S Naveen1 , P Elango2
, P Elango2 and Ramya S3*
and Ramya S3*
1Pharmacology Sri Ramachandra Medical College and Research Institute, Sri Ramachandra Institute of Higher Education and Research (SRIHER) Chennai, India.
2Department of Pharmacology, Bharath Medical College and Hospital (BMCH), Bhaarath Institute of Higher Education and Research (BIHER, Bhaarath University) Chennai, India.
3Department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Sri Ramachandra Institute of Higher Education and Research (SRIHER) Chennai, India.
Corresponding Author E-mail: ramya.s@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2685
Abstract
Background: Low back pain is a very common musculoskeletal symptom with multifactorial aetiology. Aims and objectives: Our study aimed at comparing the efficacy, safety, and tolerability of tapentadol versus tramadol in out-patients with moderate to severe chronic low back pain. Methodology: Fifty-two patients with a diagnosis of chronic low back pain for > 3 months were randomly assigned to receive either a 50 mg tablet of tapentadol (twice daily) or 50 mg tablet of tramadol (twice daily) for 1 week. The mean (±SD) difference in the reduction of pain (at end of 1 week) between the two groups was compared employing an independent student t-test for difference in mean values separately for the Visual Analogue Scale (VAS) and Roland Morris Disability Questionnaire (RMDQ) scores. The frequency of the different adverse events between the two groups was compared employing Chi-square test. Results: Except for VAS scores, the baseline demographic parameters of the two groups were comparable. The study found that tapentadol reduced VAS and RMDQ scores more than tramadol (statistically significant p<0.001) between baseline and the end of week 1. Regarding safety and tolerability, the tapentadol group experienced nausea/vomiting and dizziness/somnolence more frequently than the tramadol group, with p-values of 0.011 and 0.001 respectively. Both groups experienced similar rates of headache and constipation, with p-values of 0.668 and 0.610, respectively. Conclusion: When compared to tramadol (50 mg twice daily), tapentadol (50mg twice daily) was found to significantly improve pain and disability in patients with moderate to severe chronic low back pain, while tapentadol had greater frequencies of side effects like nausea, vomiting, dizziness, and somnolence.
Keywords
Efficacy; Low Back Pain; Safety; Tramadol; Tapentadol
Download this article as:| Copy the following to cite this article: Naveen S, Elango P, Ramya S. An Open-Label, Randomized, Parallel-Group Study to Assess the Safety, Efficacy, and Tolerability of Tapentadol Versus Tramadol in Outpatients with Moderate to Severe Chronic Low Back Pain at a Tertiary Care Hospital in South India. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Naveen S, Elango P, Ramya S. An Open-Label, Randomized, Parallel-Group Study to Assess the Safety, Efficacy, and Tolerability of Tapentadol Versus Tramadol in Outpatients with Moderate to Severe Chronic Low Back Pain at a Tertiary Care Hospital in South India. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3ZZkwmx |
Introduction
Low back pain (LBP) is a very common musculoskeletal symptom, which refers to pain or muscle stiffness or tension in the lumbosacral region of the spine.1 It affects more than 80% of people globally at some point in their lives and affects people of all ages, including young children and the elderly.2, 3
According to the Global Burden of Disease Study 2019 (GBD 2019), LBP is one of the top five factors contributing to an increase in the number of Disability-Adjusted Life Years (DALYs).4 According to GBD 2019 statistics, LBP is one of the first ten causes of DALYs and one of the prime causes of increased Years Lived in Less-than-ideal Health in India.5 It is a significant contributor to daily activity limitations and work absences, which increases the social and economic burden on families, society, businesses, and the nation.3,6
LBP can be categorized as acute, subacute, or chronic depending on how long it lasts; it is considered chronic if it lasts more than 12 weeks7. There are two types of chronic LBP: primary (non-specific or with no underlying pathology) and secondary (with underlying pathology).7
According to GBD data, occupational risks (hard physical work), physical inactivity, an increase in body mass index, alcoholism, and various psychiatric/social causes like anxiety, depression, mental stress, and somatization are the main dangers for chronic LBP in India.8
Exercises, physiotherapy, analgesics, muscle relaxants, acupuncture, behavioural therapies, transcutaneous electrical nerve stimulation, and other multidisciplinary treatments are necessary for the management of chronic LBP.9 Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) are used in the pharmacotherapy of LBP to offer short-term analgesia. Opioid analgesics including oxycodone, morphine, fentanyl, tramadol, etc. are utilized for moderate to severe LBP. Numerous recommendations for treatment include the use of benzodiazepines, opioids, selective cyclo-oxygenase II inhibitors (COXIBs), paracetamol, and NSAIDs for pain.9
Paracetamol, NSAIDs, and COXIBs are commonly involved in producing gastrointestinal side effects with relative toxicity to hepatic, cardiovascular, and renal systems. Opioids like tramadol, oxycodone, and morphine have well-documented side effects of sedation, vomiting, etc.
Tapentadol is an opioid analgesic that acts as an agonist on µ opioid receptor as well as inhibits nor-epinephrine reuptake. Due to its unique mechanism, tapentadol is thought to have extremely good analgesic efficacy with a lower rate of side effects than NSAIDs or other opioids.10
Our study has been undertaken to elucidate the efficacy, safety, and tolerability of tapentadol in comparison with tramadol in outpatients with moderate to severe chronic LBP.
Chronic LBP is a complex syndrome with multifactorial aetiology, of which the involvement of spinal and supra-spinal pain (central) pathways is vital. The study’s justification is thus provided by the usage of opioids and other drugs that affect the spinal and supraspinal pain pathways. Among the atypical opioids that affect central pain pathways include tramadol and tapentadol. Tapentadol may be more effective, more acceptable, and less likely to cause gastrointestinal adverse effects than tramadol because it is a novel molecule with a dual mode of action.
Even while analogous research comparing tramadol and tapentadol head-to-head exists in western nations, there are not many in India. Additionally, studies that have been done so far have evaluated tapentadol /tramadol in a variety of pain disorders, including post-operative pain, and diabetic neuropathy, and comparisons with NSAIDs, COXIBs, and other opioids including morphine and oxycodone. In order to choose an opioid in the context of disease presentation among the Indian population, it is crucial to examine these medications in that group. Hence the present study was undertaken among outpatients with moderate to severe chronic LBP at a tertiary care hospital in South India to
Assess the analgesic efficacy of Tapentadol in comparison with Tramadol
Assess the safety as well as tolerability of Tapentadol in comparison with Tramadol
Materials and Methods
Study setting
The study is a randomized, open-label, and parallel-group study, done at a single site i.e., at the Out Patient Department (OPD) of Orthopaedics, Sri Ramachandra Medical Hospital, Porur, Chennai.
Study participants
Following approval from the Institutional Ethical Committee (IEC) of Sri Ramachandra Medical College and Research Institute (CSP-MED/15/MAR/22/15), the study was commenced. Clinically diagnosed patients with chronic LBP for > 3 months duration satisfying complaint-based classes Ic, IIc, and IIIc (as per Mooney et al which is based on duration and location) were screened for inclusion in the study.11 Patients of either gender, aged between 30-60 years, who have been regularly using either NSAIDs or paracetamol, for at least a month before initiation of the study and with baseline Visual Analogue Scale (VAS) score of > = 40 mm were recruited after procuring written informed consent. Patients having secondary LBP due to underlying pathology, any other painful conditions that could interfere with the assessment of LBP, systemic or local infection, history or suspicion of drug/alcohol abuse based on investigator’s opinion, renal/hepatic dysfunction, history of epilepsy, history of hypersensitivity to investigational products, conditions which are contraindications for investigational drugs like acute or severe bronchial asthma and paralytic ileus, history of dependence to opioids, bowel disorders which can hinder absorption of investigational drug, history of brain injury, cerebrovascular accidents like stroke/transient ischemic attack in last 1 year, pregnant or lactating women, on concomitant drugs which can cause interactions with investigational drugs like monoamine oxidase (MAO) inhibitors, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), antipsychotics in last 90 days and who participated in any other trial in last 1 month were excluded from participating in the study.
Study procedure
Patients satisfying the eligibility criteria were recruited and randomly assigned, based on a computer-generated randomization sequence, to either of the two intervention groups. Patients in group I received a 50 mg tapentadol pill twice daily, and patients in group II received a 50 mg tramadol tablet twice daily, both to be taken respectively after meals for a week.
Visits and follow-up
The first visit (visit 0) was considered the baseline visit during which clinical examination and baseline assessment of pain was done using Visual Analogue Scale (VAS) as well as Roland Morris Disability Questionnaire (RMDQ). During follow-up visit 1, repeat assessments of pain were done using VAS as well as RMDQ and observation for occurrences of any adverse events was done.
The Visual Analogue Scale (VAS) 12
Pain perception was subjectively measured using VAS, which is a 100mm line, with one end of the line representing ‘no pain’ and the other end depicting ‘worst pain’. Patients were asked to score their pain perception by laying a mark on the 100mm line correlating to their present level of pain. To calculate a pain score out of 100, the mark on the 100 mm line was measured in relation to the “no pain” end.
The Roland-Morris Questionnaire13
The Roland-Morris Questionnaire, developed by Prof. Martin Roland, was used to determine the degree of LBP-related disability. It consists of 24 questions based on LBP-related disabilities in daily life and are prefixed with the phrase “Due to my back pain” to assist patients in differentiating between LBP-related disabilities and those caused by other conditions. Patients were asked to tick the box next to the statement if it applied to them that day, and the score was determined by adding up the total number of statements that the patient had checked. The maximum score that could be earned was 24, while the lowest was 0. Higher scores indicated a higher level of disability due to back pain.
Statistical Analysis
Statistical Package for Social Science (SPSS, software version 23) was used for all statistical analyses. All patients assigned to the treatment participated in the main analysis. Patients whose follow-up was lost were accounted for during analysis by carrying the last observation forward. Statistical analysis was done for all the variables and continuous measurements were represented as mean ± SD and categorical measurements were represented as numbers (%). All results were considered significant at a 5% level of significance or p-value of <0.05. The mean (±SD) difference in the reduction of pain between the two groups was compared employing independent student t test for the difference in mean values separately for VAS and RMDQ scores. The frequency of the various adverse events was compared between the two groups employing Chi-square test.
Results
Sixty-three patients attending the orthopedics OPD were assessed for eligibility, and based on the pre-defined eligibility criteria 52 patients were included for the study (Figure 1). Baseline characteristics with regard to age, gender, VAS and RMDQ scores of both the treatment groups are depicted in Table 1. Aside from VAS values, no significant changes were found between the groups. During the follow-up visit (1 week after treatment), individuals in both treatment groups had their VAS and RMDQ scores calculated (Table 2). The percentage of patients showing a reduction in VAS score and difference in RMDQ score from baseline to week 1 is depicted in figure 2 and 3 respectively. In comparison to tramadol, tapentadol caused a greater (statistically significant p<0.001) difference in the reduction of VAS and RMDQ ratings from baseline to the end of week 1 (Table 3).
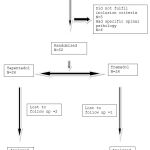 |
Figure 1: Flow chart for study participants |
Table 1: Comparison of Baseline characteristics of study patients.
|
|
Tapentadol (Group I) | Tramadol
(Group 2) |
p value |
| Age (Mean± SD) | 45.65±9.65 | 45.62±9.139 | 0.988a |
| Male/female (%) | 30.8/69.2 | 38.4/61.6 | 0.560b |
| RMDQ score at baseline (Mean ± SD) |
19.50±1.655 |
18.81±2.514 |
0.246c |
| VAS score at baseline (Mean ± SD) |
73.62±7.457 |
69.27±6.756 |
0.032d |
Note: aIndependent t test with t value= 0.015 and degree of freedom= 50; equal variances assumed
bChi-square with one degree of freedom = 1
c,d unpaired t test
Table 2: VAS score & RMDQ score after 1 week treatment
| Treatment group | VAS score | RMDQ score | ||
| Mean | Std. Deviation | Mean | Std. Deviation | |
| Tapentadol (n=26) | 54.50 | 11.656 | 16.04 | 2.068 |
| Tramadol (n=26) | 60.27 | 8.013 | 17.15 | 2.428 |
| Total (n=52) | 57.38 | 10.323 | 16.60 | 2.303 |
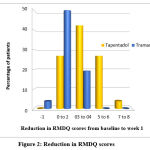 |
Figure 2: Reduction in RMDQ scores. |
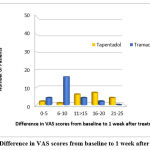 |
Figure 3: Difference in VAS scores from baseline to 1 week after treatment. |
Table 3: Difference in reduction of VAS and RMDQ scores from Base line to Week 1
| Reduction in scores from baseline-Week1 (Mean±SD) | Tapentadol
|
Tramadol
|
T value | Degrees of freedom | p value |
| VAS | 19.12±9.488 | 9±3.816 | 5.043 | 32.880 | <0.0001 |
| RMDQ | 3.46±1.923 | 1.65±1.164 | 4.1 | 41.154 | <0.0001 |
The unfavorable treatment-related adverse events as reported by the patients were recorded. The most often reported side effects included nausea/vomiting, dizziness/somnolence, headache, and constipation (Table 4). The statistical analysis using the Chi-square test revealed that the tapentadol group experienced nausea/vomiting and dizziness/somnolence more frequently than the tramadol group with p values of 0.011 and 0.001, respectively, while both groups experienced similar rates of headache and constipation, with p values of 0.668 and 0.610, respectively (Table 4). Due to the occurrence of adverse events, no participant stopped taking the study medicines or withdrew from the trial. Throughout the research period, no serious adverse events were reported.
Table 4: Treatment related adverse events among participants.
| Side effects | Tapentadol
(n=26) |
Tramadol
(n=26) |
p value | ||
| No. | % | No. | % | ||
| Nausea /
Vomiting |
20 | 77 | 11 | 42.3 | 0.011 |
| Dizziness /
Somnolence |
21 | 81 | 9 | 34.6 | 0.001 |
| Constipation | 2 | 7.7 | 4 | 15.4 | 0.668 |
| Headache | 3 | 11.5 | 1 | 3.8 | 0.610 |
Discussion
In this present one-week trial involving outpatients with moderate to severe chronic LBP, tapentadol, and tramadol were compared for their efficacy, safety, and tolerability.
There are limited clinical studies comparing tramadol and tapentadol to one another. Numerous randomized trials have compared the efficacy of tramadol to medications like morphine, oxycodone, acetaminophen, and codeine in treating a variety of severe illnesses, including post-operative pain, pain after dental extractions, myocardial infarction, and angina.14,15
With respect to chronic pain, the efficacy of tramadol has been assessed in cancer pain, chronic pain due to a variety of causes and in chronic LBP.16
Similarly, tapentadol has been compared to drugs like oxycodone and morphine in randomized control trials vastly for various painful conditions including both acute conditions like bunionectomy, dental extractions, postoperative pain etc., and chronic conditions like osteoarthritis of hip and knee and LBP.17,18
Due to its dual mode of action (opioid receptor agonism and inhibition of the reuptake of norepinephrine), tapentadol has also been evaluated for its effectiveness in treating chronic diabetic neuropathic pain (an FDA-approved indication).19 In a retrospective analysis, the efficacy of tapentadol and tramadol was compared. The study’s drawback was that baseline pain intensity scores for the patients in the control group could not be obtained.20 There is limited research examining the efficacy of tapentadol in LBP patients in terms of how it affects disability as measured by RMDQ scores.
Therefore, the goal of this study is to directly compare the efficacy and safety of tramadol with tapentadol.
In our study, analysis of age distribution showed that mean age of patients was 45.65±9.65 and 45.62±9.139 in group I and group II respectively (Table 1), and sex distribution between two the groups was found to be 30.8% males/69.2% females and 38.4% males/61.6% females in group I and group II respectively (Table 1).
The efficacy and tolerability of tapentadol and tramadol in treating chronic non-malignant pain were compared in a meta-analysis by Mercier F et al. The meta-analysis included 45 clinical trials, the majority of which were placebo-controlled trials and some of which used oxycodone or morphine as the comparator. The study’s mean (SD) duration was 9 weeks (6.8 weeks), and it revealed that 64% of the patients were female and the average age of the participants was 58 years old. 16
In contrast, our trial, which lasted for one week, was brief compared to other investigations. Our study’s patients had younger mean ages than those in earlier research, indicating a current tendency towards lower back discomfort in younger people. Additionally, 65.3% of the participants in our study were female, which is similar to the findings of the meta-analysis and suggests that the illness is more common in women.
In groups I and II, respectively, the mean baseline RMDQ scores were 19.50±1.655 and 18.81±2.514, with a p-value of 0.246 (not significant). However, the mean baseline VAS ratings in groups I and II, respectively, were 73.62±7.457 and 69.27±6.756, with a p-value of 0.032 (statistically significant). Our research work focused on evaluating the mean difference in VAS score and RMDQ score values from the baseline to the end of the study in order to account for this.
When examined using independent student t-test, the difference in mean VAS scores between the tapentadol and tramadol groups from baseline to one week after therapy was 19.12±9.488 and 9±3.816, respectively (Table 3). This shows that the tapentadol group’s improvement or decrease in VAS scores is greater or better than the tramadol group.
When compared using an independent student t-test, the means of the difference or reduction in RMDQ scores from baseline to the end of the study were 3.46±1.923 for group I and 1.65±1.164 for group II, respectively (Table: 3). Again, it shows that the tapentadol group’s improvement or decrease in RMDQ scores is greater than that of the tramadol group.
Our study’s findings diverge from those of a randomized, double-blind cross-over study by Muller FO et al., which involved 55 patients with persistent back pain and assessed the efficacy of tramadol and codeine+paracetamol (at a fixed dose), which showed similar efficacy in both arms.21
Additionally, the efficacy findings in our study are different from a meta-analysis by Mercier F et al., which revealed that in chronic non-malignant pain situations, 300mg tramadol qd was marginally more effective in lowering pain than tapentadol 100-250mg bd.
In another trial, conducted by Schnitzer TJ et al. to evaluate the effectiveness, safety, and tolerability of tramadol with placebo in patients with chronic LBP, mean VAS scores at the conclusion of treatment were 3.5 in the tramadol group and 5.1 in the placebo group.22 These results coincide with those of our current investigation. The VAS pain score at the conclusion of our study was 5.7cm/57mm. Also, the difference in RMDQ scores in tramadol patients from baseline to end of the study significantly improved (p value <0.0001).
In another study by Lee JH et al. to evaluate efficacy and tolerability of extended release (ER) form tramadol (100mg BD) in comparison to placebo in patients with chronic LBP found that pain relief was more with ER form.23
Effect of tapentadol immediate release (IR) has also been studied in phase 3 trials by Daniels SE et al. for pain associated with bunionectomy, by Hartrick C et al. for pain with degenerative joint disease and by Galvez R et al. for severe chronic LBP.17,18,24 In all of these studies tapentadol was compared with strong opioids like oxycodone and pain was assessed on a numerical rating scale and patients’ global impression of change on Likert scale and it was found that tapentadol was equally effective as oxycodone. 25
This is in accordance with the findings of our work which shows improvement in chronic LBP based on VAS and RMDQ scores.
Tapentadol has been studied for the treatment of chronic pain in a number of different disorders, including chronic osteoarthritis, chronic low back pain, and neuropathic pain. Tapentadol was proven to be a more effective analgesic than placebo and to have fewer adverse effects than oxycodone in one of the most significant studies conducted by Buynak et al. in 2010 with 965 individuals suffering from chronic LBP. 26
The side effects experienced by patients in our study in the two groups were nausea/vomiting, dizziness/somnolence, constipation and headache with the tapentadol group experiencing more adverse effects overall (Table 4).
Additionally, we discovered that the tapentadol group had nausea/vomiting and dizziness/somnolence more frequently than the tramadol group, as indicated by statistically significant p-values of 0.011 and 0.001 obtained using the Chi-Square test, respectively. Although headache and constipation were equally common in both treatment groups (p-value of 0.668 and 0.610 respectively). In a study by Daniels S et al, tapentadol IR was compared to oxycodone and placebo in the treatment of acute pain, and the results showed that tapentadol IR 50 mg had a lower incidence of nausea and/or vomiting.
Another research work by Etropolski et al. comparing the gastrointestinal tolerability of oxycodone vs. tapentadol in both acute and chronic pain, demonstrated a low incidence of nausea and vomiting with tapentadol. 27 And in this study over a 14-day period; oxycodone decreased the number of spontaneous bowel movements (p<0.001) compared to tapentadol and it was also lower (p<0.001) with tapentadol IR 50 and 75 mg compared to oxycodone IR 10 mg. The results of ER formulation of tapentadol and oxycodone were comparable with that of IR formulation in the study.
In contrast to the above-mentioned studies, in our study tapentadol is compared with tramadol which is not a strong opioid. Therefore, the incidence of treatment-emergent side effects, such as nausea/vomiting and dizziness/somnolence, is significantly higher in the tapentadol group than tramadol group. Constipation and headache frequency were comparable between the two groups (statistically not significant).
Compared to other studies, both treatment groups in our study showed a slight improvement in pain levels. This variation or relative lack of efficacy may be due to the study medication being administered less frequently and at a lower dosage. As opposed to every 4-6 hours dosing of IR formulations in other research, tapentadol IR and tramadol IR 50mg BD were the dosing schedule employed in the current study.
The studies involving tramadol and tapentadol have compared the drugs to placebo, oxycodone or NSAIDS. There have not been many trials comparing the two medications in patients with LBP; therefore an equivocal dose has not been established.
Conclusion
Results from this study showed that tapentadol is superior to tramadol in reduction of pain intensity (from VAS scores tapentadol – 19.12±9.488, tramadol – 9±3.816; p<0.0001) and also reduction of disability due to chronic LBP (from RMDQ scores tapentadol – 3.46±1.923, tramadol – 1.65±1.164; p<0.0001). But, incidences of adverse events like nausea/ vomiting (42.3%; p=0.011) and dizziness/somnolence (34.6%; p=0.001) are more common with tapentadol.
The open-label, brief duration, small sample size, and low tapentadol and tramadol doses of this trial, however, are its shortcomings. Long-term studies with a bigger population and the appropriate dosage of the medications are needed to establish a distinct and definite difference between the drugs. However, given the lack of research comparing tapentadol and tramadol head-to-head for chronic LBP in the Indian community, the findings may be useful for initial thought towards individualization of opioid choice for chronic LBP in the Indian population.
Acknowledgement
Dr. Samuel Chittaranjan Former Professor & Head Department of Orthopaedics, Sri Ramachandra Medical College & Research Institute, Sri Ramachandra Institute of Higher Education and Research (SRIHER) Chennai,
Sri Ramachandra Medical College & Research Institute, Sri Ramachandra Institute of Higher Education and Research (SRIHER) Chennai
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding source.
References
- Ehrlich G. Low back pain. Bulletin of the World Health Organization. 2003;81(9):671-676.
- Chou R. Low Back Pain (Chronic). Clinical Evidence Handbook, American Family Physician [Internet]. 2011 [cited 5 October 2021];84(4):437-438. Available from: aafp.org/afp
- Bindra S, Sinha AGK, Benjamin AI. Epidemiology of low back pain in Indian population: A review. International Journal of Basic and Applied Medical Sciences. 2015;5(1):166-179.
- Murray Christopher J L. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2015;396:1204-1222.
- org. India | Institute for Health Metrics and Evaluation [Internet]. 2021 [cited 5 October 2021]. Available from: www.healthdata.org/india
- int. Priority diseases and reasons for inclusion [Internet]. 2021 [cited 5 October 2021]. Available from: www.who.int/entity/medicines/areas/priority_medicines/Ch6_24LBP.pdf
- Zanni G, Wick J. Low Back Pain: Eliminating Myths and Elucidating Realities. J Am Pharm Assoc. 2003;43(3).
CrossRef - Furtado R. Nonspecific low back pain in young adults: Associated risk factors. Revista Brasileira de Reumatologia. 2014;54(5):371-377.
CrossRef - Delitto A. Low back pain, clinical practice guidelines linked to the international classification of functioning, disability, and health from the Orthopedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2012;42(4):A1-A57.
CrossRef - Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies H, Englberger W, et al. ( )-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (Tapentadol HCl): A novel -opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. Journal of Pharmacology and Experimental Therapeutics. 2007;323(1):265-276
CrossRef - Riddle DL. Classification and low back pain: A review of the literature and critical analysis of selected systems. Physical Therapy. 1998;78(7):708–37.
CrossRef - Hawker G. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF. Arthritis Care Res. 2011;63(S11):S240-S252.
CrossRef - Roland M, Fairbank J. The Roland Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25(24):3115-3124.
CrossRef - Houmes R, Voets MA, Verkaaik A, Erdmann W, Lachmann B. Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain with special regard to respiratory depression. Anesth Analg 1992;74:51-4.
CrossRef - Medve RA, Wang J, and Karim R. Tramadol and acetaminophen tablets for dental pain.Anesthesia Progress. 2001;48(3):79-81
- Mercier F, Claret L, Prins K, Bruno R. A Model-Based Meta-analysis to Compare Efficacy and Tolerability of Tramadol and Tapentadol for the Treatment of Chronic Non-Malignant Pain.Pain and Therapy. 2014;3(1):31-44. doi:10.1007/s40122-014-0023-5
CrossRef - Daniels SE, Upmalis D, Okamoto A, Lange C, Häeussler J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009 Mar;25(3):765–76
CrossRef - Hartrick C, Hove IV, Stegmann J, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clin Ther. 2009 Feb;31(2):260–71.
CrossRef - FDA Approves Tapentadol ER for Diabetic Neuropathy [Internet]. Medscape. [cited 5 October 2021]. Available from: medscape.com/viewarticle/769982
- Guillén-Astete CA, Cardona-Carballo C, de la Casa-Resino C. Tapentadol versus tramadol in the management of low back pain in the emergency department: Impact of use on the need for reassessments. Medicine (Baltimore). 2017;96(45):e8403.
CrossRef - Muller FO, Odendaal CL, Müller FR, Raubenheimer J, Middle MV, Kummer M. Comparison of the efficacy and tolerability of a paracetamol/codeine fixed-dose combination with tramadol in patients with refractory chronic back pain. Arzneimittelforschung. 1998 Jun;48(6):675–9.
- Schnitzer TJ, Gray WL, Paster RZ, Kamin M. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000 Mar;27(3):772–8
- Lee JH, Lee C. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of the extended-release tramadol hydrochloride/acetaminophen fixed-dose combination tablet for the treatment of chronic low back pain. Clin Ther. 2013 Nov;35(11):1830-40.
CrossRef - Galvez R, Schäfer M, Hans G, Falke D, Steigerwald I. Tapentadol prolonged release versus strong opioids for severe, chronic low back pain: results of an open-label, phase 3b study. Adv Ther. 2013 Mar;30(3):229–59.
CrossRef - Buynak R, Shapiro DY, Okamoto A, Hove IV, Rauschkolb C, Steup A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert Opin Pharmacother. 2010 Aug;11(11):1787–804.
CrossRef - Daniels S, Ed Casson, Stegmann J, Oh C, Okamoto A, Rauschkolb C, Upmalis D. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009 Jun;25(6):1551–61.
CrossRef - Etropolski M Kelly K, Okamoto A, Rauschkolbet C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther. 2011 May;28(5):401-17.
CrossRef









