Vanishree H Shivakumar1, Anand S. Tegginamani1, Nurhayati Mohamad Zain2, Avita Shanti Rath1 and Ahmad Termizi Bin Zamzuri1
1 Faculty of Dentistry, SEGi University, Malaysia.
2Faculty of Dentistry, Universiti Teknologi MARA, Malaysia.
Corresponding Author E-mail: vanishreeshivakumar@segi.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2678
Abstract
Introduction: Dental caries is a frequent chronic infectious condition in the general population. Streptococcus mutans and Lactobacillus acidophilus play a significant part in the cause of dental caries. Various antimicrobials have been tried to prevent these microorganisms. Traditional herbal medicine extracted from plants has been employed as a remedy. Among them, tea leaf extract showed promising antimicrobial properties against infections. Aims: The current study was designed to assess the antibacterial activity of various types of tea extract against cariogenic microorganisms. Methods: S. mutans and L. acidophilus were grown in this in-vitro experimental study and maintained in their respective agars. Three different concentrations of 250 mg/ml aqueous, 250 mg/ml ethanolic, and 250 mg/ml aqueous with sugar solutions were prepared from Japanese green tea, Oolong Chinese tea, and Sabah black tea. Kirby-Bauer disc diffusion method was used to assess the antibacterial activity of tea extracts. As positive and negative control groups, 0.2% chlorhexidine and 1% DMSO were employed. The zone of inhibition was determined in millimetres following a 24-hour incubation period at 37 °C. Results: Japanese green tea at its three different concentrations exhibited significantly higher mean zones of inhibition of 18.33 mm, 27.70 mm, and 18.96 mm, respectively, against S. mutans at p<0.001 compared to L. acidophilus. In contrast, chlorhexidine showed 27.53 mm and 19.39 mm for S. mutans and L. acidophilus, respectively. Conclusion: Japanese green tea demonstrated superior antibacterial activity at its ethanolic concentration compared to other concentrations and the other two types of tea.
Keywords
Antimicrobial; Dental caries; L. acidophilus; S. mutans
Download this article as:| Copy the following to cite this article: Shivakumar V. H, Tegginamani A. S, Zain N. M, Rath A. S, Zamzuri A. T. B. I. Antimicrobial Efficiency of Different forms of Tea extract (Camellia sinensis) against Cariogenic Pathogens. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Shivakumar V. H, Tegginamani A. S, Zain N. M, Rath A. S, Zamzuri A. T. B. I. Antimicrobial Efficiency of Different forms of Tea extract (Camellia sinensis) against Cariogenic Pathogens. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3CXguC4 |
Introduction
Dental caries is one of the most severe infectious conditions of the oral cavity, caused by the interaction of teeth, dietary variables, and oral flora. Streptococcus mutans and Lactobacillus acidophilus are believed to be the main etiological agents,1 as they metabolise sucrose to promote plaque biofilm development and adherence. Furthermore, in dental plaque, dietary carbohydrates breakdown occurs by the bacteria into lactic acid, which causes localised demineralisation and subsequently develops dental caries.2 Despite flossing and brushing being plaque-control procedures that aid in limiting the spread of oral microorganisms, it is still difficult to remove plaque from the oral cavity’s isolated areas. As a result, the antimicrobial agent’s application is justified to restrict the growth of cariogenic microbes and prevent dental caries. Some antimicrobial drugs, such as chlorhexidine, fluoride-based solutions, cetyl pyridinium chloride, and triclosan, have been investigated against oral microorganisms.1,3 The most popular chemical agent of preference for controlling plaque is chlorhexidine. It can function long after application due to its capacity to bond with hard and soft tissues in the mouth. However, long-term chlorhexidine use has been linked to adverse effects, including brown staining of teeth and restorative materials, taste modification, enhanced supragingival calculus maturation, ulceration of oral mucosa, and parotid enlargement.4
Although many antibacterial medicines have been suggested for preventing dental caries, they result in the death of healthy or normal oral flora. It subsequently causes abnormal variations in the oral environment, leading to the development of resistant organisms and secondary infections.5 Plants have been used as a medicine source since ancient times. Due to their low frequency of significant side effects, reported effectiveness, and inexpensive cost, herbal medicines from various plant components are gaining popularity.
According to the WHO, 80% of people worldwide still use conventional medicine. Among them tea is one of the most popular medicinal herbs and the second-most-drank beverage in the world. It also has significant cultural and economic values in many different nations.6
Tea leaves can be processed into three different types of tea: unfermented green tea, semi-fermented oolong tea, and fermented black tea.7 20% of green tea, less than 2% of oolong tea, and 78% of black tea are produced and used worldwide, and the remaining tea varieties are less common.8 Although black tea is mainly considered in Western nations and oolong tea is well-known in the Chinese province of Fujian, green tea is more prevalent among Asians.9 These teas’ anti-inflammatory, antibacterial, antioxidant, thermogenic, and anticarcinogenic qualities are well documented. Furthermore, due to their potential as antioxidants, they have been shown to lower obesity, cancer, and the risk of coronary heart disease.10,11 Considering their effectiveness, the present study was designed to assess the antibacterial activity of various types of tea extract against cariogenic microorganisms.
Materials and Methods
The current in vitro experimental investigation was conducted at the Faculty of Dentistry, SEGi University. It is part of an internally funded collaboration with Universiti Teknologi MARA, Malaysia (SEGiEC/StR/FOD/41/2021-2022). The G Power software has been employed for calculating the sample size. Using a 95% confidence interval and a study power of 80%, the sample size was calculated.
Preparation and culturing of microorganisms
The bacterial strains of Streptococcus mutans 20523, and Lactobacillus acidophilus DSM was purchased from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). S. mutans was subcultured on Brain Heart Infusion (BHI) agar and L. acidophilus on De Man, Rogosa, and Sharpe (MRS) agar, and incubation was done for 24 hours at 37oc. Before the experiment, three to five colonies from an overnight culture were suspended in saline (0.85% sodium chloride) and adjusted to match the turbidity of a McFarland 0.5 standard. This standard bacterial suspension was further used for the disc diffusion assay.
Preparation of tea extracts with different concentrations
For the experiment, four different types of tea were obtained from organic tea stores in Malaysia. Group 1: Japanese green tea from Shizuoka, Japan; Group 2: Oolong Chinese tea from Taiwan (SNC Teatime Sdn. Bhd.); and Group 3: Sabah black tea from Malaysia (Desa Tea Sdn. Bhd.). Three different concentrations of individual tea types were prepared for the experiment. Concentration:1–250 mg/ml aqueous solution, Concentration:2–250 mg/ml ethanolic solution, and Concentration:3–250 mg/ml with an aqueous sugar solution.
Aqueous extracts
Tea extracts were prepared by adding 20g of tea powder to 200 ml of boiled distilled water. The mixture was allowed to brew for ten minutes. The solution was then filtered through sterile Whatman No. 1 filter paper to get its extract. The infusion was concentrated to a fifth of the volume on a rotary evaporator, frozen, and lyophilised. The lyophilised infusion was redissolved in water to obtain a stock solution of 250 mg/ml of aqueous extracts. For extract with sugar, 5.5% (weight/volume) sugar was added during the brewing stage to obtain 250 mg/ml of aqueous with sugar extracts.12
Ethanolic extracts
20g of tea powder was soaked in 200 ml of ethanol (95%). The mixtures were then stored at room temperature for 24 hours in a securely sealed conical flask, shielded from sunlight. The mixtures were carefully stirred with sterile glass rods multiple times each day. The resulting mixes were run through Whatman No. 1 filter sheets for filtration to remove the ethanol, the extracted liquids were subjected to rotational evaporation. 13
The resulting semisolid extracts were freeze-dried for 24 hours at −60°C after being maintained overnight at −80°C in the freezer. Then, until further usage, the extracts were kept in a refrigerator at 4°C in an airtight container.
Disc diffusion method
The Kirby-Bauer disc diffusion method was used to determine the antibacterial activity of tea extracts.14 The tea extracts were redissolved in 1% DMSO to obtain a 250 mg/ml concentration and sterilised through a Millipore filter (0.22 µm). Blank antimicrobial susceptibility discs (Oxoid ™) 6 mm in diameter were used to load the 20 µl of tea extracts.
The standard drug, 0.12% chlorhexidine (CHX), was used as a positive control. A negative control with a disc of 1% DMSO was used. The standard bacterial suspension (0.5 McFarland) was applied evenly to the surface of their respective agars using a sterile cotton swab soaked in the suspension. After that, the discs loaded with various concentrations of tea extracts with positive and negative controls were placed on the surface of the agar plate. The incubation of plates was done for 24 hours at 37oc. Following incubation, a digital calliper determined the zone of inhibition’s diameter in millimetres.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) for Windows, Version 22.0, was released in 2013 to perform statistical analyses. Armonk, NY: IBM Corp. was used. The descriptive analysis includes the Zone of Inhibition (ZOI) expression in mm for different organisms in terms of mean and standard deviation for each group.
To compare the mean zone of inhibition between S. mutans and L. acidophilus in different concentrations of each group, a student-paired t-test was used. The level of significance was set at P<0.05.
Results
The mean ZOI in 250 mg/ml of aqueous solution for S. mutans in Group 1 was significantly higher [18.33 ± 0.12] as compared to L. acidophilus [9.41 ± 0.15], and a significant difference was seen at p<0.001. Likewise, the mean ZOI for S. mutans in Group 2 was significantly higher [16.44 ± 0.32] as compared to L. acidophilus [9.40 ± 0.21] and was statistically significant at p<0.001. Lastly, the mean ZOI for S. Mutans in Group 3 was significantly higher [13.26 ± 0.11] as compared to L. acidophilus [10.26 ± 0.13] and showed a significant difference at p<0.001 (Table.1, graph 1, and figures 1 and 2).
In 250 mg/ml of ethanolic solution for S. mutans, the mean ZOI in Group 1 was significantly higher [27.50 ± 0.07] as compared to L. acidophilus [14.93 ± 0.22], and the difference was significant at p<0.001. Similarly, the mean ZOI for S. mutans in Group 2 was significantly higher [27.34 ± 0.15] as compared to L. acidophilus [16.41 ± 0.15], and the difference was statistically significant at p<0.001. Whereas the mean ZOI for S. mutans in Group 3 was significantly higher [26.70 ± 0.15] as compared to L. acidophilus [14.94 ± 0.13], a statistically significant difference was seen at p<0.001 (Table.2, graph 2, and figures 1 and 2).
The mean ZOI in 250 mg/ml of sugar solution for S. mutans in Group 1 was significantly higher [18.56 ± 0.24] as compared to L. acidophilus [10.09 ± 0.36], and the significant difference was at p<0.001. Similarly, the mean ZOI for S. mutans in Group 2 was significantly higher [18.96 ± 0.28] as compared to L. acidophilus [9.49 ± 0.08] and showed a significant difference at p<0.001. Lastly, the mean ZOI for S. mutans in Group 3 was significantly higher [12.37 ± 0.07] as compared to L. acidophilus [10.32 ± 0.16] and showed a statistically significant difference at p<0.001 (Table 3, Graph 3, and Figures 1 and 2).
Table 1: Comparison of mean ZOI in 250 mg/ml of aqueous solution b/w L. acidophilus and S. mutans in each group using Student Paired t Test.
|
Groups |
Organism |
N |
Mean |
SD |
Mean Diff |
p-value |
|
Group 1 |
L. acidophilus |
9 |
9.41 |
0.15 |
-8.92 |
<0.001* |
|
S. mutans |
9 |
18.33 |
0.12 |
|||
|
Group 2 |
L. acidophilus |
9 |
9.40 |
0.21 |
-7.04 |
<0.001* |
|
S. mutans |
9 |
16.44 |
0.32 |
|||
|
Group 3 |
L. acidophilus |
9 |
10.26 |
0.13 |
-3.00 |
<0.001* |
|
S. mutans |
9 |
13.26 |
0.11 |
Table 2: Comparison of mean ZOI in 250 mg/ml of Ethanolic solution b/w L. acidophilus and S. mutans in each group using Student Paired t Test.
|
Groups |
Organism |
N |
Mean |
SD |
Mean Diff |
p-value |
|
Group 1 |
L. acidophilus |
9 |
14.93 |
0.22 |
-12.57 |
<0.001* |
|
S. mutans |
9 |
27.50 |
0.07 |
|||
|
Group 2 |
L. acidophilus |
9 |
16.41 |
0.15 |
-10.93 |
<0.001* |
|
S. mutans |
9 |
27.34 |
0.15 |
|||
|
Group 3 |
L. acidophilus |
9 |
14.94 |
0.13 |
-11.76 |
<0.001* |
|
S. mutans |
9 |
26.70 |
0.15 |
Table 3: Comparison of mean ZOI in 250 mg/ml of Sugar solution b/w L. acidophilus and S. mutans in each group using Student Paired t Test
|
Groups |
Organism |
N |
Mean |
SD |
Mean Diff |
p-value |
|
Group 1 |
L. acidophilus |
9 |
10.09 |
0.36 |
-8.47 |
<0.001* |
|
S. mutans |
9 |
18.56 |
0.24 |
|||
|
Group 2 |
L. acidophilus |
9 |
9.49 |
0.08 |
-9.47 |
<0.001* |
|
S. mutans |
9 |
18.96 |
0.28 |
|||
|
Group 3 |
L. acidophilus |
9 |
10.32 |
0.16 |
-2.05 |
<0.001* |
|
S. mutans |
9 |
12.37 |
0.07 |
Note: * – Statistically Significant
Group 1 – Japanese green Tea, Group 2 –Chinese Oolong Tea & Group 3 –Sabah Black Tea
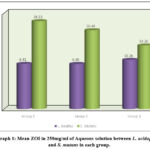 |
Graph 1: Mean ZOI in 250mg/ml of Aqueous solution between L. acidophilus and S. mutans in each group. |
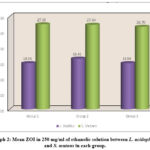 |
Graph 2: Mean ZOI in 250 mg/ml of ethanolic solution between L. acidophilus and S. mutans in each group. |
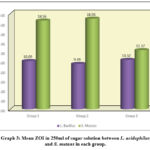 |
Graph 3: Mean ZOI in 250ml of sugar solution between L. acidophilus and S. mutans in each group. |
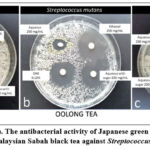 |
Figure 1: (a, b, and c). The antibacterial activity of Japanese green tea, Chinese oolong tea and Malaysian Sabah black tea against Streptococcus mutans. |
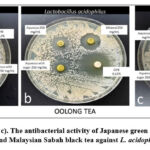 |
Figure 2: (a, b, and c). The antibacterial activity of Japanese green tea, Chinese oolong tea and Malaysian Sabah black tea against L. acidophilus. |
Discussion
The antimicrobial activity of S. mutans and L. acidophilus was investigated in the current study. Regardless of the diversity of oral microflora, there is strong evidence that these two organisms are the main causative agents in the pathogenesis of dental caries.
The present study compared the effectiveness of aqueous, ethanol, and aqueous sugar extracts of green tea, oolong tea, and black tea on S. mutans and L. acidophilus. Aqueous extract of green tea showed a better zone of inhibition of 18.33 mm for S. mutans compared to oolong (16.44mm) and black tea (13.26 mm) than against L. acidophilus. This contrasts with a study in which an aqueous oolong tea extract showed better inhibition against S. mutans than chlorhexidine.15 This might be because oolong tea contains more phytochemicals than green and black tea. Similar results were found in the study in which both aqueous and ethanolic oolong tea extracts exhibited higher inhibitory effects than black and green tea.7
In the present study, the meanzone of inhibition withan ethanolic solution of green tea for S. mutans was significantly higher (27.50mm) as compared to L. acidophilus (14.93 ± 0.22). Similarly, the mean ZOI was significantly higher than other oolong and black tea extract solutions. Similar to the present study results, a more significant zone of inhibition was seen with 300 μg/ml ethanolic extract of green tea for S. mutans (18.33mm) compared to L. acidophilus (12.67mm) and chlorhexidine. The inhibitory zones increased with increasing amounts of ethanol extracts made from green tea.1 It is attributed to the fact that the green tea polyphenols consist of significant catechins such as epigallocatechin (EGC), epicatechin EG, EGC gallate (EGCG). These major catechins disrupt the cell membrane and prevent the supercoiling of DNA, which leads to bacterial destruction. EGC interacts with proteins and distorts their tertiary structure.2 Green tea’s catechins are proven to be potential anti-cariogenic agents, which can reduce the microbial load in the oral cavity.16 In another study with black tea, the antibacterial activity was found to be minimal, and it has been mentioned that black tea’s antibacterial activity is due to the presence of polyphenols, catechins, gallic acid, and theaflavins that are modified during the fermentation of the leaves. The chemical structures of both black and green teas differ despite the presence of equal quantities of flavonoids. The production of black tea in the fermentation process involves the conversion of these flavonoids and catechins into arubigins and aflavins.15,17
Although tea has a lot of benefits for human health, catechins and tannins are present in the phenolic groups, and brownish discoloration of teeth has been seen. This is due to the fact that a lower pH causes tooth discoloration. A study demonstrated that the increased level of temperature, concentration, and frequency of drinking black tea causes discoloration of the teeth.18 However, factors such as decaffeinated or blended tea, variations in geographic location, climate, and soil may be responsible for the variations in the inhibitory effects of green tea with its chemical elements and flavonoid concentration. Compared to green and black teas, oolong tea’s features have only been the subject of a few investigations against various organisms. In the current study, oolong tea demonstrated a significantly higher ZOI of 18.96 mm for its aqueous sugar solution as compared to green tea of 18.56mm and black tea of 12.37 mm against S. mutans than L. acidophilus. Despite both organisms being treated with an aqueous sugar solution, S. mutans showed better inhibition as compared to L. acidophilus. This might be because oolong tea contains more phytochemicals than green or black tea. It has been proposed that the monomeric polyphenols synergistic impact is responsible for their antibacterial actions.19 Oolong tea comprises more alkaloids, tannins, saponins, and flavonoids with various activities. It is well known that alkaloids prevent microbial cell proliferation. Tannins and flavonoids, which have strong iron-binding abilities and reduce bacterial adhesion, are known to have anti-glucosyltransferase action and to inhibit bacterial adherence.20,21 On the other hand, 0.12% chlorhexidine was used as a positive control in the current study. It showed a significantly higher mean ZOI of 19.4 mm as compared to all other tea extracts against both S. mutans and L. acidophilus, as it has been proven to be an anti-cariogenic drug against various cariogenic organisms and is used as a mouth rinse.9 The study was constrained by the lack of an evaluation of the antibacterial activities of these tea extracts at their minimal inhibitory and minimal bacterial concentrations. The research will be continued further for the same. It is recommended to evaluate the various effects of different kinds of tea on oral health when consumed at different doses and temperatures. In addition, further comparative clinical studies on the biological properties of different Camellia sinensis species from various non-geographic areas and seasons are needed.
Clinical significance
Besides the many medicinal benefits of green tea, it might be recommended as an alternative to other black teas owing to its proven antibacterial activity. The health of the oral tissues could be well preserved by incorporating the polyphenols present in green tea into oral health products. Future clinical research should also consider assessing the antibacterial effects of various concentrations of these tea extracts in combination with other antimicrobial medications to determine whether there are any synergistic effects on various cariogenic pathogens related to oral health. The findings from these upcoming investigations may lead to the development of novel, natural formulations with low side effects potentially beneficial in preventing oral infections. These formulations might supplement commercially available antimicrobial medications.
Conclusion
Japanese green tea demonstrated superior antibacterial activity at a concentration of 250 mg/mL ethanolic solution against S. mutans and L. acidophilus when compared to its other concentrations and the concentrations of the other two types of tea.
Acknowledgement
We would like to thank the Research and Innovation Management Centre, SEGi University Kota Damansara, Malaysia for the support. Special gratitude to Professor Dato’ Dr. Mohamed Ibrahim Abu Hassan, Former Dean Faculty of Dentistry, Universiti Teknologi MARA for his support.
Conflict of Interest
The authors report no conflict of interest.
Funding Sources
This study was financially supported by SEGiIRF/2018-3/FoD-13/77 grant.
References
- Anita P, Sivasamy S, Madan Kumar PD, Balan IN, Ethiraj S. In vitro antibacterial activity of Camellia sinensis extract against cariogenic microorganisms. J Basic Clin Pharm. 2014;6(1):35-39.
- Namita P, Mukesh R, and Vijay K. Camellia sinensis (Green tea): A review. Glob J Pharmacol. 2012;6 (2):52‑9.
- Parikh‑Das AM, Sharma NC, Du Q, and Charles CA. Superiority of essential oils versus 0.075% CPC‑containing mouth rinse: a two‑week randomised clinical trial. J Clin Dent. 2013;24(3):94-99.
- Parwani SR, Parwani RN, Chitnis PJ, Dadlani HP, and Prasad SV. Comparative evaluation of anti‑plaque efficacy of herbal and 0.2% Chlorhexidine gluconate mouthwash in a 4‑day plaque re‑growth study. J Indian Soc Periodontol. 2013;17(1):72-77.
- Segura-Egea JJ, Gould K, Şen BH, Jonasson P, Cotti E, Mazzoni A, et al. Antibiotics in Endodontics: a review. Int Endod J. 2017;50(12):1169-1184.
- Tahani B, Sabzian R. Effect of Camellia sinensis plant on decreasing the level of halitosis: A systematic review. Dent Res J (Isfahan). 2018;15(6):379-384.
- George DE, Shetty R, Shetty PJ, and Gomes LA. An In vitro Study to Compare the Effect of Different Types of Tea with Chlorhexidine on Streptococcus mutans. J Clin Diagn Res. 2017;11(9): ZC05-ZC07.
- Yang CS, and Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130 (10):2409-2412.
- Wang, S., Zeng, T., Zhao, S., Zhu, Y., Feng, C., Zhan, J., et al. Multifunctional health-promoting effects of oolong tea and its products. Food Sci. Hum. Wellness. 2022;11(3):512-523.
- Vyas T, Nagi R, Bhatia A, and Bains SK. Therapeutic effects of green tea as an antioxidant on oral health-A review. J Family Med Prim Care. 2021;10(11):3998-4001.
- Teixeira AM, and Sousa C. A Review on the Biological Activity of Camellia Species. Molecules. 2021;26(8):2178.
- Voina, C, Delean, A, Muresan, A, Valeanu, M, Mazilu Moldovan, A Popescu., et al. Antimicrobial Activity and the Effect of Green Tea Experimental Gels on Teeth Surfaces. Coatings. 2020;10(6):537.
- Truong D, Nguyen DH, Ta NT, Bui AV, Do T, and Nguyen H. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti‑inflammatory activities of severinia buxifolia. J Food Qual. 2019; 1:1‑9.
- Bauer AW, Kirby WM, Sherris JC, and Turck M. Antibiotic susceptibility testing by a standardised single disk method. Am J Clin Pathol. 1966; 45(4):493‑496.
- Subramaniam P, Eswara U, and Maheshwar Reddy KR. Effect of different types of tea on Streptococcus mutans: an in vitro study. Indian J Dent Res. 2012;23(1):43-48.
- Hattarki SA, Bogar C, and Bhat KG. Green tea catechins showed antibacterial activity on streptococcus mutans -An in vitro study. Indian J Dent Res. 2021;32(2):226-229.
- Rasheed A, Haider M. Antibacterial activity of Camellia sinensis extracts against dental caries. Arch Pharm Res. 1998; 21(3): 348-352.
- Hidayah A, Redjeki S, and Gunawan, A H. The Influence of Temperature to Change Email Color after Application of Black Tea (Camellia sinensis L.). J Int Dent Med Res. 2018; 11(3): 988-993.
- Sasaki H, Matsumoto M, Tanaka T, Maeda M, Nakai M, Hamada S, and Ooshima T. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Res. 2004;38(1):2-8.
- Kamrani YY, Amanlou M, Esmaeelian B, Bidhendi SM, and Jamei MS. Inhibitory effects of a flavonoid-rich extract of Pistacia vera hull on growth and acid production of bacteria involved in dental plaque. Int J Pharmacol. 2007; 3:219-226.
- Kolliyavar B, Shettar L, and Thakur S. Chlorhexidine: The gold standard mouth wash. J Pharm Biomed Sci. 2016;06(02):106–109.








