
, Tzania el Izz Avinda1 , Ahmad Musafi Hasan1
, Ahmad Musafi Hasan1 , Kresna Mukti1
, Kresna Mukti1 and Dias Rudi Haryadi1
and Dias Rudi Haryadi1
1Departement of Pharmacology, Universitas Jenderal Soedirman, Purwokerto, Indonesia.
2Departement of Physiology, Universitas Jenderal Soedirman, Purwokerto, Indonesia.
3Departement of Genetic and Molecular Biology, Universitas Jenderal Soedirman, Purwokerto, Indonesia.
Corresponding Author E-mail: afifah@unsoed.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2686
Abstract
Background: Acute kidney injury (AKI) is one of the health problems. Kidney ischemia-reperfusion injury (IRI) contributes to pathological conditions of AKI. An imbalance between renal vasoconstriction and vasodilatation mediators was played a role in IRI and its chronic complications. Stress oxidative and inflammation were major pathomechanism of IRI. Administration of celery ethanol extract is one of the efforts to prevent kidney damage caused by IRI. This study aimed to investigate the time effect of celery ethanol extract administration on inhibition of kidney IRI. Methods: Twenty male Sprague Dawley rats with a weight range of 190-210 g were selected for the study. The rats were divided into five groups randomly: sham operation (SO, n=4) group, IRI group (ischemia-reperfusion injury, n=4), IRI+S7 (celery ethanol extract 1000 mg/kg BW 7 days orally+ischemia-reperfusion injury, n=4), IRI+S14 (celery ethanol extract 1000 mg/kg BW 14 days orally+ischemia-reperfusion injury, n=4), IRI+S28 (celery ethanol extract 1000 mg/kg BW 28 days orally+ischemia-reperfusion injury, n=4). Serum samples were collected for creatinine serum, NO, SOD, and TNF-α measurement. mRNA expression of ET-1 and ETAR was quantified using reverse transcriptase-PCR. Result: Serum creatinine, NO, and SOD level in rats with celery ethanol extract 1000 mg/kg BW for 7 and 14 days administration before IRI induction lower than IRI group (p<0.05) and increase in 28 days administration. Meanwhile, the TNF-α level, ET-1, and ETAR gen expression lower than the IRI group but not significantly different (p>0.05). Conclusion: Administration of celery ethanol extract 1000 mg/kg BW for 7 days and 14 days prevents renal ischemia-reperfusion injury via increasing NO and SOD. Administration more than 28 days is not recommended.
Keywords
Celery; Endothelin-1; Ischemia-reperfusion injury; Nitrite oxide; Superoxide dismutase
Download this article as:| Copy the following to cite this article: Afifah A, Muflikhah K, Ati V. R. B, Sutrisna E, Pribadi F. W, Rujito L, Avinda T. E. L, Hasan A. M, Mukti K, Haryadi D. R. Celery Ethanol Extract Prevents Renal Ischemia-Reperfusion Injury via Increasing Nitrite Oxide and Superoxide Dismutase. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Afifah A, Muflikhah K, Ati V. R. B, Sutrisna E, Pribadi F. W, Rujito L, Avinda T. E. L, Hasan A. M, Mukti K, Haryadi D. R. Celery Ethanol Extract Prevents Renal Ischemia-Reperfusion Injury via Increasing Nitrite Oxide and Superoxide Dismutase. Biomed Pharmacol J 2023;16(2). |
Introduction
Chronic Renal Failure, the final phase of the AKI condition, occurs in 20% of cases turning to increase the risk of morbidity, mortality and hospitalization which produce an impact on the costs required 1–4. Among several types of AKI, Acute tubular necrosis caused by ischemia-reperfusion injury (IRI) is considered as the most common cause of AKI 5. IRI contributes to pathological conditions of AKI using some pathological pathways such as hemodynamic changes, tubular epithelial injury, activation of neutrophils, the release of reactive oxygen species and other inflammatory mediators. In addition, adhesion molecules and a variety of cytokines are also involved 6,7.
The balance between renal vasoconstrictor and vasodilator mediators has been implicated in IRI and its chronic complications. Nitrite oxide (NO) is a vasodilator produced by NO synthase (NOS). NO is known as a renoprotective factor against renal ischemia-reperfusion injury due to its anti-inflammatory, vasodilatory, and antioxidant properties 8.
Endothelin-1 (ET-1), a potent vasoconstrictor in blood mechanism proven to be upregulated in renal tubules in IRI 9. The vasoconstriction effect of ET-1 is mediated by ETAR 10. Inhibition of the endothelin system and reducing ET-1 production is efficient in reducing the kidney damage caused by IRI. The lack of endothelium-derived ET-1 not only attenuated proximal tubular injury in response to IRI but also decreased inflammatory and oxidative stress responses. Due to the difficulty of AKI therapy, it is necessary to take preventive action to prevent kidney damage due to IRI. One effort that can be done is to utilize natural ingredients that have been used as food ingredients.
Celery is known that was contained carbohydrates, flavonoids, alkaloids, steroids, glycosides, phenols, furocoumarins volatile oils sesquiterpene alcohols, fatty acids, and a wide range of trace elements 11. Celery is known to prevent kidney damage due to IRI 12. There has never been done a study on the time effect of celery ethanol extract administration on preventing mechanism renal ischemia-reperfusion injury. This study purposed to investigate the time effect of celery ethanol extract administration on inhibition of ET-1 and ETAR gene expression, NO, SOD, and TNF-α level in renal ischemia-reperfusion injury.
Materials and methods
The Experiment of Animal and the IRI model
This experimental study was performed from July to September 2019 after obtaining Ethical clearance from the ethics committee of the Faculty of Medicine Universitas Jenderal Soedirman, Ref: 3052/KEPK/VII/2019. Twenty male Sprague Dawley rats with a weight range of 190-210 g were selected for the study. The rats were divided into five groups with random assignment: a sham operation (SO) group, IRI group, IRI+S7 (the extract of celery 1000 mg/kg BW 7 days orally followed by IRI), IRI+S14 (the extract of celery 1000 mg/kg BW 14 days orally followed by IRI), IRI+S28 (the extract of celery 1000 mg/kg BW 28 days orally followed by IRI). The IRI was performed to induce acute kidney injury. Following ketamine (100 mg/kg BW) intramuscular injection, the abdomen then was opened to perform a clamping method on both renal pedicles, using a non-traumatic vascular clamp for 45 minutes. Then, both clamps were get off. We used silk surgical thread 3/0 (OneMed®) for closing the site of incision. Sham-operated (SO) rats were subjected to a similar technique but no renal hilus clamping was performed. All groups were terminated on day 1 after surgery. The Rats were put in cages with natural light and dark cycles of 12:12 hours, temperature 25oC and humidity of 40%-60%. The rats were given standard feed and dringking water ad libitum.
Collection of Samples
Rats were euthanized on day 1 after the operation using ketamine (100 mg/kg BW) for anesthetized. Blood collected by hematocrit tube in retro-orbital sinus was centrifuged and the resulting serum was stored at -20oC before analysis. The abdomen and thorax were opened to visualize the heart and kidney. The organs were perfused with 0.9% NaCl from the left ventricle. The left kidney was harvested and kept in RNA later® for RNA extraction.
Preparation of 70% Ethanolic Extract of Celery
Celery stems and leaves were collected from Pratin, Purbalingga, Central Java, and identified in Taxonomy Laboratory in the Faculty of Biology, Universitas Jenderal Soedirman. Celery was washed thoroughly then dried using an oven at 45oC. Celery was crushed in a disk mill with a 60 mesh sieve. Then put in a container for extraction. Celery powder was added to 70% ethanol and macerated for 24 hours. The mixture is filtered using a Buchner funnel attached to a vacuum pump. Furthemore, evaporation was carried out using a vacuum rotary evaporator at a temperature of 45oC and a rotational speed of 90 rpm so that was obtained rough extract. We prepared the extract solution freshly each time and given 1000 mg/kg BW once daily for 7, 14, and 28 days before induction of IRI on the treatment groups.
Measurement of Serum Creatinine Level
Serum creatinine level was measure to evaluate kidney function. Serum creatinine examination was performed using related kits in Medico Laboratory, Purwokerto.
Gene Expression Examination
The extraction of RNA obtained from kidney of rats using RNA solution. The concentration of RNA was quantified with a nanodrop system. The ReverTra-Ace (Toyobo, Japan, Cat No. TRT-101) was used for cDNA synthesizing and was added with random primers and dNTP. Reverse transcription-polymerase chain reaction was carried out to amplify the following specific cDNAs: ET-1 (forward: GTCGTCCCGTATGGACTAGG, and reverse: ACTGGCATCTGTTCCCTTGG), ETAR (forward: GGAATCGGGATCCCCTTGAT, and reverse: GTGCTGCTCGCCCTTGTATT). The following conditions were used for amplification: 94oC for 2 s (initial denaturation), 94oC for 10 s (denaturation), 55oC for 30 s (annealing), 72oC for 1 s (extension) and 72oC for 10 s (last extension). The gene expression was quantified using Image J software. GAPDH was used as a housekeeping gene.
Measurement of SOD Level
The level of SOD was measure using a serum sample. The ransod method was used to determine the level of SOD. The absorbance was assessed using a spectrophotometer 595 nm wavelength
Measurement of NO level
NO level was measure using a serum sample. The level of NO was determined using a Griess method. The absorbance was assessed using a spectrophotometer 530 nm wavelength.
Measurement of TNF-α levels
An ELISA was used to measure TNF-α levels. Bioassay technology laboratory rat TNF-α ELISA kit with catalog number E0764Ra was used.
Statistical analysis
The results were expressed as the mean±standard deviation. Statistical analysis was performed using One Way ANOVA followed by post hoc least significance (LSD) test for NO and ET-1 gene expression. Kruskal Wallis test for serum creatinine level, SOD, and ETAR gene expression followed by post hoc Mann-Whitney. Differences were considered to be significant at p<0.05.
Results
Celery Extract Attenuates Kidney Function
The IRI model represents acute kidney injury marked by the elevation of serum creatinine level. Based on the Kruskal Wallis test, serum creatinine level was significant differences between the group (p=0.004). Serum creatinine level in IRI group were higher than SO group (1.61±0.38 mg/dL vs 0.68±0.26 mg/dL, p=0.02). Serum creatinine in the intervention group was lower than the IRI group. The giving celery ethanol extract for 7, 14, and 28 days before IRI induction prevented the increase of serum creatinine significantly compare to IRI group (1.17±0.06 mg/dL, p=0.43), (0.99±0.15 mg/dL, p=0.021) (1.09±0.06, mg/dL p=0.021), respectively, vs (1.61±0.38 mg/dL). The mean serum creatinine level in 14 days of giving celery extract before IRI induction was the lowest compare to other intervention groups. While the serum creatinine level of the giving celery extract for 28 days before IRI induction higher than 14 days.
Effect of Celery Ethanol Extract on NO level
The result of measurement NO level was significant differences between groups using a one-way ANOVA test (p=0.000). The NO level in the IRI group (3.05±0.51 µmol/L) was lower than the SO group (9.86±0.56 µmol/L) (p=0.000). The giving of celery ethanol extract for 7, 14, and 28 days before IRI induction prevented the decrease of NO level significantly compared to the IRI group ((5.99±0.16 µmol/L, p=0.000) (8.07±0.062 µmol/L, p=0.000) (7.38±0.26 µmol/L, p=0.000), respectively, vs (3.05±0.51 µmol/L)). The NO level in the IRI+S14 group was the highest compare to IR+S7 and IR+S28.
Effect of Celery Ethanol Extract on SOD level
The result of the SOD level using the Kruskal Wallis test was significant differences between groups (p=0.003). The level of SOD in the IRI group (30.34±5.51 U/mL) was lower than the SO group (59.54±3.15 U/mL) (p=0.021). The giving of celery ethanol extract for 7, 14, and 28 days before IRI induction prevented the decrease of SOD level significantly compared to the IRI group (50.26±0.66 U/mL, p=0.021) (55.69±3.84 U/mL, p=0.021) (53.86±2.28 U/mL, p=0.021), respectively, vs (30.34±5.51 U/mL)). The level of SOD in IRI+S14 (55.69 U/mL) was the highest compare to other intervention groups.
Effect of Celery Ethanol Extract on TNF-α level
The result of TNF-α using one way ANOVA test was no significant differences between groups (p=0.111). The mean of TNF-α level in the IRI group was higher (39.28±2.42 U/mL) than in the SO group (34.58±8.47 U/mL). The level of TNF-α in celery ethanol extract 1000 mg/BW for 7 days group was the lowest.
Effect of Celery Ethanol Extract on ET-1 and ETAR gene expression
ET-1 gene expression in the IRI group (0.97±0.29) was higher than the SO group (0.58±0.13). ET-1 gene expression in IRI+S7, IRI+S14, IRI+S28 were 0.70±0.25, 0.68±0.23, and 0.83±0.24 respectively. Based on the One-way ANOVA test there were no significant differences between groups (p=0.194). ETAR gene expression in the IRI group (1.20±0.12) was higher than the SO group (0.68±0.12) (p=0.182). ETAR gene expression in IRI+S7, IRI+S14, IRI+S28 were 0.72±0.12, 0.72±0.19, and 0.89±0.33 respectively. Based on the Kruskal Wallis test there were no significant differences between groups (p=0.182).
Table 1: The level of serum creatinine, NO, SOD, and TNF-α.
|
|
SO group |
IRI group |
Celery ethanol extract 1000 mg/kgBW for 7 days |
Celery ethanol extract 1000 mg/kgBW for 14 days |
Celery ethanol extract 1000 mg/kgBW for 28 days |
|
Creatinine |
0.66(0.46-0.95)* |
1.56(1.22-2.12)# |
1.17(1.10-1.23) |
0.98(0.85-1.13) * |
1.08(1.03-1.16) * |
|
NO (µmol/L) |
9.86±0.56* |
3.05±0.51# |
5.99±0.16* |
8.07±0.62* |
7.38±0.26* |
|
SOD (U/ml) |
60.09(55.46-62.51) * |
28.49(26.12-38.25) # |
50.21(49.50-51.10) * |
55.67(51.50-59.94) * |
53,54(51.65-56.70) * |
|
TNF-α (U/ml) |
34.58±8.47 |
39.28±2.42 |
23.33±8.64 |
30.76±6.61 |
32.04±4.29 |
Data presented as mean±SD, exept for serum creatinine and SOD as median. *=p<0.05 vs IRI group. # =p<0.05 vs SO group. SO (Sham operation), IRI (Ischemia-reperfusion injury).
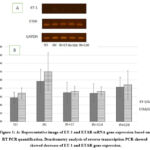 |
Figure 1: A: Representative image of ET-1 and ETAR mRNA gene expression based on RT PCR quantification. |
Discussion
This research determined the time effect of celery ethanol extract administration on preventing the increase of ET-1 and ETAR gene expression, the decrease of NO and SOD level in renal ischemia-reperfusion injury.Our previous study showed that ethanol extract of celery prevents kidney damage caused by IRI at a dose of 1000 mg/kg BW for 14 days before IRI induction 12. IRI is a major cause of acute kidney injury (AKI). Acute kidney injury assessed by kidney function. Serum creatinine levels were assessed to evaluate kidney function. In this study, the serum creatinine level in IRI groups higher than in the SO group. This study is in line with Arfian et al., (2012) that creatinine serum level in IRI higher than SO group. In this study, the administration of celery ethanol extracts 1000 mg/kg BW for 7, 14, and 28 days before IRI prevents the increase of serum creatinine level. According to Afifah et al. (2019), celery ethanol extract has a protective effect on kidney damage in the IRI rat model.12 The lowest serum creatinine level in this study is in 14 days before IRI induction. However, the administration of celery ethanol extract 1000 mg/kg BW for 7, 14, and 28 days before induction prevents the increase of serum creatinine, the administration for 28 days showed serum creatinine level higher than 7 and 14 days. This phenomenon showed that giving celery ethanol extract 1000 mg/kg BW for more than 28 days increase the creatinine serum level. It’s a concern for us that giving celery ethanol extract for a long time can worsen renal function disturbance.
The damage to the kidney caused by IRI fundamentally occur in two stages. The first is during ischemia, cell energy depletion is the main factor. The second is during reperfusion, the interactions occur between the oxidative and microcirculatory stress parallel with inflammation and apoptosis 13.
Celery contained carbohydrates, flavonoids, alkaloids, steroids, glycosides, phenols, furocoumarins, volatile oils, sesquiterpene alcohols, fatty acids, and a wide range of trace elements 11. Celery has anti-inflammatory 14 and antioxidant effect 15 which were the major mechanism of the pathophysiology of IRI. IRI induced kidney failure and increase ET-1 and ETAR expression. ET-1 is a potent vasoconstrictor that contributes to the pathogenesis of ischemia-reperfusion injury-induced acute kidney injury. The therapeutic strategy in the management of AKI can be done with blocking ET-1.
In this study, we observe the effect of celery ethanol extract on the ET-1 and ETAR gene expression in kidney IRI rat model. The ET-1 gene expression in the IR group was higher than the SO group but there were no significant differences based on statistical analysis. The increase of ET-1 one day after AKI has been already reported. Vasoconstriction cause of ET-1 in the renal vascular system is mediated by ETAR. In this study, ETAR gene expression in the IR group was higher than the SO group, however, it is not significant on statistics. ETAR gene expression was no significant differences, although, the study showed decrease ETAR gene expression after giving celery ethanol extract for 7 days and 14 days before IRI, but increased for 28 days.
ET-1 and NO regulate vessels’ tonus balance which was influenced by vessel remodeling. Endothelial damage due to vasoconstriction can be reduced by reducing NO (6). It showed that the level of NO in the IRI group lower than the SO group. The downregulation of the NO/sGC/cGMP pathway and low NO level is commonly found in kidney disease 16.
The administration of celery 1000 mg/kg BW for 7, 14, and 28 days before IRI induction prevents the decrease of NO level. The highest NO level in this study is in 14 days of giving celery ethanol extract. NO has been recognized as tissue protective through physiological regulation of vascular tone, platelet aggregation inhibition, attenuation of leukocyte adherence to the endothelium, scavenging of oxygen-derived free radicals, maintenance of normal vascular permeability, inhibition of smooth muscle proliferation, immune defense, and stimulation of endothelial cell regeneration (8). NO inhibits vasoconstriction and represents a counter regulator ET-1 system. NO production in the kidney has been shown to be important for the regulation and protection of many kidney functions 16.
The oxidative stress and inflammation were the major factor that influenced the kidney damage in the ischemia-reperfusion injury. IRI generated a huge amount of ROS 17. SOD is an antioxidant endogen against oxidative injury because of this enzyme dismutase superoxide to hydrogen peroxide, which is then detoxified by catalase or glutathione peroxidase. Therefore, administration of SOD may be a potential therapeutic approach to decrease oxidative stress and injury in ischemic reperfusion of the kidney (18). Administration of celery ethanol extract in this study prevents the decrease of SOD level in ischemia-reperfusion injury rat model. Administration 1000 mg/kg BW of celery ethanol extract for 14 days before IRI was the most prevent decrease of SOD level. There were three isoforms of SOD. SOD3 is the isoform that is most abundantly expressed in the kidney and the bloof vessels. Thus it can be considered as the most relevant isoform for protection against ischemia-induced renal impairment 18.
Acute kidney injury is an inflammatory disease 19. The innate and adaptive immune systems participate in the inflammatory response to IRI 6. TNF-α is a proinflammatory cytokine that has been implicated in the pathobiology of AKI. The result of the TNF-α level in this study was no significant differences using the one-way ANOVA test (p>0.05). Nevertheless, the mean TNF-α level in the treatment group lower than the IRI group. The lowest is in celery ethanol extract 1000 mg/kg BW for 7 days group and in 14 days and also 28 days groups were higher than 7 days but still lower than IRI group.
Conclusion
Administration of celery ethanol extract 1000 mg/kg BW for 7 and 14 days prevents renal ischemia-reperfusion injury via increasing nitrite oxide and superoxide dismutase. The giving of celery ethanol extract 1000 mg/BW for more than 28 days is not recommended.
Acknowledgment
The author thanks to the Research Institutions and Community Service, Universitas Jenderal Soedirman which was supported by research grants (BLU). We thank all staff of the Pharmacology Departement and Research Laboratory of Medical Faculty, Universitas Jenderal Soedirman for assisting in performing this project.
Conflicts of Interest
The authors declared no conflict of interest.
Funding Source
This study supported financially by Research institution and community service, Universitas Jenderal Soedirman. Grant number is 2387/ UN23.14/ PN/ 2018
References
- Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. Basic Med J. 2015;5(1):e006497–e006497.
CrossRef - Kam Tao Li P, Burdmann E a, Mehta RL. Acute kidney injury: Global health alert. J Nephropathol. 2013;2(2):90–7.
CrossRef - Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR. Contribution of Acute Kidney Injury Toward Morbidity and Mortality in Burns: A Contemporary Analysis. Am J Kidney Dis. 2007;49(4):517–23.
CrossRef - Kerr M, Bedford M, Matthews B, O’donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29(7):1362–8.
CrossRef - Coca SG, S. Si, Parikh CR. Chronic Kidney Disease after Acute Kidney Injury: A Systematic Review and Meta-analysis. Kidney Int. 2012;29(6):997–1003.
CrossRef - Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–21.
CrossRef - Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Ren Inj Prev. 2015;4(2):20–7.
- Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22(1):46–55.
CrossRef - Miguel C De, Speed JS, Kasztan M, Gohar EY, Pollock M. Endothelin-1 and the kidney: new perspectives and recent findings. Curr Opin Nephrol Hypertens. 2016;25(1):35–41.
CrossRef - Arfian N, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Hirata K ichi. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem Biophys Res Commun [Internet]. 2012;425(2):443–9.
CrossRef - Al-snafi AE. The Pharmacology of Apium graveolens-A Review. Int J Pharm Res Sch. 2014;3(1–1):671–7.
- Afifah A, Muflikhah K, Ati ViRB, Tsani RM, Khasanah D, Maulana W. Protective effect of ethanol extract of celery (Apium graveolens L) on kidney damage in ischemia/reperfusion injury rats model. Molekul. 2019;14(1):11–7.
CrossRef - Soares ROS, Losada DM, Jordani MC, Évora P, Castro-E-Silva O. Ischemia/reperfusion injury revisited: An overview of the latest pharmacological strategies. Int J Mol Sci. 2019;20(20).
CrossRef - Baananou S, Borgi W, Mahmoud A, Boukef K, Chouchane N, Aouam K, et al. Anti-inflammatory and Analgesic Activities of Tunisian Apium graveolens L. Leaves Extracts in Rats: J Biol Act Prod from Nat. 2012;2(4):225–31.
CrossRef - Sameh B, Ibtissem B, Mahmoud A, Boukef K, Naceur A. Antioxidant Activity of Apium graveolens Extracts. J Biol Act Prod from Nat. 2011;(August 2014):340–3.
CrossRef - Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci. 2018;19(9).
CrossRef - Kim J, Jung KJ, Park KM. Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol – Ren Physiol. 2010;298(5):1118–29.
CrossRef - Dias, Vera Junn, Eunsung Mouradian MM. 基因的改变NIH Public Access. 2008;23(1):374–81.
- Bonventre J V., Zuk A.Ischemic acute renal failure: An inflammatory disease? Vol. 66, Kidney International. 2004. p. 480–5.
CrossRef







