Essie Octiara1* , Chintya Pricilia Meliala2
, Chintya Pricilia Meliala2  and Lisdayanti Sikumbang2
and Lisdayanti Sikumbang2
1Departement of Pediatric Dentistry, Faculty of Dentistry, Universitas Sumatera Utara, Medan, Indonesia.
2 Faculty of Dentistry, Universitas Sumatera Utara, Medan, Indonesia.
Corresponding Author E-mail: essie.octiara@usu.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2670
Abstract
Dental caries and root canal infections are common dental and oral diseases due to the dominance of S.mutans and E.faecalis. Despite not having any economic value and becoming a waste, durian peel is claimed as an antibacterial. This study aims to analyze durian peel ethanol extract antibacterial activity against S.mutans and E.faecalis based on the inhibition zone diameter, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC). This type of research is a laboratory experiment using Post-test Only Control Group Design. There were 7 concentrations of durian peel ethanol extract (50%, 25%, 12.5%, 6.25%, 3.125%, 1.56%, and 0.78%), both positive and negative control with three times repetitions. Moreover, the bacterial suspensions used were S.mutans ATCC 25175 and E.faecalis ATCC 29212. Data analysis used One Way Anova, while data that were not normally distributed used Kruskal-Wallis. Post Hoc analysis used LSD for normal data and Mann-Whitney for abnormal data. Durian peel ethanol extract shows the antibacterial activity of S.mutans with MIC at a concentration of 0.78%, while against E.faecalis is 12.5%. The higher the concentration, the larger the inhibition zone formed. The best-tested concentration of ethanol extract from durian peel is 6.25-50% to inhibit the growth of S. mutans and 50% for E.faecalis. Durian peel ethanol extract can prevent dental caries since it is antibacterial against S.mutans and E.faecalis.
Keywords
Durio zibethinus Murr.; E.faecalis; MBC; MIC; S.mutans
Download this article as:| Copy the following to cite this article: Octiara E, Meliala C. P, Sikumbang L. Antibacterial Activity of Durian Peel Ethanol Extract (Durio zibethinus Murr.) Against Streptococcus mutans and Enterococcus faecalis. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Octiara E, Meliala C. P, Sikumbang L. Antibacterial Activity of Durian Peel Ethanol Extract (Durio zibethinus Murr.) Against Streptococcus mutans and Enterococcus faecalis. Biomed Pharmacol J 2023;16(2). |
Introduction
An oral cavity is a place for various microorganisms to live as normal flora, which plays a role in human physiological development and defense. Components of these microorganisms can be pathogenic if the environment is disturbed or in places that are not supposed to be, so it can cause infection in the oral cavity.1 S.mutans and E.faecalis are bacteria that are often associated with infections of the oral cavity.2-3
S.mutans play an essential role in the formation of dental caries. Caries cause damage to the hard tissues of the teeth. If ignored, it can be an entry point for microorganisms related to endodontic infection, leading to dental pulp infection and ends up with causing pulp necrosis.2 E.faecalis is a microorganism that is often associated with endodontic infections. E.faecalis is a persistent bacteria in root canal infections and can be a major cause of endodontic treatment failure.4-5 Besides S.mutans and E.faecalis, S.aureus is a gram-positive bacteria that cause caries and endodontic infections.6
The use of natural materials in dentistry has been conducted since ancient times and their use has increased significantly. Natural ingredients have been widely used as antibiotics, analgesics, anti-inflammatories, Etc. Furthermore, the limitations of most commercial drugs, such as cytotoxicity, have resulted in the emergence of new treatment developments using natural ingredients, attracting a lot of attention.4 Based on the Regulation of the Minister of Health of the Republic of Indonesia Number 88 of 2013 concerning the master plan for the development of traditional medicines raw materials,7 it is expected to be able to utilize the wealth of Indonesia’s biological resources and the wealth of traditional health to be used as medicines to replace conventional medicines.
Durian (Durio zibhetinus Murr.) is an Indonesian fruit plant in great demand. Based on its structure, it consists of three parts; durian seeds (5-15%), durian flesh (20-30%), and durian skin (60-75%), which is the most significant part of durian and yet considered to have no economic value. Therefore, it is not utilized and ends up being waste which can cause environmental problems. Arlofa8 reported that based on the results of phytochemical tests, durian peel contains tannins, alkaloids, triterpenoids, and flavonoids that can act as antibacterial ingredients. Pratiwi et al.9 reported that durian peel extract with a 1.1% concentration of ethyl acetate solvent could inhibit the growth of P.acnes, which causes acne with an inhibition zone diameter of 11.17 mm. In addition, Arlofa et al.10 reported that a 1% concentration of durian peel ethanol extract could inhibit the growth of E.coli and S.aureus.
Based on previous research,8-10 durian peel extract has the potential to be antibacterial. Research on using durian peel extract against bacteria that cause oral infections is still limited, so further research on S.mutans and E.faecalis is needed. This research is a preliminary study to analyze the antibacterial activity of durian peel ethanol extract against S.mutans and E.faecalis based on the inhibition zone diameter, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC).
Materials and Methods
Ethical clearance has been approved by the Health Research Ethics Committee of the University Sumatera Utara with No. 238/KEPK/USU/2022. The ethanol extract of durian peel was made at the Research and Development Laboratory of Medicinal Plants (ASPETRI) Medan, and then continued with antibacterial activity testing conducted at the Microbiology Laboratory of the Faculty of Pharmacy, Universitas Sumatra Utara.
This study was a laboratory experiment using Post-test Only Control Group Design. The sample size was calculated using the Federer formula, and three replications were obtained. The test group consisted of: The concentrations of the ethanol extract of durian peel (50%, 25%, 12.5%, 6.25%, 3.125%, 1.56%, 0.78%), a positive control that was treated with chlorhexidine 0.2%, and a negative control which is given treatment by giving Dimethyl Sulfoxide (DMSO) solvent. The bacteria, S.mutans ATCC 25175 and E.faecalis ATCC 29212 used in this study, were obtained from Microbiologics USA.
Making durian peel ethanol extract
The durian peel of Durio zibethinus Murr. species from Sorkam District, Central Tapanuli Regency, North Sumatera, Indonesia, was collected as much as 500 grams. The durian peel was cleaned, cut into small pieces, and dried in a drying cabinet to dry (for 7 days). The durian peel was mashed to obtain simplicia. Then, the simplicia was mixed with 1 liter of 70% ethanol using the maceration method in a closed vessel and soaked for 24 hours while stirring occasionally. After 24 hours, the bath was filtered with cotton and filter paper to obtain the filtrate and dregs. The dregs were soaked again with 500 ml of 70% ethanol for 24 hours, stirred occasionally and filtered again. The filtrate was combined and evaporated to obtain a thick extract. The thick extract from durian skin was then diluted with DMSO to obtain durian peel extract with concentrations of 50%, 25%, 12.5%, 6.25%, 3.125%, 1.56%, and 0.78%.
Antibacterial activity test of durian peel ethanol extract
The durian peel extract (Durio zibethinus Murr.) was first subjected to phytochemical screening before testing the growth of S.mutans and E.faecalis to determine the secondary metabolites contained in the durian peel ethanol extract which can act as an antibacterial. Two different species of bacteria were used in this study, including S.mutans (ATCC 25175) and E.faecalis (ATCC 29212). The bacterial suspension is done by taking the bacteria stock cultures (S.mutans, E.faecalis) and suspended in 10 mL of 0.9% NaCl solution to obtain the same turbidity as McFarland 0.5.
In a petri dish containing Mueller Hinton Agar (HiMedia®, India) media and the suspension of bacteria, 25 µL of different extract concentrations (50 %, 25%, 12.5%, 6.25%, 3.125%, 1.56%, 0.78%) were impregnated into 6 mm diameter of sterile blank discs. DMSO-loaded discs were then used as a negative control. The positive control are chlorhexidine 0.2% discs. Chlorhexidine 0.2% is used because it is a potent antiseptic for chemical plaque control in the oral cavity.11
The discs were applied to the agar using sterile tweezers. The discs were placed on four discs per plate to avoid overlapping the inhibition zones. Petri dishes were incubated for 24 hours at 37°C. After 24 hours, observations were conducted by measuring the inhibition zone diameter, which is the diameter of the bacterial colony-free (clear zone) formed around the paper disc using a caliper. The lowest concentration capable of providing inhibition is defined as the MIC.
The MBC is determined by the streaking method from the clear zone formed at the diffusion method and then subculturing on Plate Count Agar (HiMedia®, India) media to calculate the number of colonies and the percentage of reduction. Furthermore, the clear zone formed from each concentration was streaked using a sterile cotton swab and then it was allowed to stand for 10 minutes in a test tube containing Mueller Hinton Broth (HiMedia®, India) media. After that, 1 ml of MHB was taken from each tube and transferred to a sterile petri dish, then PCA media was added and homogenized. All petri dishes were incubated at 37°C for 24 hours. After 24 hours, the number of colonies formed in all petri dishes was calculated by using a colony counter machine (Interscience Scan®300Interlab, France). The lowest concentration, which can reduce 98 – 99% of bacterial colonies of the initial number of bacteria, is considered MBC.12-13
The data obtained from all examinations were processed and analyzed using the Statistical Product Service Solutions version 20 for Windows. Data analysis used One Way Anova, while data that were not normally distributed used Kruskal-Wallis. In addition, post Hoc analysis used LSD for normal data and Mann-Whitney for abnormal data.
Results
The phytochemical screening of durian peel ethanol extract shows that tannins, flavonoids, triterpenoids, and saponins act as antibacterial. Moreover, forming a clear zone around the paper disc indicates that the ethanol extract of the durian peel in this study can inhibit the bacteria tested (Figure 1). The results of measuring the average diameter of the inhibition zone formed from the ethanol extract of durian peel against the bacteria S.mutans and E.faecalis can be seen in Table 1.
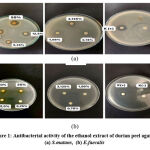 |
Figure 1: Antibacterial activity of the ethanol extract of durian peel against (a) S.mutans, (b) E.faecalis |
Table 1: Antibacterial activity of durian peel ethanol extract against S.mutans and E.faecalis
| Test Group | Diameter of inhibition zones (mm) | |||
| S.mutans | E.faecalis | |||
| Mean±SD | p-value | Mean±SD | p-value | |
| Extract 50% | 13.42±0.55 | 0.002* | 10.26±0.72 | 0.001* |
| Extract 25% | 12.75±0.61 | 9.50±0.26 | ||
| Extract 12.5% | 12.35±0.35 | 8.70±0.10 | ||
| Extract 6.25% | 11.53±0.25 | 0 | ||
| Extract 3.125% | 11.28±0.43 | 0 | ||
| Extract 1.56% | 10.75±0.70 | 0 | ||
| Extract 0.78% | 9.95±1.06 | 0 | ||
| Positive control | 18.48±0.14 | 14.30±0.34 | ||
| Negative control | 0 | 0 | ||
*Significant (p≤0.05); Kruskal-Wallis Test
Based on the results of the measurement of the diameter of the inhibition zone against S.mutans, the ethanol extract of durian peel shows the ability to inhibit the growth of S.mutans starting at a concentration of 0.78%, while against E.faecalis the bacterial inhibitory activity starting from the extract with a concentration of 12.5%. Therefore, these two concentrations are determined as the MIC value of the durian peel ethanol extract for each bacteria tested (Table 1).
The results of the calculation of the number of colonies formed from the bacterial subculture procedure formed from the clear zone in this study can be seen in Tables 2 and 3. MBC is determined from the lowest extract concentration, reducing as much as 98-99% of the initial bacterial colonies (negative control). The percentage reduction is calculated by the formula:13-14
Where:
A : Number of bacterial colonies in each test group
B : Number of initial bacterial colonies (negative control)
Table 2: The MBC of durian peel ethanol extract against S.mutans.
| Test Group | Number of Colonies
(Mean±SD) (CFU/ml) |
Differences
(B-A) |
% Reduction | p-value |
| Extract 50% | 312±11.93 | 3717 | 92.26% | 0.000* |
| Extract 25% | 683±44.91 | 3346 | 83.05% | |
| Extract 12.5% | 1132±47.23 | 2897 | 71.90% | |
| Extract 6.25% | 1388±90.74 | 2641 | 62.55% | |
| Extract 3.125% | 1893±43.00 | 2136 | 53.02% | |
| Extract 1.56% | 2115±30.14 | 1914 | 47.50% | |
| Extract 0.78% | 2735±102.48 | 1294 | 32.12% | |
| Positive control | 0 | 4029 | 100% | |
| Negative control | 4029±141.10 | 0 | 0% |
*Significant (p≤0.05); One Way Anova Test
Table 3: The MBC of durian peel ethanol extract against E.faecalis
| Test Group | Number of Colonies
(Mean±SD) (CFU/ml) |
Differences
(B-A) |
% Reduction | p-value |
| Extract 50% | 241.00±31.04 | 3347 | 93.28% | 0.002* |
| Extract 25% | 554.33±60.74 | 3033.67 | 84.55% | |
| Extract 12.5% | 742.33±202.05 | 2845.67 | 79.31% | |
| Extract 6.25% | 1111.00±179.30 | 2477 | 69.04% | |
| Extract 3.125% | 1542.67±407.65 | 2045.33 | 57% | |
| Extract 1.56% | 1653.00±304.47 | 1935 | 53.93% | |
| Extract 0.78% | 2093.33±284.31 | 1494.67 | 41.66% | |
| Positive control | 0 | 3588 | 100% | |
| Negative control | 3588.00±278.14 | 0 | 0% |
*Significant (p≤0.05); Kruskal-Wallis Test
Based on the percentage reduction of durian peel extract on the number of S.mutans and E.faecalis colonies, none of the concentration groups tested can reduce the number of initial colonies by 98-99% other than the positive control group. The highest concentration of durian peel ethanol extract, which is 50% concentration against S.mutans bacteria, can reduce the number of initial colonies by as much as 92.26%, while against E.faecalis is 93.28%. All examination data, when tested statistically, show significant results. It means there is the ability to reduce the number of colonies from the ethanol extract of durian peel.
Discussion
The results of this study prove that the ethanol extract of durian peel has an antibacterial effect against S.mutans and E.faecalis bacteria. It is because durian peels contain compounds that have the potential as antibacterial. Furthermore, several studies8,14 have reported that durian peel contains tannins, alkaloids, flavonoids, saponins, and triterpenoids as antibacterial compounds. According to the results of the phytochemical screening, the researchers also found tannins, flavonoids, triterpenoids, and saponins. Tannins, the active ingredients with antibacterial properties, are the largest in durian peel, if they bind to the bacterial cell wall, they will be toxic to prevent bacterial growth. Moreover, flavonoids can form complex compounds against extracellular proteins, disrupting the integrity of the bacterial cell membrane so that the cell membrane is damaged. Alkaloids interfere with peptidoglycan constituent components in bacterial cells, so the cell wall layer is not fully formed and causes cell death. However, in this study, no alkaloid compounds are found in the durian peel ethanol extract. Triterpenoids cause damage to purines, which are the entrance and exit of compounds, so bacterial cells lack nutrients and experience cell death. Saponins break down or lyse bacterial walls and play a role in removing debris.14-15
S.mutans and E.faecalis are gram-positive bacteria with teichoic acid found in the peptidoglycan of the bacterial wall.16 This teichoic acid serves as an exit and entry of ions into and out of bacterial cells. One type of teichoic acid is lipoteichoic acid, which can bind to tannins, so bacterial growth is more easily inhibited.
If it is associated with the antibacterial activity criteria by David and Stout7, where the inhibition zone formed ≥ 20 mm is categorized as having very strong bacterial inhibition, 10 – 20 mm is categorized as having strong bacterial inhibition, 5-10 mm is categorized as having moderate bacterial inhibition and ≤5 mm are categorized as having weak bacterial inhibition. Therefore, the antibacterial activity of the ethanol extract of durian peel (Durio zibethinus Murr.) against S.mutans in this study is at a concentration of 0.78% with an average diameter of the inhibition zone of 9.95 mm which is considered a moderate bacterial inhibition while at concentrations of 1.56%, 3.125%, 6.25%, 12.5%, 25%, and 50% (inhibition zone diameter 13.42 mm) are in the strong category. However, for antibacterial activity against the growth of E.faecalis, concentrations of 12.5% (inhibition zone diameter 8.70 mm) and 25% have moderate bacterial inhibitory. In comparison, concentrations of 50% (inhibition zone diameter 10.26 mm) are strong bacterial inhibitory categories.
The results of the Post Hoc test show significant differences in the inhibition zone of S.mutans in the ethanol extract of durian peel with a concentration of 6.25% against 0.78%. Durian peel ethanol extract with a concentration of 6.25% is the lowest concentration extract as an antibacterial S.mutans with a strong antibacterial category. In E.faecalis, there are significant differences in inhibition zones in the ethanol extract of durian peel with a concentration of 50% against a concentration of 12.5%. Durian peel ethanol extract with a concentration of 50% is the lowest concentration extract as an antibacterial E.faecalis with a strong antibacterial category. Therefore, based on this study, it is recommended to use the ethanol extract of durian peel with a concentration of 6.25% as an antibacterial S.mutans and a concentration of 50% as an antibacterial E.faecalis.
This study is in line with research which had conducted by Permatasari et al.,17 who reported that durian peel extract with a concentration of 0.78% could inhibit the growth of bacteria, causing supragingival plaque with an inhibition zone diameter of 1.37 mm. Another study conducted by Safitri et al.18 stated that durian peel extract with 70% ethanol solvent could inhibit the growth of S.aureus with a minimum inhibitory value at a concentration of 10%. In addition, research conducted by Muawanah et al.19 reported that the ethanol extract of durian peel with 96% ethanol solvent could inhibit the growth of S.aureus at a concentration of 5% (inhibitory zone diameter 12 mm) and against E.coli at a concentration of 5% (11 mm inhibition zone diameter). The study’s results still show the activity of durian peel extract, which can inhibit the growth of bacteria even in different bacteria. Differences in methods, solvents, and types of bacteria can cause differences in the results of antibacterial effects with previous studies.
From the results of this study, the ethanol extract of durian peel can inhibit caries-causing bacteria, that are S.mutans and E.faecalis. Moreover, another study10,18,19 showed that durian peel extract could inhibit other caries-causing bacteria, S.aureus. Therefore, durian peel extract can be used as an alternative natural medicinal ingredient to prevent dental caries. The active ingredient of durian peel extract can be used in mouthwash, root canal irrigation, and topical application.
Conclusion
The MIC of the ethanol extract of durian peel (Durio zibethinus Murr.) against the growth of S.mutans is at a concentration of 0.78% with an average inhibition zone diameter of 9.95 mm and against E.faecalis is 12.5% with an average inhibition zone diameter of 8.70 mm. The MBC in this study has not been found among the concentrations of the tested extracts. Further research is needed to prove that the concentration of 6.25%-50% is antibacterial against S.mutans and 50% against E.faecalis.
Conflict of Interest
The author declares no conflict of interest.
Funding Source
There is no funding sources.
References
- Astuti P, Meilawaty Z. Efek antibakteri pasta gigi yang mengandung tea tree oil terhadap bakteri S. aureus, S. mutans dan S. viridans. Stomatognatic., 2013;10(3):121–124.
- Blancas B, Lanzagorta ML, Jiménez-Garcia LF, Lara R, Molinari JL, Fernández AM. Study of the ultrastructure of Enterococcus faecalis and Streptococcus mutans incubated with salivary antimicrobial peptides. Clin Exp Dent Res., 2021;7(3):365–375. doi:10.1002/cre2.430
CrossRef - Damayanti A, Kaswindiarti S. Perawatan pulpektomi non vital pada gigi desidui anterior maksila. JIKG.. 2017;1(1):58–63. doi:10.23917/jikg.v1i1.4159f
- Ramachandra JA, Nihal NK, Nagarathna C, Vora MS. Root canal irrigants in primary teeth. World J Dent., 2015;6(4):229–234. doi:10.5005/jp-journals-10015-1349
CrossRef - Hafizha, Suardita K, Pribadi N. Daya antibakteri ekstrak batang pisang ambon (Musa paradisiaca var. sapientum) terhadap pertumbuhan Enterococcus faecalis. Conserv Dent J., 2018;8(2):85. doi:10.20473/cdj.v8i2.2018.85-90
CrossRef - Al-Akwa AAY, Zabara AQMQ, Al-Shamahy HA, Al-labani MA, Al-Ghaffari KM, Al-Mortada AM, et al. Prevalence of Staphylococcus aureus in dental infections and the occurrence of MRSA in isolates. Univers J Pharm Res., 2020;5(2):23-27. doi:10.22270/ujpr.v5i2.384
CrossRef - Kementerian Kesehatan Republik Indonesia. Berita negara Republik Indonesia: Rencana induk pengembangan bahan baku obat tradisional. URL: https://www.kemhan.go.id/itjen/wp-content/uploads/migrasi/peraturan/88.pdf. Accesed July 22, 2022.
- Arlofa N. Uji kandungan senyawa fitokimia kulit durian sebagai bahan aktif pembuatan sabun. J Chemtech., 2015;1(1):18–22.
- Pratiwi MM, Kawuri R, Ardhana IPG. Potensi antibakteri limbah kulit durian (Durio zibethinus Murr.) terhadap Propionibacterium acnes penyebab jerawat. J Biol Udayana., 2019;23(1):8–15. doi:10.24843/jbiounud.2019.v23.i01.p02
CrossRef - Arlofa N, Ismiyati, Kosasih M, Fitriyah NH. Effectiveness of durian peel extract as a natural anti-bacterial agent. J Chem Eng Env., 2019;14(2):163–170. doi:10.23955/rkl.v14i2.14275
CrossRef - Paul J. Recent trends in irrigation in endodontics. Int J Curr Microbiol App Sci., 2014;3(12):941–952.
- Ashakirin SN, Tripathy M, Patil UK, Majeed ABA. Antimicrobial activity of essential oils: exploration on mechanism of bioactivity. Int J Pharm Sci Res., 2017;8(8):3187–3193. doi:10.13040/IJPSR.0975-8232.8(8).3187-93
CrossRef - Nasri, Harahap U, Silalahi J, Satria D. Antibacterial activity of lactic acid bacteria isolated from dengke naniura of carp (Cyprinus carpio) against diarrhea-causing pathogenic bacteria. Biodiversitas., 2021;22(8):3098–3104. doi: 10.13057/biodiv/d220802
CrossRef - Anggraeni EV, Anam K. Identifikasi kandungan kimia dan uji aktivitas antimikroba kulit durian (Durio zibethinus Murr.). J Kimia Sains dan Aplikasi., 2016;19(3):87–93. doi: 10.14710/jksa.19.3.87-93
CrossRef - Pratiwi AA, Syafnir L, Alhakimi TA. Penelusuran pustaka uji aktivitas ekstrak kulit buah durian (Durio zibethinus Murray) sebagai antibakteri terhadap bakteri Propionibacterium acnes dan bakteri Staphylococcus epidermidis. In: Prosiding Farmasi. 2021. p. 53–59. doi: 10.29313/.v7i1.26022
CrossRef - Azzahra F, Hayati M. Uji aktivitas ekstrak daun pegagan (Centella asiatica (L). urb) terhadap pertumbuhan S.mutans. B-Dent J., 2019;5(1):9–19. doi: 10.33854/jbd.v5i1.133
CrossRef - Permatasari RI, Krismariono A, Ulfah N. Daya hambat ekstrak kulit durian (Durio zibethinus Murray ) terhadap plak supragingiva. Periodontic., 2015;7(2):21–24.
- Safitri A. Uji aktivitas ekstrak etanol kulit durian (Durio zibethinus Murr.) terhadap bakteri Propionibacterium acnes dan Staphylococcus aureus. J Farm Udayana., 2020;9(2):66-71. doi: 10.24843/JFU.2020.v09.i02.p01
CrossRef - Muawanah N, Jaudah H, Ramadhanti TD. Pemanfaatan limbah kulit durian sebagai anti bakteri pada sabun transparan. In: Seminar Nasional Sains dan Teknologi. Jakarta: Fakultas Teknik Universitas Muhammadiyah Jakarta; 2019. p. 1–10.









