Manuscript accepted on :09-03-2023
Published online on: 18-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ramlah Kadir, Dr. Raja Azman Raja Awang
Second Review by: Dr. Mario Vincenzo Russo
Final Approval by: Dr. Patorn Piromchai
Asma Mohammed Salim ALshuili1, Shadia Al-Sinawi2, Radiya Al-Ajmi2, Asem Shalaby2,3 and Mohamed Mabruk1*
1Sultan Qaboos University , Department of Allied Health Sciences, College of Medicine and Health Sciences, Muscat, Oman
2Sultan Qaboos University , Department of Pathology, College of Medicine and Health Sciences, , Muscat, Oman
3Mansoura University ,Pathology Department, College of Medicine, Mansoura, Egypt.
Corresponding Author E-mail: mabruk@squ.edu.om
DOI : https://dx.doi.org/10.13005/bpj/2683
Abstract
Background: Hepatitis B virus (HBV) infection is considered a major global health problem. The main objectives of the current study is to determine the patterns of expression of the hepatitis B surface antigen (HBsAg) and HBV core antigen (HBcAg) in liver tissue samples obtained from hepatitis B virus-infected Omani patients and to associate between the pattern of the expression of HBsAg and HBcAg with the other clinical parameters and anti-viral therapy. Methods : The expression patterns of HBsAg and HBV core antigen HBcAg were determined by immunohistochemistry (IHC), in 58 formalin-fixed paraffin-embedded liver tissue biopsies obtained from chronic hepatitis B Omani patients. The association between positivity for HBV antigens with gender, age group, histological appearances, and antiviral therapy was determined. Results: IHC-positive staining of HBsAg was demonstrated in 28 patients (48.3%) patients, of whom 4 (6.9%) also showed HBcAg expression. The expression pattern of HBsAg was predominantly cytoplasmic and was seen in 25 (89.3%) of patients, whereas expression of HBcAg was nuclear in 3 (75%) of patients. HBsAg and HBcAg IHC positivity were more common among males than females and among those aged 39–58 years (P = 0.130 and 0.569, respectively). The presence of lymphocytic infiltration in the majority of liver biopsies examined in the present study indicates that the liver damage could be attributed to immunologically mediated events, especially because HBV is a non-cytopathogenic virus. No significant statistical association was found between positivity for HBsAg/HBcAg by IHC and antiviral treatment. Conclusions: Determination of the expression patterns of HBV antigens in liver biopsies obtained from chronic hepatitis B Omani patients and the association between these expression patterns with other clinical histopathological parameters and anti-viral therapy, will contribute greatly to a better understanding of the pathogenesis of HBV in this unique cohort group of infected individuals.
Keywords
Antiviral therapy; Clinicopathological parameters; Hepatitis B virus; hepatitis B surface antigen; hepatitis B core antigen; Immunohistochemistry
Download this article as:| Copy the following to cite this article: ALshuili A. M. S, Al-Sinawi S, Al-Ajmi R, Shalaby A, Mabruk M. Association between the Expression Patterns of Hepatitis B Surface Antigen and Hepatitis B Core Antigen with Clinicopathological Parameters and Antiviral Therapy in Liver Biopsies Obtained from Chronically Infected Hepatitis B Positive Omani Patients. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: ALshuili A. M. S, Al-Sinawi S, Al-Ajmi R, Shalaby A, Mabruk M. Association between the Expression Patterns of Hepatitis B Surface Antigen and Hepatitis B Core Antigen with Clinicopathological Parameters and Antiviral Therapy in Liver Biopsies Obtained from Chronically Infected Hepatitis B Positive Omani Patients. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3BIwdUF |
Introduction
Hepatitis B virus (HBV) is one of the viruses that cause viral hepatitis 1. The infections range from acute hepatitis to fulminant liver failure. Around 15–40% of infected patients will develop life-threatening liver diseases, including cirrhosis and hepatocellular carcinoma 2,3. Moreover, around 350 million infected people are considered chronic carriers 2. It has been estimated that one million people die each year from HBV infection, most of whom die from liver failure or hepatocellular carcinoma (HCC), the latter is considered as the third-leading cause of cancer-related mortality globally is hepatocellular carcinoma (HCC)3.
After acute hepatitis B infection, most infected individuals completely clear the virus from the blood and liver, accompanied by seroconversion from surface antigens to subsequent antibodies in acute infections 4,5. This immune response is carried out by the non-cytolytic cytokines (e.g., interferon-gamma [IFN] and tumor necrosis factor-alpha [TNF]), which are used to clear the virus in such situations 4,5. Further research has revealed that virus clearance is aided by natural killer cells, various IFNs and receptors, CD4+ and CD8+ T cells Fas ligand, and the TNF receptor 1 6. Patients with chronic infection have a weakened adaptive immune system that is tightly based, implying that HBV clearance is T cell-dependent 5,6.
Pegylated-interferon (Peg-IFN) and nucleotide analogs are the 2 main medications for chronic HBV infection 7. Their mechanism of action is to suppress polymerase activity and thereby slow the progression of the disease, but neither is sufficiently effective in delivering a functional cure 5,6,7.
Vaccination against HBV has proven to be highly effective in reducing disease burden, carrier state development, and HB-related morbidity and mortality 8-10.
The aim of the present study is to determine the expression patterns of the hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBcAg) in formalin-fixed paraffin-embedded liver biopsy tissue samples obtained from chronically infected hepatitis B positive Omani patients. Moreover, this study aimed to determine the association between the immunohistochemical positivity of HBVAg and other clinical parameters, such as liver fibrosis and cirrhosis, and with clinicoopathological features of the HBV-infected liver, as well as to evaluate HBsAg and HBcAg positivity with antiviral therapy. This approach may contribute to understanding the pathogenesis of HBV in Omani patients. A better understanding of the pathogenesis of this virus will be of great help in the future management of hepatitis B positive patients in Oman.
Materials and Methods
Ethical approval
Ethical approval for this research was obtained from the Medical Research Ethics Committee, Sultan Qaboos University, Muscat, Oman.
REF.NO. SQU-EC/197/2020; MERC# 2242.
Specimens
In the present study, 58 formalin-fixed paraffin-embedded liver biopsies were obtained from 58 Omani patients who had been histologically diagnosed with chronic HBV infection. The inclusion criteria included all Omani patients with chronic HBV infection who attended Sultan Qaboos University Hospital (SQUH). The exclusion criteria were non-Omani patients and patients with missing data. The formalin-fixed paraffin-embedded liver biopsy specimens were obtained from the Pathology Department at SQUH. In addition, clinicopathological data were collected from the information system at SQUH, including age, gender, histopathological data, and antiviral therapy. The antiviral therapy that had been given to the patients included tenofovir alafenamide, oseltamivir phosphate, acyclovir, daclatasvir, eltrombopag, and entecavir.
Immunohistochemistry
Immunohistochemistry was performed on Bench Mark Ultra from Ventana (Ventana Medical System Ltd., USA). The detection kit was an Ultra View Universal DAB detection kit (Ventana Medical System Ltd., USA). The monoclonal antibodies used were NCL-HBcAg-506 Mouse Monoclonal (Leica Biosystems, UK) and NCL-HBsAg-2 Mouse Monoclonal (Leica Biosystems, UK). For each experiment, positive and negative controls were included., one section was used as a test section and the parallel section was used as a negative control in which the primary antibodies was omitted.
Briefly, the formalin-fixed paraffin-embedded liver biopsy tissue samples were cut at 3μm thickness and mounted on superfrost plus slides and kept overnight at 37°C. As per the protocol created in Bench Mark Ultra (Ventana), the negative and positive controls and the test slides were baked at 72°C for 4 minutes, followed by deparaffinization at 72°C for 8 minutes. The slides were subjected to antigen retrieval by using Cell Conditioning-1 for 64 minutes. After washing, the liver biopsy sections were exposed for NCL-HBcAg-506 Mouse Monoclonal (Leica Biosystems, UK) and NCL-HBsAg-2 Mouse Monoclonal (Leica Biosystems, UK) for 36 minutes at 37°C.
The sections were counterstained by hematoxylin-11 for 12 minutes and post- counterstained by bluing reagent for 4 minutes. The slides were removed from Bench Mark Ultra and rinsed in running water with mild detergent. The slides were then dehydrated in graded alcohol, cleared in Xylene, and mounted in a Sukara cover slipper. The slides were examined by 2 independent pathologists. The patterns of the expression of HBV antigens in relation to the histopathological features in each section was recorded.
Statistical analysis
SPSS (Statistical Package for Social Sciences) version 23 was used for statistical analysis. The proportion and frequency of clinicopathological and histopathological data collected were projected. Most of the variables were evaluated in pairs, with HBsAg positive expression versus HBcAg positive expression. Pearson’s Chi-square and Fisher’s exact tests were used to analyze classified variables. A pie chart and cross tabulation were used to display the data. A statistically relevant P-value of 0.05 was used.
Results
Expression of HBsAg and HBcAg in liver biopsies
Of the 58 liver biopsies obtained from the HBV seropositive Omani patients and analyzed by IHC, 28 (48.3%) were positive for HBVsAg expression, and 4 (6.9%) were positive for HBcAg expression. Of the 28 (48.3%) HBsAg positive patients, 21 (52.5%) were male and 7 (38.9%) were female (P = 0.402). Moreover, cases that were positive for HBsAg were most common among those aged 39–58 years (71.4%), followed by those aged 19–38 years (21.4%) (Table 1). Two (7.1%) of the HBsAg positive cases were aged ≥ 59 years (P = 0.130). None of those aged 8–18 years was positive for HBsAg expression. On the other hand, all those who were positive for HBVcAg expression were male 4 (100.0%) (P = 0.300). Of these, 2 (50.0%) were aged 39–58 years, 1 (25.0%) was aged 19–38, and 1 (25.0%) was aged ≥ 59 years. Those in the group aged 8–18 years were negative for HBVcAg expression (P = 0.569). The results for the expression of HBsAg and HBcAg in liver biopsies are summarized in Table 1.
Table 1: HBsAg and HBcAg positivity expression by IHC according to gender and age groups
| Hepatitis B surface antigen(HBsAg)
(n=28) |
P-value | Hepatitis B core antigen(HBcAg)
(n=4) |
p-value | |
| Sex
male female |
21 (52.5%) 7 (38.9%) |
0.402 |
4 (10.0%) 0 (0.0%) |
0.300 |
| Age(years)
8-18 19-38 39-58 >=59 |
0 (0.0%) 6 (21.4%) 20 (71.4%) 2 (7.1%) |
0.130 |
0 (0.0%) 1 (25.0%) 2 (50.0%) 1 (25.0%) |
0.569 |
| Total | 28 (100%) | 4 (100%) |
n: number of cases
Patterns of HBsAg and HBcAg expression in liver biopsies obtained from Omani patients
A total of 25 (89.3%) of the 28 (48.3%) HBsAg positive liver biopsies showed a cytoplasmic pattern of staining, as shown in Figure 1A, while the remaining 3 cases (10.7%) showed mixed cytoplasmic and membrane patterns of expression (Figure 1B).
Of the 4 (6.9%) patients positive for HBcAg expression, 3 (75.0%) showed a nuclear pattern of staining (Figure 1C). The fourth positive case for HBcAg expression 1 (25.0%) showed mixed cytoplasmic and nuclear patterns of HBcAg expression (Figure 1D). An example of a negative result for HBV immunostaining are shown in Figure 1E.
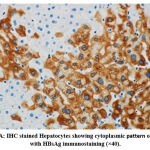 |
Figure 1A: IHC stained Hepatocytes showing cytoplasmic pattern of staining with HBsAg immunostaining (×40). |
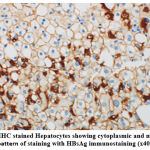 |
Figure 1B:IHC stained Hepatocytes showing cytoplasmic and membranous pattern of staining with HBsAg immunostaining (x40). |
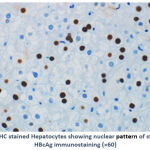 |
Figure 1C:IHC stained Hepatocytes showing nuclear pattern of staining with HBcAg immunostaining (×60) |
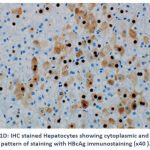 |
Figure 1D: IHC stained Hepatocytes showing cytoplasmic and nuclear pattern of staining with HBcAg immunostaining (x40 ). |
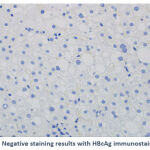 |
Figure 1E: Negative staining results with HBcAg immunostaining (x40 ). |
HBsAg and HBcAg expression in association with clinical and histopathological features
Of the 58 HBV-infected patients, 8 had liver cirrhosis and 36 had bridging fibrosis. Of the 8 patients with liver cirrhosis, only 1 (12.5%) expressed HBsAg (P = 0.053) and 1 (12.5%) expressed HBcAg (P = 0.457). Of the 36 patients with fibrosis, 16 (44.4%) were positive for HBsAg expression (P = 0.455) and 3 (8.3%) expressed HBcAg (P = 1.00) (Table 2).
Twenty-five (89.3%) of the 28 cases expressing HBsAg showed lymphocytic infiltration (P = 0.707). Moreover, all of the 4 positive patients for the expression of HBcAg showed lymphocytic infiltration (P = 1.00). Of the 8 patients with liver cirrhosis, 6 (75.0%) showed lymphocytic infiltration (P = 0.303). Of the 36 with bridging fibrosis, 33 (91.7%) showed lymphocytic infiltration (P = 0.238).
Table 2: The frequency of liver cirrhosis, bridging fibrosis in 58 HBV infected patients and were positive for HBsAg or HBcAg expression.
| Patient with liver cirrhosis(N*:8) | P-value | Patient with bridging fibrosis(N;36) | p-value | |
| Positive for HBsAg (IHC) | 1 (12.5%) | 0.053 | 16 (44.4%) | 0.455 |
| Positive for HBcAg (IHC) | 1 (12.5%) | 0.457 | 3 (8.3%) | 1.00 |
HBsAg and HBcAg expression in relation to antiviral therapy
Of the 28 patients who were positive for HBsAg expression, 8 were given antiviral therapy and the rest (20) were not (P = 0.098). Of the 30 patients who were negative for HBsAg, 3 (10%) received antiviral therapy. Of 54 patients who were negative for HBcAg, 10 (18.5%) received antiviral therapy.
Discussion
The findings of the present study revealed that HBsAg was expressed in the liver tissues of 28 (48.3%) of the 58 chronically HBV-infected Omani patients. These findings are concordant with a study conducted by Sharma and others in 2002, where HBsAg was positive in 48 out of 100 patients (48%)11. In the present study, using IHC staining patterns for HBsAg, 25 of 28 patients (89.3%) showed a cytoplasmic pattern of staining for HBsAg, while the remaining 3 (10.7%) showed mixed cytoplasmic and membrane patterns of expression. A previously published study revealed a similar finding of the cytoplasmic expression of HBsAg in a high number of infected hepatocytes 12. The finding of the present study that HBcAg expression was positive in 4 (6.9%) cases, with a predominantly nuclear pattern of staining (75.0%), is compatible with a previously published study 11 in which 13 of 100 (13%) of the HBV positive patients exhibited nuclear patterns of HBcAg expression.
The present study showed that HBsAg and HBcAg positivity by IHC was more common among males than females, but there was no significant association between gender and IHC positivity for HBsAg and HBcAg (P = 0.402 and 0.300, respectively). In addition, IHC positivity of HBsAg and HBcAg increased with age until the age of 59 years, with no significant association between age group and HBsAg and HBcAg expression (P = 0.130 and 0.569, respectively). A higher rate of HBV infection in males in comparison to females in the present study could be due to the fact that Omani males are more frequently exposed to the risk factors than females are. These findings of the present study align with those of a study conducted in Pakistan, which showed that 68.15% of HBV-infected individuals were males and 31.85% were females 13.
The degree of the intrahepatic inflammatory lymphocytic infiltrate is recognized as the histologic hallmark of the severity of chronic hepatitis B virus infection because HBV is considered a non-cytopathic virus 14. In the present study, the majority of positive liver biopsies for the expression of HBsAg and HBcAg showed lymphocytic infiltration of (89.3%) and (100.0%) of cases, respectively.
Cirrhosis caused by the HBV was once thought to be incurable. It is linked to a higher risk of death and a higher chance of developing hepatocellular carcinoma (HCC) 15. As is demonstrated in the present study, 8 of the 58 patients had liver cirrhosis, with only 1 positive for both HBsAg and HBcAg expression, with no significant association between the development of liver cirrhosis and IHC positivity for HBsAg and HBcAg. This could be due to the fact that HBV is a non-cytopathic virus, and it is most likely that the pathogenesis of HBV infection, including liver damage, is mostly regulated through immunologically mediated events 16. In the present study, the presence of HBVAg in only 2 patients with liver cirrhosis and the finding that the majority of patients with liver cirrhosis (75.0%) showed lymphocytic infiltration may enforce the view that liver damage and cirrhosis in these patients could be attributed to immune response rather than the presence of the HBV. It has been shown that there is a close link between the progression of cirrhosis and the degree of HBV replication, indicating that long-term antiviral therapy could reduce the risk of the prognosis to cirrhosis in these chronically HBV-infected Omani patients 17. In relation to the study’s findings, 2 of the 8 patients (25.0%) with liver cirrhosis were given antiviral treatment without significant association.
Son et al. [18] showed that there is no correlation between hepatitis histological activity and HBsAg intracellular expression 18. This finding is clearly confirmed by the present study, in which 36 cases had bridging fibrosis, of which 16 (44.4%) were positive for HBsAg expression and 3 (8.3%) were positive for HBcAg expression, with no statistical association. Moreover, the majority of the cases with bridging fibrosis showed lymphocytic infiltration without a statistical association. In addition, 9 of 36 (25.0%) of the cases with bridging fibrosis were given antiviral therapy but there was no statistical association between antiviral therapy and the development of bridging fibrosis. A previous study showed that a large percentage of patients who received a direct-acting antiviral drug saw their liver inflammation and fibrosis reversed 19.
HBcAg expression has been identified as a key target for antiviral immune responses 20. Previously published studies showed that antiviral therapy responses can be predicted using measurements of HBsAg and HBV DNA 21,22. According to the results of the present study, of the 28 cases positive for HBsAg, only 8 (28.6%) were given antiviral treatment. Moreover, of the 4 cases that were positive for HBcAg, 1 patient (25.0%) was given antiviral therapy with no significant association (P = 0.098 and 1.00 respectively).
In summary, the current study showed that HBsAg and HBcAg IHC positivity were more common among male than female patients and among those aged 39–58 years. Moreover, the presence of lymphocytic infiltration in the majority of liver biopsies examined indicate that liver damage could be immune modulated rather than the result of direct liver damage caused by HBV infection, especially since HBV is a non-cytopathogenic virus. No significant statistical association was found between positivity for HBsAg/HBcAg by IHC and antiviral treatment
Conclusions
The expression patterns of HBsAg and HBcAg in liver biopsies obtained from 58 HBV chronically infected Omani patients were detected by immunohistochemistry. The expression patterns of both HBsAg and HBcAg and their association with clinicopathological parameters in liver biopsies obtained from chronically infected hepatitis B positive individuals gave further insight into the pathogenesis of HBV infection in Omani patients.
Acknowledgement
The authors would like to thank the head and the staff of the Department of Pathology at Sultan Qaboos University for allowing us to use their laboratory facilities.
Conflict of Interest
The authors declare no conflict of interest
Funding Source
There are no funding for this project
References
- Liu, C.J.; Kao, J.H.; Shih, C; Yang; C.C.; Choijilsuren, G.; Chang, C.H.; Liou, A.T. Hepatitis B Virus. Trends Microbiol. 2018; 26(4):386-7.
CrossRef - Hasan, I. Epidemiology of hepatitis B. Acta Med Indones. 2005;37(4),231- 4.
- Xie, Y.; Hepatitis B Virus-Associated Hepatocellular Carcinoma. Adv Exp Med Biol. 2017;1018:11-21.
CrossRef - Wu, J.; Han, M.; Li, J.; Yang, X.; Yang, D. Immunopathogenesis of HBV Infection. Adv Exp Med Biol. 2020;1179:71-7.
- Zhan, Q.; Xu, J.H.; Yu, Y.Y.; Lo, K.k. Felicianna, E.; El-Nezami, H.; Zeng, Z. Human immune repertoire in hepatitis B virus infection. World J Gastroenterol. 2021; 7,27(25):3790-01.
CrossRef - Schuch,A.; Hoh,A.; Thimme, R. The Role of Natural Killer Cells and CD8+ T Cells in Hepatitis B Virus Infection. Front Immunol 2014; 5: 258.
CrossRef - Tan, G.; Song, H.; Xu, F.; Cheng, G. When Hepatitis B Virus Meets Interferons. Front Microbiol. 2018; 9:1-12.
- Stevenson, C.; Youn, J.H.; Hayney, M.S.; David C. Preventing hepatitis B virus infection among healthcare professionals: potential impact of a 2-dose versus 3-dose vaccine..Hum Vaccin Immunother. 2021; 17(11):4567-77.
CrossRef - Mohanty, P.; Jena, P.; Patnaik, L. Vaccination against Hepatitis B: A Scoping Review.Asian Pac J Cancer Prev. 2020; 21(12):3453-59.
CrossRef - Cargill, T.; Barnes, E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin Exp Immunol. 2021; 205(2):106-18.
- Sharma, R.R.; Dhiman, R.K.; Chawla, Y.; Vasistha, R.K. Immunohistochemistry for core and surface antigens in chronic hepatitis. Trop Gastroenterol. 2002; 23(1):16-9.
- Nakopouloul ,L.; Adraskelasl,N.;Stefanakil,K.;Zacharoulisl,D.; and Hadziyannis, S.T. Expression of HBsAg and HBcAg in liver tissue: correlation with disease activity. Histol Histopath 1992; 7: 493-9
- Khan,F.; Shams,S.; Din Qureshi,I.; Israr, M., Khan,H.; Sarwar,M.T.; Ilyas,M. Hepatitis B virus infection among different sex and age groups in Pakistani Punjab. Virol J. 2011; 8: 225.
CrossRef - Rehermann, B. Intrahepatic T cells in hepatitis B: Viral control versus liver cell injury, Journal of Experimental Medicine. 2000;191(8): 1263–8.
CrossRef - Chu, C.M.; Liaw, Y.F.; Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis. 2006; 26(2): 142-2.
CrossRef - Oh, I. S.; Park,S.H. Immune-mediated Liver Injury in Hepatitis B Virus Infection, Immune Network 2015;15(4): 191.
CrossRef - Van Bömmel, F; Berg, T. Treatment of HBV related cirrhosis, Liver International, 2013; 33(1): 176–1.
CrossRef - Son, M. S., Yoo, J. H., Kwon, C.-I., Ko, K. H., Hong, S. P., Hwang, S. G., Park, P. W., Park, C. K. and Rim, K. S. Associations of Expressions of HBcAg and HBsAg with the Histologic Activity of Liver Disease and Viral Replication, Gut and Liver. 2008; 2(3): 166–73.
CrossRef - Cheng, C. H., Chu, C. Y., Chen, H. L., Lin, I. T., Wu, C. H., Lee, Y. K., Hu, P. J. and Bair, M. J. Direct-acting antiviral therapy of chronic hepatitis C improves liver fibrosis, assessed by histological examination and laboratory markers, Journal of the Formosan Medical Association. 2020; 120 (5): 1259-68.
CrossRef - Uzun, Y.; Bozkaya, H.; Erden, E.; Cinar, K.; Idilman, R.; Yurdaydin, C.; Uzunalimoglu, O. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J Gastroenterol Hepatol. 2006;21(6),977-81
CrossRef - Song,J.C.; Min,B.Y.; Kim,J.W.; Kim,J.Y.; Kim Y.M.;Shin, C.M., Lee, S.H.; Hwang,J.H.; Jeong S.H.;, Kim, N.; Lee, D.H. Pretreatment serum HBsAg-to-HBV DNA ratio predicts a virologic response to entecavir in chronic hepatitis B. Korean J Hepatol. 2011; 17(4):268–73.
CrossRef - Caviglia, G.P.; Troshina, Y.; Garro, E. Usefulness of a Hepatitis B Surface Antigen-Based Model for the Prediction of Functional Cure in Patients with Chronic Hepatitis B Virus Infection Treated with Nucleos(t)ide Analogues: A Real-World Study. Clin. Med. 2021, 10: 3308-11







