Manuscript accepted on :27 Jan 2023
Published online on: 18-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Carla Guerreiro
Second Review by: Dr. Amal Khalil
Final Approval by: Dr. Patorn Promchai
Anit Treesa Joy1 , Harish. M2*
, Harish. M2* and Rishad K. S1
and Rishad K. S1
1UniBiosys Biotech Research Labs, Sahaan Arcade, Cochin University Road (CUSAT Jn.), South Kalamassery, Cochin, Kerala India.
2SCMS School of Technology and Management, Biotechnology Division -SIBB R and D, S. Kalamassery, Kochi, Kerala, India
Corresponding Author E-mail: harishmadhav20@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2698
Abstract
Ginseng is a plant’s root of the Panax family that is characterized by the presence of ginsenosides. It is used as a traditional medicine for many years in East Asian regions generally as an adaptogenic medicine to make the body resistant to homeostasis and other adverse environmental factors. Inflammation and lipid signaling are intermixed modulators of homeostasis and immunity. Cyclooxygenase is a key enzyme in lipid signalling. The present study focused on the anti-inflammatory analysis of phytoconstituents of the ginseng plant against COX1 and COX2 genes. In this study we approached the study of the interaction of phytoconstituents of ginseng plant with COX-1 and COX-2 using an insilico approach. It is done in 2 main stages: docking between COX1 and COX2 with phytoconstituents of ginseng plant and the ADMET analysis. . The drug-likeness of phytoconstituents were predicted and the ADMET properties. Molecular docking studies were done using the Autodock server and MyPresto program to explore the binding pattern with COX-1 and COX-2. The result showed that phytoconstituents gallic acid and myricetin have high anti-inflammatory action due to the electrostatic force of attraction of COX1 and COX2. Quercetin, and apigenin due to high binding affinity due to the attraction of COX2, epicatechin, and chlorogenic acid on COX1. The phytoconstituents gallic acid, myricetin, apigenin, chlorogenic acid, epicatechin and quercetin can potentially be used as anti-inflammatory agents.
Keywords
Anti-Inflammatory Drugs; ADMET Analysis; Cyclooxygenases; Ginseng; Molecular Docking
Download this article as:| Copy the following to cite this article: Joy A. T, Harish M, Rishad K. S. Anti-Inflammatory Activity of Phytoconstituents of Ginseng Plant- Insilico Approach. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Joy A. T, Harish M, Rishad K. S. Anti-Inflammatory Activity of Phytoconstituents of Ginseng Plant- Insilico Approach. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3MbFhqd |
Introduction
Ginseng is an herbal traditional plant that is a short, slow-growing plant with fleshy roots having 11 different varieties. It is an herb that is having light-colored, cleft-shaped roots, long stems, and green oval-shaped leaves1. American ginseng (Panax quinquefolius) and Asian ginseng (Panax ginseng) are the most popular types of ginsengs. Other types are Korean ginseng and South China ginseng. Ginseng can be used as a component in health drinks, hair tonics, and cosmetic products. Ginseng can be also used as a possibly effective aid in lowering blood pressure and respiratory infections. In most cases, ginseng can be used as alternative medicine, other uses include breast cancer, fatigue, menopausal symptoms, memory loss, bleeding disorders, and digestive disorders.
The major bioactive compound produced by ginseng is a saponin with a structure of dimmerene terpenoid ie., ginsenosides 2. Ginsenosides are used to maintain stable blood pressure, mental stress reduction, and boost immune function3. The ginseng saponins can exert various pharmacological effects like anti-inflammatory, antiviral, cardiovascular activity, and immunomodulatory effects4. Polysaccharides present in ginseng have immunomodulation, anti-fatigue, antitumor, antiadhesive, antioxidant, antiulcer, hepatoprotective, hypoglycemic, and antihyperlipidemic activities5. The effects of remaining volatile and non-volatile components that are present in ginseng include anti-inflammatory, cardioprotective, neuroprotective, antiaging, anti-tumour, anti-coagulation, and treatment of diabetes mellites6.
According to Saleem et al., in Pharmacological analysis of Indian ginseng (Withania somnifera), withanolide a pharmacologically active steroidal lactone, an alkaloid isolated from the root of the plant is present. The principal withanolide extracted from the plant is withanolides A and D, found in India, which have antitumor, and cytotoxic properties. Withaferin A, an alkaloid extracted from the root of Withania somnifera, extracted and purified, exhibits anti-inflammatory activity by inhibiting NFкβ activity and targeting CYS 179 8. In addition to withanolide, Indian Ginseng contains other bioactive compounds such as glycosides, phytophenols, flavanoids, steroids, and phenols 9,10. Also, it is used in traditional medicine formulations as an antiinflammatory, adaptogenic, and antipyretic agent 11,12. W. somnifera has been shown to possess antiinflammatory properties in many animal models of inflammation like carrageenan-induced inflammation, and cotton pellet granuloma13. But no docking studies are still carried out for establishing the data.
Anti-inflammatory drugs can interact with the pathogenesis of inflammation seeking to provide patient comfort with a variety of actions such as non steroidal anti-inflammatory drugs, corticosteroids, colchimes, penicillamines, and immunosuppressive agents. The most difficult and essential step in drug discovery and development is to execute drug metabolism and pharmacokinetics (DMPK) studies, often referred to as ADMET14. In pharmacology, ADMET stands for “absorption, distribution, metabolism, excretion, and toxicity”.ADMET properties have a pertinent role in determining the effectiveness of clinical candidates that can act as good standard as a drug. Non-steroidal anti-inflammatory drugs can be used worldwide, used to treat pain resulting from the inflammatory process 15. The main mechanism of NSAIDs is the inhibition of COX action in a selectively in the production of thromboxane and prostaglandins which have side effects 16. Specific modifications of anti-inflammatory effects and side effects are associated with the existence of COX 1 and COX 2 genes 17. Thus, in this scenario, the present study focused on the interaction of phytoconstituents of ginseng plants other than ginsenosides with COX 1 and COX 2 genes using the insilico approach.
The main objective of the study is to examine the Inhibitory action of phytoconstituents against COX-1 and COX-2, an in silico approach, Drug-likeness prediction, and ADMET analysis of phytoconstituents of ginseng plant.
Methodology
Ligand molecule preparation
The ginseng plant’s phytoconstituent’s three-dimensional structure was retrieved from the National Library of Medicine PubChem in SDF format. It was converted using Open Babel GUI software to PDB format.
Preparation of the receptor molecule structure
The FASTA format of cyclooxygenase 1 (P23219) and cyclooxygenase 2 (P35354)was obtained from UniProt (https://www.uniprot.org). The FASTA formats were copied to the Swiss model and were searched for templates. The PDB structure of the receptor was downloaded. The repository of COX 1 was 6Y3C (Human COX-1 Crystal Structure)and COX2 was 4RRW.
ADMET and drug-likeness evaluation
The simplified molecular-input line-entry systems (SMILE) of phytoconstituents of plant were submitted to the SwissADME tool to evaluate molecular properties and in silico pharmacokinetic parameters18. The ADME predictions were computed for Log Kp of skin permeation value, blood-brain barrier permeability, cytochrome-P inhibitors, gastro-intestine absorption, and P-GP substrate. The toxicological endpoints and organ toxicities of the ligands like hepatotoxicity, immunotoxicity, carcinogenicity, cytotoxicity, mutagenicity, LD50and irritant properties were predicted using Osiris software and Pro Tox II.
Receptor-ligand docking
In-silico docking studies were performed using the AutoDock server (https://vina.scripps.edu/)19. The autodock result was opened in the MyPresto program. The desired ligand in the structure was selected and was run for the delta G value. The scores of dockings were documented and the poses were visualized. The hydrogen bonds and other interactions involved in docking and their respective amino acid positions and distances were evaluated by using chimera 1.5.3.
Results
The ADME/Toxicity analysis showed that the investigated phytoconstituents possessed several favourable drug-likeness properties (Table.1), ADME properties like blood-brain barrier permeability, P-glycoprotein gastrointestinal absorption, cytochrome-P inhibitor (Table.2), and toxicity properties (Table.3).
Table 1: Drug Likeness Prediction of Compounds
| Molecule | Formula | MW(g/m
ol) |
NR
B |
NH
A |
NH
D |
TPSA | iLOGP | LR |
| Catechin | C15H14O6 | 290.27 | 1 | 6 | 5 | 110.38 | 1.33 | 0 |
| Kaempferol | C15H10O6 | 286.24 | 1 | 6 | 4 | 111.13 | 1.70 | 0 |
| Apigenin | C15H10O5 | 270.24 | 1 | 5 | 3 | 90.90 | 1.89 | 0 |
| Quercetin | C15H10O7 | 302.24 | 1 | 7 | 5 | 131.36 | 1.63 | 0 |
| Myricetin | C15H10O8 | 318.24 | 1 | 8 | 6 | 151.59 | 1.08 | 1 |
| Resveratrol | C14H12O3 | 228.24 | 2 | 3 | 3 | 60.69 | 1.71 | 0 |
| Epicatechin | C22H18O10 | 442.37 | 4 | 10 | 7 | 177.14 | 1.70 | 1 |
| Protocatechuic acid | C7H6O4 | 154.12 | 1 | 4 | 3 | 77.76 | 0.66 | 0 |
| Ferulic acid | C10H10O4 | 194.18 | 3 | 4 | 2 | 66.76 | 1.62 | 0 |
| Cinnamic acid | C9H8O2 | 148.16 | 2 | 2 | 1 | 37.30 | 1.55 | 0 |
| Syringic acid | C9H10O5 | 198.17 | 3 | 5 | 2 | 75.99 | 1.54 | 0 |
| Caryophyllene | C15H24 | 204.35 | 0 | 0 | 0 | 0.00 | 3.29 | 1 |
| Falcarinol | C17H24O | 244.37 | 8 | 1 | 1 | 20.23 | 4.19 | 1 |
| Spathulenol | C15H24O | 220.35 | 0 | 1 | 1 | 20.23 | 3.04 | 0 |
| Menadione | C11H8O2 | 172.18 | 0 | 2 | 0 | 34.14 | 1.74 | 0 |
| Hydroxycinnamic acid | C9H8O3 | 164.16 | 2 | 3 | 2 | 57.53 | 0.95 | 0 |
| Caffeic acid | C9H8O4 | 180.16 | 2 | 4 | 3 | 77.76 | 0.97 | 0 |
| Chlorogenic acid | C16H18O9 | 354.31 | 5 | 9 | 6 | 164.75 | 0.87 | 1 |
| Gallic acid | C7H6O5 | 170.12 | 1 | 5 | 4 | 97.99 | 0.21 | 0 |
| Hydroxybenzoic acid | C7H6O3 | 138.12 | 1 | 3 | 2 | 57.53 | 0.85 | 0 |
| Vanillic acid | C8H8O4 | 168.15 | 2 | 4 | 2 | 66.76 | 1.40 | 0 |
| Falcarindiol | C17H24O2 | 260.37 | 8 | 2 | 2 | 40.46 | 4.01 | 0 |
| Stigmasterol | C29H48O | 412.69 | 5 | 1 | 1 | 20.23 | 5.01 | 1 |
| Sitosterol | C29H50O | 414.71 | 6 | 1 | 1 | 20.23 | 4.79 | 1 |
| Pectin | C6H10O7 | 194.14 | 1 | 7 | 5 | 127.45 | -0.19 | 0 |
| D galactose | C6H12O6 | 180.16 | 1 | 6 | 5 | 110.38 | 0.24 | 0 |
| L rhamnose | C6H12O5 | 164.16 | 0 | 5 | 4 | 90.15 | 0.66 | 0 |
(MW: Molecular weight, NHD: Number of Hydrogen Donor, NRB: Number of rotatable bonds, NHA: Number of Hydrogen Acceptor, TPSA: Total polar surface area, LR: Lipinski rule of five violations).
Table 2: Adme Predictions of Selected Phytoconstituents
| Molecule | Formula | |||||||||
| Catechin | C15H14O6 | HIGH | NO | YES | NO | NO | NO | NO | NO | -7.82 |
| Kaempferol | C15H10O6 | HIGH | NO | NO | YES | NO | NO | YES | YES | -6.70 |
| Apigenin | C15H10O5 | HIGH | NO | NO | YES | NO | NO | YES | YES | -5.80 |
| Quercetin | C15H10O7 | HIGH | NO | NO | YES | NO | NO | YES | YES | -7.05 |
| Myricetin | C15H10O8 | LOW | NO | NO | YES | NO | NO | NO | YES | -7.40 |
| Resveratrol | C14H12O3 | HIGH | YES | NO | YES | NO | YES | NO | YES | -5.47 |
| Epicatechin | C22H18O10 | LOW | NO | NO | NO | NO | NO | NO | NO | -7.91 |
| Protocatechuic acid | C7H6O4 | HIGH | NO | NO | NO | NO | NO | NO | YES | -6.42 |
| Ferulic acid | C10H10O4 | HIGH | YES | NO | NO | NO | NO | NO | NO | -6.41 |
| Cinnamic acid | C9H8O2 | HIGH | YES | NO | NO | NO | NO | NO | NO | -5.69 |
| Syringic acid | C9H10O5 | HIGH | NO | NO | NO | NO | NO | NO | NO | -6.77 |
| Caryophyllene | C15H24 | LOW | NO | NO | NO | YES | YES | NO | NO | -4.44 |
| Falcarinol | C17H24O | HIGH | YES | NO | YES | NO | YES | NO | NO | -3.89 |
| Spathulenol | C15H24O | HIGH | YES | NO | NO | YES | NO | NO | NO | -5.44 |
| Menadione | C11H8O2 | HIGH | YES | NO | YES | NO | NO | NO | NO | -5.79 |
| Hydroxycinnamic acid | C9H8O3 | HIGH | YES | NO | NO | NO | NO | NO | NO | -6.26 |
| Caffeic acid | C9H8O4 | HIGH | NO | NO | NO | NO | NO | NO | NO | -6.58 |
| Chlorogenic acid | C16H18O9 | LOW | NO | NO | NO | NO | NO | NO | NO | -8.76 |
| Gallic acid | C7H6O5 | HIGH | NO | NO | NO | NO | NO | NO | YES | -6.84 |
| Hydroxybenzoic acid | C7H6O3 | HIGH | YES | NO | NO | NO | NO | NO | NO | -6.02 |
| Vanillic acid | C8H8O4 | HIGH | NO | NO | NO | NO | NO | NO | NO | -6.31 |
| Falcarindiol | C17H24O2 | HIGH | YES | NO | NO | NO | YES | NO | NO | -4.78 |
| Stigmasterol | C29H48O | LOW | NO | NO | NO | NO | YES | NO | NO | -2.74 |
| Sitosterol | C29H50O | LOW | NO | NO | NO | NO | NO | NO | NO | -2.20 |
| Pectin | C6H10O7 | LOW | NO | YES | NO | NO | NO | NO | NO | -9.15 |
| D Galactose | C6H12O6 | LOW | NO | YES | NO | NO | NO | NO | NO | -9.70 |
| L rhamnose | C6H12O5 | HIGH | NO | YES | NO | NO | NO | NO | NO | -8.79 |
(Log Kp, skin permeation value; GI, gastro-intestinal; BBB-p, blood-brain barrier permeability; PGP, P-glycoprotein; CYP, cytochrome-P).
 |
Table 3: Prediction Of Toxicity Of Phytoconstituents |
The role of COX genes in inflammation has been studied for decades. Although anti-inflammatory drugs are available for treatment, the standard drugs are reported to have adverse effects on long time usage. Studies are being done to find improved anti-inflammatory drug quality.
The binding energy of myricetin to COX 1 is -6.13kcal/mol. Epicatechin requires -5.22kcal/mol to bind to COX-1. The binding energy required by the chlorogenic acid to bind to COX-1 was -4.54kcal/mol while gallic acid requires -2.82kcal/mol to bind to COX-1. The interaction of Myricetin against COX-1 resulted in two hydrogen bonds with amino acid residues CYS47 with a bond length of 2.98Ao and GLN461 with a bond length of 3.36Ao. Two amino acid residues GLN465 with a bond length of 2.22Ao and CYS41 with a bond length of 2.21Aowere bound to epicatechin. Interaction of chlorogenic acid against COX-1 resulted in two hydrogen bonds with amino acid residues GLY45 with a bond length of 2.9Ao and CYS47 with a bond length of 1.6Ao while in the case of gallic acid, two amino acid residues SER530 with a bond length of 2.20Aoand 3.10Ao were bound to COX-1. (Figure.1).
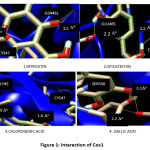 |
Figure 1: Interaction of Cox1 |
The binding energy required by the gallic acid to bind to COX-2 was -5.2kcal/mol. The binding energy of quercetin to COX-2 was -6.19kcal/mol while myricetin required -4.94kcal/mol. Apigenin required 4.64kcal/mol to bind to COX-2. The interaction of gallic acid against COX-2 resulted in two hydrogen bonds with amino acid residues GLN241 with a bond length of 3.40Ao and ARG333 with a bond length of 3.24Ao. Three amino acid residues GLN461 with a bond length of 3.51Ao, TYR130 with a bond length of 2.48Ao, and CYS159 with a bond length of 77.49Aowere bound to quercetin. Myricetin was bound to amino acid residues CYS159 with a bond length of 57.59Aoand ARG44 with a bond length of 2.65Aowhile apigenin is bound to amino acid residues ASP229 with a bond length of 2.49Aoand CYS42 with a bond length of 28.20Ao(Figure 2).
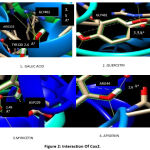 |
Figure 2: Interaction of Cox2. |
Discussions
In this study, investigated molecules possessed several favorable drug-likeness properties (Table 1). The molecular weights of all the phytoconstituents were found to be less than 500 and thus these molecules can easily be transported, distributed, and immersed. The number of hydrogen bonds acceptors except for epicatechin and the number of hydrogen bond donors for myricetin, epicatechin, and chlorogenic acid were by Lipinski’s rule of five, which describes it should be less than 10 and 5 respectively. Thus it can be predicted that according to Lipinski’s rule of five these compounds are likely to be orally active. TPSA values were higher than the default range. Except for stigmasterol, log P values of all the compounds were found to be less than 5 and are in acceptance of Lipinski’s rule of five, suggesting permeability across cell membrane justifying that they can be orally used (TABLE 1).
All the phytoconstituents of the ginseng plant except myricetin, epicatechin, caryophyllene,
chlorogenic acid, stigmasterol, sitosterol, pectin, and galactose showed high gastrointestinal (GI) absorption. All the phytoconstituents except resveratrol, ferulic acid, cinnamic acid, falcarinol, spathulenol, menadione, hydroxycinnamic acid, hydroxybenzoic acid, and falcarindiol showed no BBB permeability showing undefined penetration across the Central Nervous System, hence lessening the side effects linked to CNS. All the compounds except catechin, pectin, galactose, and rhamnose are not Pgp-substrate. Kaempferol, apigenin, quercetin, myricetin, resveratrol, menadione and falcarinol were predicted as CYP1A2 inhibitors. Resveratrol, caryophyllene, falcarinol, falcarindiol, and stigmasterol were reported as CYP2C9 inhibitors. Caryophyllene and spathulenol were CYP2C19 inhibitors. Kaempferol, apigenin and quercetin were CPY2D6 inhibitors while gallic acid, kaempferol, apigenin, quercetin, myricetin, resveratrol, and protocatechuic acid were CPY3A4 inhibitors. Except for pectin and galactose, all other compounds are rather a skin permeable, revealing relatively good permeability values (Table 2).
The toxicity of a chemical can be measured in terms of toxicity endpoints, and toxicity parameters
such as mutagenicity, carcinogenicity, mutagenicity, and many other endpoints. It can be further measured both quantitatively and qualitatively. ProTox, a web server published in 2014 for rodent oral toxicity. The web server classifies the different levels of toxicities such as carcinotoxicity, cytotoxicity, toxicological endpoints (such as mutagenicity, and immunotoxicity), oral toxicity, organ toxicity (hepatotoxicity), toxicological pathways (AOPs), and toxicity targets, which provide a deep idea about the possible molecular mechanisms and its toxic responses20.
Only cinnamic acid showed hepatotoxicity. Quercetin, myricetin, protocatechuic acid, hydroxycinnamic acid, caffeic acid, and gallic acid were shown to have carcinogenic properties. Ferulic acid, caryophyllene, chlorogenic acid, stigmasterol, and sitosterol were predicted to have immunotoxic properties. Phytoconstituents of ginseng plant except for quercetin, epicatechin, ferulic acid, caryophyllene, spathulenol, falcarinol, chlorogenic acid, gallic acid, hydroxybenzoic acid, stigmasterol, sitosterol, and pectin show irritant property. Quercetin, myricetin, and menadione showed mutagenic properties. No compounds exhibited cytotoxic effects. (Table 3).
In the above study, the phytoconstituents gallic acid and myricetin showed high anti-inflammatory action against cells among various phytoconstituents in the ginseng plant and it is evident that these phytoconstituents showed high binding affinity may be due to the electrostatic force of attraction on COX1 and COX2 genes. Even though the other phytoconstituents that show anti-inflammatory action are quercetin and apigenin showed high binding energy due to the attraction on COX2 genes and epicatechin and chlorogenic acid on COX1 genes.
According to Cheo et al.,2006, radical scavenging of gallic acid – linolenic acid was compared to those of gallic acid and ascorbic acid and tyrosine inhibition effect. Gallic acid did not show tyrosinase activity and the result of the COX inhibition effect showed that gallic acid have higher selectivity in COX1 inhibition, thus it could be used as a functional reagent for anti-inflammatory effects. According to Ratna et al.,2020, based on the study of molecular docking of chlorogenic acid and its isomers in atherosclerosis, it is reported that the strongest bond is found in the docking result of chlorogenic acid with COX2 and the smallest binding energy value was also obtained from the result of COX2 docking with chlorogenic acid compared to its isomers, so that it has the potential as an anti-inflammatory agent. According to Peng and Yun, 2017, based on the study on the anti-inflammatory effect of myricetin and other plant compounds in neonatal rats, it is reported that Myricetin, and fisetin formed strong bonds and interactions with the ligand-binding sites of TNF-α, COX-1 and COX-2 and can suppress the enzymes responsible for inflammation. Therefore, myricetin, and fisetin can be used as alternatives to existing NSAIDs and an anti-inflammatory agents. According to Jee et al.,2007, the study on the Inhibition of Cyclooxygenase-2 Expression, Adhesion of Monocytes to Human Umbilical Vein Endothelial Cells, and Expression of Cellular Adhesion Molecules on apigenin, it is showed that apigenin inhibited Nitric Oxide production and COX-2 expression, and collagenase activity involved in rheumatoid arthritis. These inhibitory activities of apigenin on the inflammatory responses suggest that it may be useful as an alternative medicine to help treat inflammatory symptoms. According to Subramaniya et al.,2017, based on the study of Differential cytotoxic activity of Quercetin on colonic cancer cells depending on ROS generation through COX-2 expression, it is reported that increased generation of reactive oxygen species (ROS) was observed only in Quercetin treated cells, which is due to overexpression of COX–2, as COX-2 silencing inhibited Quercetin induced apoptosis and ROS generation. Insilico analysis provided evidence that Quercetin could partially inhibit COX-2 enzyme by binding to subunit A which has peroxidase activity and serves as a source of ROS. Quercetin depends on COX-2-dependent ROS generation that induces apoptosis and inhibits cell survival, thus quercetin and its derivatives can be used as an anti-inflammatory agent. According to Rajesh et al.,2019, in the case of epicatechin, they can effectively inhibit the LPS inhibited the release of TNF alpha, IL6, NO and PGE2 production mediated by the LPS-stimulated macrophages suggesting that the epicatechin has anti-inflammatory properties.
According to Lestari, it is studied that, according to molecular docking studies, aspirin showed higher binding affinity towards COX2 and the presence of a hydrogen bond of ARG120, which is important for COX2 interaction. Similarly, in this study, the myricetin and gallic acid showed higher binding energy and there is the presence of hydrogen bonds of ARG44 and ARG333 was observed, which indicates the interaction of COX2. According to Lestari, Aspirin had higher effectiveness as an inhibitor of COX1 and COX2. The interaction of COX2 with aspirin formed 1 hydrogen bond with GLN529 and the interaction with COX1 formed hydrogen with SER, GLU, ARG, and TRP. In this study, it is indicated that the quercetin interact with COX2 forming a hydrogen bond with GLN461 and the gallic acid and epicatechin interacts with COX1 and a formed hydrogen bond with SER 530 and GLU461 respectively. In the process of competitive binding, it is found that alginate is more easily bound to COX2 due to smaller binding energy, thus it is considered as an excellent potential as an inhibitor of COX227. In this study it is thought that apigenin more easily interacts with COX2 because of the smaller binding energy compared to quercetin, myricetin and gallic acid; likewise, chlorogenic acid easily interact with COX1 because of the smaller binding energy compared to myricetin and epicatechin, thus these are considered as excellent potential for the interaction of COX1 and COX2. According to Lestari, alginate interacts with COX1 formed a hydrogen bond with GLN374 thus alginate is considered one of the inhibitors of COX1; Similarly, in this current study, it is considered that the myricetin bind to GLN461 by hydrogen bond, thus it is also considered as one of the inhibitors of COX1.
Conclusion
Ginseng has been widely used as a traditional medicine for many years in East Asian Regions generally as a stimulant, and adaptogenic medicine. Though all the parts such as fruit, stem, leaves, flowers, and roots of the ginseng plants have medicinal value, the roots are used most extensively for medicinal purposes, especially for their remedial properties. The phytoconstituents of the ginseng plant have several properties such as anti-inflammatory, antibacterial, antiviral, anti-diabetic, etc. To evaluate the efficiency and safety of ginseng plant consumption, more and more ginseng clinical trials have been conducted recently. From the modern research studies, ginseng possesses a variety of bioactive compounds including ginsenosides, polysaccharides, and peptides that have been used effectively for neuroprotective, Immunomodulatory, antiinflammatory, anti-diabetic, antiglycaemic, and anticancer effects. In the present study, the phytoconstituents present in the ginseng plant were studied for their anti-inflammatory action against COX genes. Precisely, the in-silico approach showed that the ginseng plant and its phytoconstituents show anti-inflammatory properties against COX genes. The phytoconstituents like gallic acid, myricetin, apigenin, epicatechin, chlorogenic acid, and quercetin can potentially be used as anti-inflammatory agents. Even though the phytoconstituent of ginseng plant myricetin and apigenin show irritation, further more studies are needed to conclude the anti-inflammatory property of these compounds. The interaction of these phytoconstituents may provide useful insight for efforts to design new NSAIDs with novel properties providing an important field for future research on drug development.
Acknowledgment
The authors extend their sincere thanks to Bineesh Skaria of UniBiosys Biotech Research Labs for providing all the infrastructure for doing the research work.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding sources.
Reference
- Debra MacDonald S, Terstege DJ, Tasker RA. Standardized ginseng extract G115® potentiates the antidepressant-like properties of fluoxetine in the forced swim test. ActaNeuropsychiatr2021;33(3):141-147.
CrossRef - Hao, D.; Gu, X.; Xiao, P.; Peng, Y. Chemical and biological research of Clematis medicinal resources. Sci. Bull. 2013, 58, 1120–1129
CrossRef - Sellami M, Slimeni O, Pokrywka A. Herbal medicine for sports. a review. J Int Soc Sports Nutr. 2018; 15: 14
CrossRef - Alice Sze Tsai Wong, Ze Yu Shi and Jin Zhang Zheng. Chemical structure and pharmacological profile of ginseng saponins. 2019.
- Sang M, Bae BS, Park HW, Ahn NG, Cho BG, Cho YL, Kwak YS. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng 2015;39(4):384-91.
CrossRef - Hanbing Liu, Xiaoyan Lu, Yang Hu, Xiaohui Fan. chemical constituents of Panax ginseng and P.notoginseng. explain why they differ in therapeutic efficiency. 2020.
- Saleem S, Muhammad G, Hussain MA, Altaf M Bukhari SNA. Withaniasomnifera L. Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci 2020; 23:1501-1526.
- Khare CP. Indian Medicinal Plants–An Illustrated Dictionary First Indian Reprint. Springer (India), Pvt.Ltd.New Delhi ; 2007.p.717-718
- Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. High catechin concentrations detectedinWithaniasomnifera (ashwagandha) by high performance liquid chromatography analysis. Altr Med 2011; 11:65-69.
CrossRef - Divisha R, Ranganathan V, Vijayakaran K, Elamaran A, Senthil KP. Quantifying phytophenols in Andrographis paniculata and Withaniasomnifera leaf extract. J Pharam 2018; 7:477-479.
CrossRef - Kalra R, Kaushik N. Withaniasomnifera (Linn.) Dunal.A review of chemical and pharmacological diversity. Phytochem Rev 2017; 16:953-987.
CrossRef - Das S, Saraf A, Sharma D, Sohal JK. Qualitative screnning of bioactive secondary metabolites present in Withaniasomnifera and Rauwolfia serpentina root and stem extract with pharmacological importance. Int J Res Ana Rev 2019; 6:69-74.
- Rasool M and Varalakshmi P. Immunomodulatory role of Withaniasomnifera root powder of experimental induced inflammation; An in-vivo and in-vitro Vas Pharma 2006;44:406-410.
CrossRef - Mishra S, and Dahima R. In vitro ADME studies of TUG-891, a GPR-120 inhibitor using SWISS ADME predictor. Journal of Drug Delivery and Therapeutics2019; 9(2): 366-369.
- Lima A.S, Alvim H.G.O. Review on non-steroid anti-inflammatory: Acetylsalicylic acid. Rev. Inc. Ciente. Ext 2018; 1:169–174.
- Sandoval A.C, Fernandes D.R, Silva E.A, Terra Júnior A.T. The indiscriminated use of non-steroid anti-inflammatory (NSAID). Rev. Cient. FAEMA 2017; 8: 165–176.
CrossRef - Hayashi S, Sumi Y, Ueno N, Murase A, Takada J. Discovery of a novel COX-2 inhibitor as an orally potent antipyretic and anti-inflammatory drug: Design, synthesis, and structure-activity relationship. Biochem. Pharmacol. 2011; 82: 755–768.
CrossRef - Lipinski C. A, Lombardo F, Dominy B. W, and Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews1997; 23(1-3): 3-25.
CrossRef - Trott O, and Olson A. J.AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry2010; 31(2): 455-461.
CrossRef - Banerjee P, Eckert A. O, Schrey A. K, and Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic acids research2018; 46(W1): W257-W263.
CrossRef - Cheo-Run Jo,III-Yun Jeong,Na- Young Lee, Kwan-Soo Kim,Myung Woo Byun.Synthesis of a novel compound from gallic acid and linolenic acid and its biological functions, Food Science and Biotechnology 15 (2): 2006; 317-320.
- Ratna Nurarifa, Devi Era Rachmawati, Dwi Utari.Molecular Docking Chlorogenic Acid and Isomer Compound as Cyclooxygenase-2 (COX-2) Inhibitor in Atherosclerosis, Journal of Smart Bioprospecting and Technology (Volume 02): 2020; 2714-7894.
- Peng Guo and Yun-Yun Feng. Anti-inflammatory effects of kaempferol, myricetin, fisetin and ibuprofen in neonatal rats,Tropical Journal of Pharmaceutical Research: 2017; 16 (8): 1819-1826.
CrossRef - Je-Hyuk Lee, Hong Yu Zhou 1, So Yean Cho 1, Yeong Shik Kim 1, Yong Soo Lee 2, and Choon Sik Jeong. Anti-inflammatory Mechanisms of Apigenin: Inhibition of Cyclooxygenase-2 Expression, Adhesion of Monocytes to Human Umbilical Vein Endothelial Cells, and Expression of Cellular Adhesion Molecules, Arch Pharm Res Vol 30: 2007; 10 : 1318-1327.
CrossRef - Subramaniya Bharathi Raja, Vijayabharathi Rajendiran, Nirmal Kumar Kasinathan, Amrithalakshmi P, Sivaramakrishnan Venkatabalasubramanian, Malliga Raman Murali, Halagowder Devaraj, Sivasithamparam Niranjali Devaraj. Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression, Food and Chemical Toxicology, Volume 106, Part A: 2017; 92-106.
CrossRef - Rajesh Shukla, Vikas Pandey, Gautam P. Vadnere, Santram Lodhi. Role of Flavonoids in Management of Inflammatory Disorders, Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases (Second Edition): 2019; 18: 293-322.
CrossRef - Lestari Dewi. In Silico Analysis of the Potential of the Active Compounds Fucoidan and Alginate Derived from Sargassum Sp. as Inhibitors of COX-1 and COX-2.2016; 70(3): 172-176.
CrossRef







