Manuscript accepted on :24-03-2023
Published online on: 12-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ankur Kumar Arya and Dr. Md. Sarwar Hossain
Second Review by: Dr. Debasmita Duttapramanick
Final Approval by: Dr. Patorn Promchai
Abd. Malik1* , Rifka Amaliah2
, Rifka Amaliah2 , Vathimah Zahra1 and Aktsar Roskiana Ahmad2
, Vathimah Zahra1 and Aktsar Roskiana Ahmad2
1Department of Phytochemistry, Faculty of Pharmacy, Universitas Muslim Indonesia, Makassar, Indonesia
2Department of Pharmacognosy, Faculty of Pharmacy, Universitas Muslim Indonesia, Makassar, Indonesia.
Corresponding Author E-mail:aktsar.roskiana@umi.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2661
Abstract
Passion fruit (Passiflora edulis Sims.) is a tropical plant that grows widely in South East Asian region, including on Sulawesi Island. The plant is important in the food sector and for medicinal purposes. The fruits of the Passion plant are made as a popular syrup beverage in Makassar produced by several companies. The production process results in an unusable seed containing potential natural oil. Passion Seed Oil (PSO) has several benefits in health and cosmetics. The oil contains chemically active compounds such as phenolic and flavonoids with antioxidant activities. This study was performed by comparison of several solvents acetone, ethyl acetate, chloroform, methanol, and n-hexane. The antioxidant activity, total phenolic and flavonoid content of all extracts were then examined by the researchers. The potential antioxidant activity of PSO was shown by a methanol extract of 71.67 µg/mL. The highest flavonoid content was ethyl acetate extract of 35.40 mg RE/g crude extract, and phenolic was acetone extract of 193.80 mg GAE/g crude extract.
Keywords
Antioxidant; Flavonoid; Natural Oil; Passion Seed Oil; Phenolic
Download this article as:| Copy the following to cite this article: Malik A, Amaliah R, Zahra V, Ahmad A. R. Antioxidant Activity, Phenolic and Flavonoid Content of Passion Fruit Seed Oil. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Malik A, Amaliah R, Zahra V, Ahmad A. R. Antioxidant Activity, Phenolic and Flavonoid Content of Passion Fruit Seed Oil. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3DlV3uJ |
Introduction
Passion fruit belongs to the family Passifloraceae and one of the species with high economic value1. Passion fruit is one of the important commodities in South Sulawesi, especially in Makassar, since it is one of the present popular icons of passion fruit syrup. The syrup-making process produces waste in the form of skins and seeds. Passion fruit contains as much as 13.6% seeds; therefore, the more syrup produced, the more seed waste will be produced. The passion fruit syrup industry makes about 40% of seed waste, and 100% is unusable.
Meanwhile, passion fruit seeds contain high fiber and nutrients and plant-based vegetables that can be used for cosmetics and food.2,6 However, passion fruit waste is only disposed of and unusable; therefore, this is an opportunity to be managed and developed into an economically valuable product. Passion seeds oil (PSO) contains flavonoids and piceatannol, which can inhibit the tyrosinase enzyme and melanin biosynthesis.2
The tyrosinase enzyme converts tyrosine into 3,4-dihydroxyphenylalanine (DOPA) and dopaquinone which is further synthesized into melanin pigment characterized by black/brown spots on the skin.13 In addition, PSO contains 87.59% unsaturated fatty acids and other compounds, like tocopherol (499.30 mg/Kg), phenolic (314.13 mg GAE/kg), and vitamin C (1.90 mg/L).12 The PSO has a high antioxidant activity with a radical scavenging mechanism of up to 82.81% and an EC50 value of 10.62 g oil/g DPPH.10,15 & 17 Therefore, PSO can potentially prevent free radicals that trigger premature skin aging. Environmental influences such as ultraviolet light, cigarette smoke, pollutants, temperature, nutrition, and an unhealthy lifestyle can form free radicals and Reactive Oxygen Species (ROS). This stimulates skin inflammation, triggering a series of biochemical reactions in the skin that damage the collagen network in the epidermis layer, leading to premature aging (photoaging/premature skin aging). It can depigment skin by directly inhibiting tyrosinase activity in the process. The binding of flavonoids to copper and their antioxidant activity have been reported to play an important role in inhibiting the action of tyrosinase enzymes.2 Tocopherol compounds in passion fruit are known as antioxidant agent. In this section, it is necessary to describe the specific specifications related to the scheme. It is strong and able to reduce skin damage due to UV B light. It can also inhibit photocarcinogenesis by preventing the formation of cyclopyrimidine dimmers in the epidermal P-53 gene and inhibiting the process of melanogenesis.19 Combining tocopherol and phenolic compounds in passion fruit oil will effectively overcome skin damage leading to premature aging (photo-aging) and hyperpigmentation (melasma). Furthermore, Huda et al (2017) reported that passion fruit (Passiflora edulis) seed extract prevented an increase in the amount of skin melanin equivalent to 4% hydroquinone in guinea pigs (Cavia porcellus) exposed to UV B4 light.
Based on the description, passion fruit seeds oil has potential in the pharmaceutical field for developing pharmaceutical preparations, especially herbal cosmetics. Still, selecting extraction methods is essential for successfully extracting active compounds. Furthermore, the type and amount of solvent used can also affect the number of active compounds that can be drawn, where compounds with polar properties will dissolve in polar solvents, and non-polar compounds will dissolve in non-polar solvents.
Materials and methods
Materials
The passion fruit seed samples were obtained from the passion fruit syrup industry waste in Makassar. The passion fruit seeds were dried and powdered. AlCl3, Acetone, ethyl acetate, methanol, n-hexane, chloroform, gallic acid, folin-ciocalteau, potassium acetate, sodium carbonate, quercetin, and rutin were purchased from Merck (Merck KGaA, Darmstadt, Germany).
Extraction
The passion seeds oil was extracted by maceration method with assisted ultrasonication. As much as 500 g of powder passion seeds were added with various solvents, including acetone, ethyl acetate, chloroform, methanol, and n-hexane. The maceration assisted-ultrasonicator method was sonicated for 30 minutes.
Antioxidant assay
The antioxidant assay was performed according to the method by Laura Gonzalez with minor modifications. Briefly, a fresh solution of DPPH was prepared by dissolving 10.0 mg of DPPH powder in 100 mL of methanol. Different concentrations of extraction solution (2.5 ml) and DPPH solution (2.5 ml) were mixed together and incubated at room temperature in the dark for 30 minutes. A UV-VIS spectrophotometer was used to detect the absorbance at 517 nm. The inhibition rates of free radical scavengers were estimated using the following formula:
Total phenolic content
The total phenolic content (TPC) of extracts was determined using the folin-ciocalteu (FC) method, which refers to Nugroho, et al. (2012) with a slight modification. Each extract was dissolved in distilled water to a concentration of 50.0 g/mL. Gallic acid (0-60 g/mL) and standard curve was created. Diluted gallic acid (1.6 mL) was mixed well with 0.2 mL of FC reagent (diluted 5-fold with distilled water) for 3 minutes. The mixture was treated with sodium carbonate (0.2 mL, 10% w/v) and allowed to stand at room temperature for 30 minutes. The absorbance of the mixture was measured at 760 nm using a UV-VIS spectrophotometer. TPC was expressed as milligrams of Gallic acid equivalents per gram defatted P. edulis (mgGAE/g).
Total Flavonoid Content
The total flavonoid content (TFC) of each extract was analyzed by a colorimetric method using aluminium chloride reagent 14. By diluting rutin with methanol (0-100 g/mL), a standard curve was created. Diluted extracts and rutin standards (2.0 ml) were combined with 0.1 ml of 10% (w/v) aluminum chloride solution and 0.1 mM potassium acetate solution. For 30 minutes, the mixture was left at room temperature. A UV-VIS spectrophotometer was then used to detect the mixture’s maximum absorbance at 415 nm. TFC was calculated using milligrams of rutin equivalents per gram of extract.
Results and Discussion
Extraction
Passion oil extracts were obtained using solvents such as acetone, ethyl acetate, chloroform, methanol, and n-hexane (Figure 1). The results of extraction with different solvents are presented in the following order: chloroform 18.19%, acetone 14.02%, n-hexane 10.79%, methanol 5.45 %, and ethyl acetate 4.93% (Table 1).
 |
Figure 1: The extracted amount of PSO in different solvent. |
Table 1: Extract the amount of PSO in different solvent
|
Solvents |
Extract amount (%) |
|
Acetone |
14.02 |
|
Ethyl acetate |
4.93 |
|
Cholorform |
18.19 |
|
Methanol |
5.45 |
|
n-Hexane |
10.79 |
Based on the data, the extraction yield of chloroform (18.19%) was higher than other solvents. These results indicated that increasing the extraction yield correlates to the chemical compounds of the passion fruit seed. The maximum yield was found in compounds with intermediate polarity. This could be due to the increased solubility of moderate polarity in ethyl acetate. Extraction is the primary method of obtaining and separating phytochemicals from plant material referred to Laura Gonzalez et al. (2019). The effectivity of extraction is affected by the chemical nature of phytochemicals, extraction method, sample particle size, solvent, and the presence of interfering substances. The extraction yield depends on the solvents used, pH, temperature, and extraction time. The same solvent and composition of the sample are known as the most important parameters.
Antioxidant activity
The antioxidant activity of each extract using the DPPH method is shown in Figure 2. The most potent antioxidant activity was methanolic extract with an IC50 value of 71.67 µg/mL, followed by acetone at 147.29 µg/mL, chloroform at 147.65 µg/mL, ethyl acetate with 158.66 µg/mL, n-hexane extract with 962.5 µg/mL, respectively.
 |
Figure 2: The IC50 value of PSO. |
The antioxidant activity of PSO is a potential source of antioxidants from natural oil. However, the activity is lower than quercetin as standard with IC50 3.67 µg/mL. In 2011, Ferreira et. al. reported the antioxidant activity of PSO extracted by petroleum ether with EC50 > 1000 µg/mL. Gonzalez et. al. (2019), reported antioxidants using the DPPH method and found IC50 was 82.81 TEAC mmol/100 g. This finding showed that the higher activity of PSO extracted by methanol has higher antioxidant activity than other solvents. The activity has a direct correlation with phenolic content in methanol. This was different from acetone extract with lower antioxidants compared to methanol extract.
Total Phenolic and Flavonoid Content
Table 1. shows the TPC of the extracts determined by the FC technique. TPC values were calculated using the calibration curve y = 0.0047x + 0.123 (R2=0.9894), where x represents absorbance and concentration of a gallic acid solution (mg/mL) reported as mg GAE/g. The TFC of the extracts is shown in Table 2. All extracts were measured by aluminum chloride method and rutin as a standard.
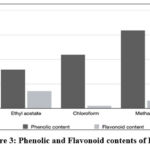 |
Figure 3: Phenolic and Flavonoid contents of PSO |
Table 2: Phenolic and Flavonoid contents of PSO
|
Extract |
Phenolic content (mgGAE/g extract) |
Flavonoid content (mgRE/g extract) |
|
Acetone |
193.60 |
23.08 |
|
Ethyl acetate |
79.00 |
35.40 |
|
Chloroform |
109.80 |
5.48 |
|
Methanol |
158.90 |
15.89 |
|
N-hexane |
26.10 |
21.00 |
Note:
RE = Rutin Equivalent
GAE = Gallic Acid Equivalent
The results showed that the phenolic compounds were mainly dissolved in acetone and methanol, and the flavonoids were dissolved in ethyl acetate. This could be because the water extract contains more non-phenolic components like carbohydrates and terpenes than other extracts. It can also be caused by the complicated synthesis of some phenolic compounds, which can have more phenolic groups or a larger molecular weight than phenol in water extract. Previous reports indicated that the main compounds were fatty acids, phenols, and carotenoids.1,4,7,8,18
The result showed that ethyl acetate with 35.40 mgRE/g extract had the highest flavonoid content. It was observed that the medium solvent polarity was more effective in TFC extraction. The effectivity of the ethyl acetate solvent was followed by the acetone extract, n-hexane, methanol, and chloroform, respectively. Gonzalez, et., al. (2019), reported that PSO contains 5.32 gRE/100 g samples.
Conclusion
We conclude that PSO has the potential as an antioxidant and source of phenolic and flavonoid constituents. Methanolic extract of passion seed has the most potent antioxidant activity with an IC50 value of 71.67 µg/mL.
Conflict of Interest
There is no conflict of interest
Acknowledgement
The authors thank Universitas Muslim Indonesia for their financial support.
References
- Chóez-Guaranda, I., Ortega, A., Miranda, M., & Manzano, P. Chemical composition of essential oils of Passiflora edulis f. flavicarpa agroindustrial waste. Emirates Journal of Food and Agriculture., 2017; 29 (6): 458–462. https://doi.org/10.9755/ejfa.2016-10-1542
CrossRef - Charissa, M., Djajadisastra, J. and Elya, B. Uji aktivitas antioksidan dan penghambatan tirosinase serta uji manfaat gel Extract kulit batang taya (Nauclea subdita) terhadap kulit. Jurnal Kefarmasian Indonesia., 2016; 6 (2): 98-107.
CrossRef - De Paula, R. C. M., Soares, A. G., & Freitas, S. P. Volatile compounds in passion fruit seed oil (Passiflora setacea BRS Pérola do Cerrado and Passiflora alata BRS Doce Mel). Chemical Engineering Transactions, 2015; 44: 103–108. https://doi.org/10.3303/CET1544018
- Ferreira, B. S., De Almeida, C. G., Faza, L. P., De Almeida, A., Diniz, C. G., Da Silva, V. L., Grazul, R. M., & Le Hyaric, M. Comparative properties of amazonian oils obtained by different extraction methods. Molecules, 2011; 16 (7): 5874–5885. https://doi.org/10.3390/molecules16075875
CrossRef - Huda, S. M. N., Wiraguna, AAGP., dan Pangkahila, W.. Krim Extract Biji Markisa (Passiflora edulis) Sama Efektifnya dengan Krim Hidrokuinon 4% dalam Menghambat Peningkatan Jumlah Melanin pada Kulit Marmut 88 Jantan (Cavia porcelus) yang Dipapar Sinar UV-B. Jurnal Biomedik (JBM), 2017; 9 (1): 1-6.
CrossRef - Karsinah, Hutabarat R.C dan Manshur A. Buah Eksotik Kaya Manfaat. Balai Litbang Pertanian, Kementrian Pertanian, Iptek hortikultura. Aripan Solok: Balitbu, 2010.
- Laura Gonzalez, Andree Ál Varez, Elizabeth Murillo, Carlos Guerra, & Jonh Mendez. Potential Uses of the Peel and Seed of Passiflora edulis Sims F. Edulis (Gulupa) From Its Chemical Characterization, Antioxidant, and Antihypertensive Functionalities. Asian Journal of Pharmaceutical and Clinical Research, 2019; 12 (10): 104–112. https://doi.org/10.22159/ajpcr.2019.v12i10.33828
CrossRef - Lima-Neto, A. B. M., Marques, M. M. M., Mendes, F. N. P., Vieira, Í. G. P., Diniz, D. B., & Guedes, M. I. F. Antioxidant activity and physicochemical analysis of passion fruit (Passiflora glandulosa Cav.) pulp native to Cariri region. Acta Scientiarum – Biological Sciences, 2017; 39 (4): 417–422. https://doi.org/10.4025/actascibiolsci.v39i4.34045.
CrossRef - Lucarini, M., Durazzo, A., Raffo, A., Giovannini, A., & Kiefer, J. Passion Fruit (Passiflora sp.) Seed Oil. Fruit Oils: Chemistry and Functionality, 2019; 577–603. https://doi.org/10.1007/978-3-030-12473-1_29
CrossRef - Malacrida, C.R. and Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Brazilian Archives of Biology and Technology, 2012; 55 (1): 127-134.
CrossRef - Mamede, A. M. G. N., Soares, A. G., Oliveira, E. J., & Farah, A. Volatile Composition of Sweet Passion Fruit (Passiflora alata Curtis). Journal of Chemistry, 2017. https://doi.org/10.1155/2017/3497216
CrossRef - Ngginak, J., Rupidara, A. and Daud, Y. Analisis Kandungan Vitamin C dari Extract Buah Ara (Ficus carica L) dan Markisa Hutan (Passiflora foetida L). Jurnal Sains dan Edukasi Sains, 2019; 2(2): 54-59.
CrossRef - Nguyen. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chemistry Central Journal, 2016; 4–9
CrossRef - Nugroho, A.E, Malik, A, Pramono, S. Total Phenolic and Flavonoid Contents, and in vitro Antihypertension Activity of Purified Extract of Indonesian Cashew Leaves (Anacardium occidentale L.). IFRJ; 2012; 20(1): 299-305.
- Pereira, M.G., Maciel, G.M., Haminiuk, C.W.I., Bach, F., Hamerski, F., de Paula Scheer, A. and Corazza, M.L. Effect of extraction process on composition, antioxidant and antibacterial activity of oil from yellow passion fruit (Passiflora edulis Var. Flavicarpa) seeds. Waste and Biomass Valorization, 2019; 10 (9): 2611-2625.
CrossRef - Promono, A. A. dan Rustam, E. Perubahan Kondisi Fisik, Fisiologis, dan Biokimia Benih Michelia champaca pada Berbagai Tingkat Kemasakan. Pros. Sem. Nas. Masy. Biodiv. Indon. 2017; 3 (3): 368-375.
CrossRef - Silva, R.M., Plácido, G.R., Silva, M.A.P.D., Castro, C.F.D.S., Lima, M.S. and Caliari, M. Chemical characterization of passion fruit (Passiflora edulis f. flavicarpa) seeds. African journal of biotechnology, 2015; 14 (14): 1230-1233.
CrossRef - Surlehan, H. F., Noor Azman, N. A., Zakaria, R., & Mohd Amin, N. A. Extraction of oil from passion fruit seeds using surfactant-assisted aqueous extraction. Food Research, 2019; 3(4): 348–356. https://doi.org/10.26656/fr.2017.3(4).146
CrossRef - Woolery-Lloyd H, Kammer JN. Treatment of Hyperpigmentation. Semin Cutan Med Surg. NewYork. 2011; 30 (3): 171-175.
CrossRef - Zeraik, M. L., Pereira, C. A., Zuin, V. G., & Yariwake, J. H. Maracujá: um alimento funcional. Revista Brasileira de farmacognosia, 2010; 20 (3): 459-471.
CrossRef







