Saba Aws Hashem1* , Luay Abu-Qatouseh1
, Luay Abu-Qatouseh1 , Eyad Mallah2
, Eyad Mallah2 , Kenza Mansoor2
, Kenza Mansoor2 , Feras Darwish El-Hajji4
, Feras Darwish El-Hajji4 , Mohammed Malkawy1, Mona Bustami1
, Mohammed Malkawy1, Mona Bustami1 , Nasir Idkaidek3
, Nasir Idkaidek3 and Ahmad M Al Masalmeh1
and Ahmad M Al Masalmeh1
1Department of Pharmacology and Biomedical Sciences, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan
2Department of Pharmaceutical Medicinal Chemistry and Pharmacognosy, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan
3Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan
4Department of Clinical Pharmacy and Therapeutics, Faculty of Pharmacy and Medical Sciences, Applied Science University, Amman, Jordan
Corresponding Author E-mail: sabaaws1411@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2613
Abstract
Objectives: Metformin is the most widely given medication for type 2 diabetes mellitus (T2DM). Annona muricata L. is a medicinal plant that belongs to the family Annonaceae, popularly known as graviola. Graviola leaves extract was found useful against diabetes, headache, insomnia, cystitis, inflammation, cancer, and other health benefits. The objectives of the current study are to investigate the effect of graviola leaves extract on metformin pharmacokinetics in rat plasma by applying high performance liquid chromatography (HPLC) method as well as its pharmacological effects on breast cancer (MCF-7) cells and prostate cancer (DU-145) cells. Methods: Wistar rats were classified into two groups; the first group (control group) received metformin (20 mg/kg) alone by oral gavage, while the second group, was administered a combination of metformin (20 mg/kg) and graviola leaves extract (20 mg/kg). Blood samples were collected at different time intervals to be analyzed using a validated HPLC method. Plasma profile and pharmacokinetic parameters were determined for each group. In addition, blood glucose levels at 0 hours and after 2 hours of metformin administration were measured in both groups. Breast cancer (MCF-7) cells and prostate cancer (DU-145) cells were used to investigate the anticancer effect of metformin (40 mg/ml), graviola leaves extract (20 mg/ml) and their combination by the standard MTT assay. Results: In the first group, metformin maximum plasma concentration (Cmax) and the area under the curve (AUC0-last) were (1509.25 ng/ml and 8705.59 h*ng/ml) respectively. In the second group, Pre-administration of graviola leaves extract significantly reduced MET (Cmax) and (AUC0-last), (701.88 ng/ml and 3467.72 h*ng/ml), respectively (P ≤0.05). Further, the use of metformin and graviola leaves extract separately showed strong anticancer activity on (MCF-7) cell lines with IC50 values of (10 and 20 mg/ml), respectively as well as on (DU-145) cell lines with IC50 value of (0.3125 and 5 mg/ml), respectively. In addition, the combination of metformin and graviola leaves extract showed a synergistic effect on (MCF-7) cells since the fractional inhibitory concentration value (FIC = 0.375) was less than 0.5, while it showed an additive effect on (DU-145) cells since the fractional inhibitory concentration value (FIC = 1.5) was between (0.5 and 4). Conclusion: In the current study, pre-administration of graviola leaves extract significantly reduced efficacy of metformin In vivo. The combination of metformin and graviola leaves extract showed a synergistic anticancer effect on breast cancer in vitro, while the combination has an additive effect on prostate cancer. The combination could be a potential therapeutic option to help treat breast cancer. The result achieved in this study is very encouraging to be considered for further investigation.
Keywords
Cell lines; Graviola; Interaction; Metformin; Pharmacokinetics
Download this article as:| Copy the following to cite this article: Hashem S. A, Mallah E, Mansoor K, El-Hajji F. D, Malkawy M, Bustami M, Idkaidek N, Al-Masalmeh A. M, Qatouseh L. A. The Effect of Graviola Leaves Extract (Annona muricata L.) on Pharmacokinetic of Metformin in Rats’ Plasma and Pharmacological Activity of their Combination on Breast and Prostate Cancer Cell Lines. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Hashem S. A, Mallah E, Mansoor K, El-Hajji F. D, Malkawy M, Bustami M, Idkaidek N, Al-Masalmeh A. M, Qatouseh L. A. The Effect of Graviola Leaves Extract (Annona muricata L.) on Pharmacokinetic of Metformin in Rats’ Plasma and Pharmacological Activity of their Combination on Breast and Prostate Cancer Cell Lines. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3wXV0Sy |
Introduction
In nature, there are no molecules that have no effect. As a result, this diversity enlarges the variety of products available while also increases the likelihood of interaction. An interaction occurs when the impact of one drug is affected qualitatively or quantitatively by another substance (herbal medicine/product/ingredient)1. Interactions between herbal treatments and prescription medications can happen, and they can have serious clinical effects2. Interactions are often deliberately formed to enhance the therapeutic efficacy of one drug with another or to minimize its side effects, which are both good things. In other situations, an unfavorable interaction may arise because of unauthorized medication usage or when a patient begins therapy with a certain medication1. Herb-drug interactions may be more common than drug-drug interactions because drugs typically contain single active ingredient, but nearly all HMPs (including single-herb formulations) contain combinations of pharmacologically active compounds3. Many pharmaceutical medications and medicinal herbs are beneficial at one dose but harmful at another. Herb-drug interactions can increase or reduce the pharmacological or toxicological effects of each substance4.
Metformin is the first-line therapy for (T2DM) either used alone or in combination with other treatments5.The origins of metformin (dimethyl-biguanide) came from the French Lilac (Galega officinalis) as an herbal traditional medicine in medieval Europe to treat several diseases6. Metformin is an antihyperglycemic drug that lowers both basal and postprandial blood glucose levels. It does not cause hypoglycemia since it does not stimulate insulin secretion7. Besides metformin is mostly used to treat diabetes mellitus (DM), but it also has anticancer effect by suppressing cancer cell growth and tumor progression. This is because activating AMPK (AMP-activated protein kinase) has been found to be beneficial in cancer treatment since it can diminish tumors8. In recent years, various studies have presented evidence showing that metformin may have other possible roles besides glucose lowering, such as antitumor, antiaging, cardiovascular protective, neuroprotective, or as a remedy for polycystic ovarian syndrome (PCOS)9.
Annona muricata L., popularly known as Guabana, Soursop, or Graviola. The family Annonaceae includes the tropical evergreen fruiting tree called Graviola10. Graviola is native to North and South America’s hottest tropical regions. It is commonly available in Central America, Venezuela, Peru, Columbia, Brazil, Mexico, Cuba, and India. Graviola is popular in the United States, where it is sold as capsules (leaf and stem powder) and as a tea under a diversity of brand names11. It has been demonstrated that all components of the graviola tree are effective against several ailments, including human parasite infections and cancer. Graviola leaves have been found to be particularly effective in treating diabetes, headache, sleeplessness, cystitis, and inflammation10.
Materials and Methods
Materials and Reagents
Metformin HCl Powder was kindly donated by Dar Aldawa Pharmaceutical Co., Amman-Jordan. Graviola leaves extract (DER 5: 1) was obtained from Biotikon. Dr. med. Michalzik®, Germany. Metronidazole benzoate was obtained from Jordan Pharmaceutical Manufacturing (JPM) Co., Amman-Jordan.Deionized water and acetonitrile advanced gradient grade got from (Fisher Scientific). Phosphoric acid was got from (Laboratory Rasayan, India) and potassium dihydrogen phosphate from (Grainland Chemical Company, UK).
The cells of human breast cancer (MCF-7) the cells of human prostate cancer (DU-145) were obtained by the American Type Culture Collection (ATCC, Rockville, MD, USA). Amphotericin b, penicillin streptomycin, L-glutamine, and trypsin Ethylenediaminetetraacetic Acid (EDTA) were obtained from (EuroClone S.P.A. Via Figino 20/22-20016 Pero (MI) Italy). Dulbecco’s Minimum Essential High-Glucose Medium (DMEM), Dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), and Roswell Park Memorial Institute (RPMI-1640) were purchased from (Capricorn Scientific GmbH). Trypan blue solution (0.4%) and 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from (Sigma). “ELISA Plate Reader (Pharma-RL-ER-01)” was used12.
Instrumentation
Analyses of the samples were performed using reversed-phase high-performance liquid chromatography (HPLC) and quantified using ultraviolet detection at 234 nm, detector (UV-VIS Plus), (ChromQuest Software 4.2.34), and a Finnigan Surveyor System (Thermo Electron Corporation, San Jose, CA, USA), on a Hypersil BDS C-18 column (150 mm * 4.6 mm) with an average particle size of 5 micrometer13. The pH of the mobile phase was adjusted using a pH meter. (Thermo scientific, USA). A computer system operating under windows “XP, SP3” was used to do the analysis process of the chromatographic data. The experiment was performed at Instrumental laboratory at Pharmaceutical Center of Petra University.
Chromatographic Conditions
For the buffer preparation, potassium dihydrogen phosphate (KH2PO4) was dissolved in 1 liter of water at a concentration of 6.8 g/l. Phosphoric acid was then used to adjust the pH to 3.0. Acetonitrile and buffer solution made up the mobile phase. (35%: 65%).
Table 1
| Mobile Phase | 35% of water contains (6.8 g/l) potassium dihydrogen phosphate buffer and 65% of acetonitrile.
pH= 3.0, adjust with H3PO4 |
| Column Type | Hypersil Thermo Electron Corporation, BDS C-18 Column (150 mm x 4.6 mm, 5 µm) |
| HPLC Conditions | |
| Pump Flow Rate | 0.9 ml/min |
| Column Oven Temperature | 25 °C |
| Auto-sampler Temperature | 5 °C |
| Auto-sampler Injection Volume | 20 µl |
| Wavelength | 234 nm |
| Expected Retention Times (Minutes) | |
| Metformin | 2.8 |
| Metronidazole Benzoate (IS) | 4.4 |
Validation of HPLC Method
The analytical method was developed based on the European Medicines Agency (EMA) guidelines. Selectivity, stability, linearity, recovery, accuracy, and precision were evaluated during the validation process. Concerning linearity, seven calibration points (40, 156, 312, 625, 1250, 2500 and 5000) ng/ml were prepared and utilized to determine linearity. The calibration curve of metformin was plotted in the Y-axis as the peak area ration (PAR), the analyte peak area to the IS peak area versus the nominal standards concentrations. The plotted curve’s linearity was assessed using the correlation coefficient (R²), this one was greater than 0.99. Six replicate quality control (QC) samples were analyzed to determine the accuracy and precision. The relative error (RE%) was utilized to evaluate accuracy, whereas the variation coefficient (CV%) was utilized to evaluate precision.
Preclinical Protocol
The animal study protocol was approved by the Ethical Committee of the High Research Council, approval number (1A/1/2020) at the Faculty of Pharmacy and Medical Sciences, University of Petra (Amman, Jordan). Male and female Wistar laboratory rats weighing 200 g on average were provided by the Applied Science Private University’s animal house (Amman, Jordan). Rats were housed in climate-controlled conditions with a 12-hour light/dark cycle, humidity of 55–65%, and temperatures of 22–24°C.14. Rats were given free access to water while they fasted overnight. Rats (n=8) were weighed and randomly divided into two groups. On the day of the trial, a control group was given a single dosage of (20 mg/kg) metformin solution via oral gavage. The other group received a combination of graviola leaves extract and metformin as follows: three days before the experiment (20 mg/kg) of graviola leaves extract was given by oral gavage, and on the experiment day graviola leaves extract was given again half an hour before metformin administration 15.
Study Design
On the day of the experiment, distilled water was freshly made and mixed with (20 mg/kg) of metformin. A stainless-steel oral gavage needle was used to administer a calculated volume to the rats. Randomly, two groups of rats were formed: metformin (8 rats), metformin and graviola (8 rats). Graviola leaves extract were given for 3 days before experiment and 30 minutes before the metformin dose. At the following intervals: (0.0, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.5, 7.5, and 24.0) hours, blood samples were drawn from the rat’s ocular vein and put into containers containing EDTA. To prepare plasma for HPLC analysis, plasma was separated, put immediately into labeled Eppendorf tubes, and stored at (-20°C) at University of Petra (Amman, Jordan).
Measurement of Blood Glucose Levels in Normoglycemic Rats
Sixteen rats were divided into two groups of eight at random. A measurement of basal blood glucose was taken after a 15-hour overnight fast that included only water. The following therapies were given orally: The first group received metformin alone (20 mg/kg), while in the second group, pre-administration of graviola leaves extract (20 mg/kg), was given a three days ago before the experiment day as well as a half-hour before giving metformin (20 mg/kg). From the tail vein, blood samples were taken at 0 hours and 2 hours after metformin administration, and an Accu-Chek Active® glucometer from Roche Diagnostics in Germany was used to measure blood sugar levels.
Cell Culture
The current study was performed at the pharmaceutical biotechnology laboratory at the University of Petra Pharmaceutical Center. Two types of human cell lines, breast cancer cells (MCF-7 ATCC HTB-22) and human prostate cancer cells (DU-145 ATCC HTB-81) were used. Metformin (40 mg/ml) was prepared by dissolving (40 mg) of metformin in (1ml) of specific media, (DMEM) for (MCF-7) cells and (RPMI-1640) for (DU-145) cells and other serial dilutions of 2-folds until 8 dilutions were reached. In addition, graviola leaves extract (20 mg/ml) was prepared by dissolving (20 mg) of graviola leaves extract in (1ml) of specific media, (DMEM) for (MCF-7) cells and (RPMI-1640) for (DU-145) cells and other serial dilutions of 2-folds until 8 dilutions were reached. Furthermore, the combination of metformin with graviola leaves extract was applied according to the checkerboard assay.
 |
Figure 1: The combination of metformin and graviola leaves extract |
Statistical Analysis
IBM SPSS statistics 26 was used to analyze the data, pharmacokinetic parameters were determined using WINNONLIN (Version 5.2) software and Microsoft Excel 365. (P ≤0.05) was considered significant.
Results and Discussion
Validation results
To illustrate the previously described HPLC method for determining metformin analysis in rat plasma, EMA guidelines were used to carry out a partial validation method. Utilizing the specified chromatographic procedure, metformin and metronidazole were divided in the three-minute running duration (Figure 2). More than 0.99 was the correlation coefficient (R2) for the calibration curve of metformin for each run. Furthermore, the averages of the accuracy for the QCL, QCM and QCH were (98.33%, 103.52% and 102.67%) respectively. While the precision values were (2.24%, 2.76% and 3.36%), respectively. As illustrated, since all of the results were determined to be within the acceptable limits of the validation parameters, the present assay offers reasonable accuracy, precision, and linearity for metformin over the concentration range examined.
 |
Figure 2: Chromatograms of blank (A). Plasma sample containing metformin 1600 ng/ml and internal standard 20 µg/ml (B). |
Graviola leaves extract reduces Cmax of metformin
Plasma profile of metformin and its pharmacokinetic parameters were illustrated in (Figure 3). Metformin alone reached its maximum plasma concentration Cmax (1509 ng/ml) after 1.5 hours of administration. Pre-administration of graviola leaves extract significantly reduced Cmax of metformin (701 ng/ml) after 1.5 hours.
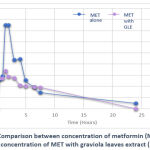 |
Figure 3: Comparison between concentration of metformin (MET) alone and concentration of MET with graviola leaves extract (GLE) |
According to the current findings, pre-administration of graviola leaves extract significantly reduced Cmax of metformin. The possible explanation for the reduction of metformin plasma level with graviola leaves extract is due to graviola leaves extract impact on the absorption of metformin. The current findings are consistent with previous study by (Awad et al., 2016) on the pharmacokinetic interactions between metformin and pomegranate juice in which pomegranate juice significantly reduced Cmax of metformin, the author explained this result is due to metformin absorption mechanism because several studies have showed that its absorption mechanism is mostly a saturable transporter-mediated process, with some of it passing through the membrane by passive diffusion from the small intestine.13 Metformin is a polar compound that is extremely soluble. Permeation is generally the rate-limiting step in the absorption process, which makes the time to achieve maximum concentration in plasma take a lengthy period. However, because renal excretion is often rapid, the form of the plasma level-time profile is mostly determined by absorption variables16,17. Metformin exhibits flip-flop kinetics, meaning that its slow absorption is the rate-limiting factor in its disposal. Clinical trials with metformin have shown decreasing bioavailability at higher doses, suggesting saturable intestinal absorption 18,19. Tmax is an indicator that shows bioavailability rate. The Tmax was found to have decreased slightly, this could result from physiological GIT parameters, including the stomach emptying rate 17.
Measurement of Blood Glucose Levels in Normoglycemic Rats
The results of the blood glucose levels showed no significant change by comparing the first group tests with the second group at 0.0 hours and after 2 hours of metformin administration.
In vitro part
The (IC50) values of the compounds are described in table (2).
Table 2
| Compounds | IC50 (mg/ml) on (MCF-7) | IC50 (mg/ml) on (DU-145) |
| Metformin | 10 | 0.3125 |
| Graviola Leaves Extract | 20 | 5 |
To quantify the combined interactions between the two compounds being tested (the FIC index), the mentioned equation was applied:
Table 3: FIC value classification
| FIC Value | |
| Synergy | <0.5 |
| Antagonism | >4 |
| Additive or indifference | 0.5-4 |
According to the (FIC) value classification, a synergistic activity was obtained between metformin and graviola leaves extract on (MCF-7) cell lines since the (FIC) value <0.5. While an added activity was obtained between metformin and graviola leaves extract on (DU-145) cell lines since the (FIC) value between (0.5 and 4).
The result of this study highlighted the anti-tumor effect of metformin and/or graviola leaves extract on human cancer (MCF-7) and (DU-145) cells. These results of (MCF-7) cells are consistent with those from previous study by (Falah et al., 2017) which demonstrated the inhibitory effect of metformin, Curcumin, and their combination on the proliferation of different cell lines for breast cancer, such as (MCF-7)20. Metformin has been shown in recent studies to have direct anticancer properties or to decrease tumor proliferation indirectly by enhancing insulin sensitivity21. By referring to previous study by (Sahra et al., 2008), In transformed tumor cells, AMPK activation has been shown to have a potent antiproliferative effect. The growth of cancer cells (DU145, PC-3, and LNCaP) was suppressed by metformin because it blocked cell cycle in G0/G122. In addition, according to (Queiroz et al., 2014), By inducing cell cycle arrest in the G0-G1 phase, apoptosis, and cell death, metformin reduces the growth of (MCF-7) cells. Oxidative stress, AMPK, and FOXO3a activation were all found to be linked to these effects. According to several studies, in addition to directly limiting cell proliferation, metformin can also do so indirectly by altering the cell cycle, activating tumor suppressor genes, and causing cell death brought on by elevated oxidative stress21.
Graviola leaves extract evoked suppressive effects. However, it has been found that Annona muricata L. has a richness of phytoconstituents that contribute to its antioxidant capabilities, including annonaceous acetogenins, essential oils and fatty acids, as well as other phytochemicals like flavonoids, alkaloids, polyphenols, and tannins23.
In addition, a strong anticancer medication should weaken cancer cells without harming healthy cells, decreasing the adverse effects. Human prostate cancer cells (PCa) and breast cancer cells (MCF-7, 4T1, MDA-MB-468) undergo apoptosis when subjected to graviola and its active components, however normal cells such breast epithelial cells (MCF-10A) and prostate epithelial cells do not (PWR-1E). As a result, Graviola has a high selectivity for cancer cells. In addition, unlike conventional anticancer medications, which exhibited high toxicity in animals, in vivo investigations confirmed the safety of Graviola on animals 24.
Conclusion
In conclusion, there is a significant inhibitory effect of graviola leaves extract on pharmacokinetic of metformin. Synergistic activity between metformin and graviola leaves extract on breast cancer in vitro is reported, while graviola leaves extract has an additive effect with metformin on prostate cancer.
Acknowledgements
The authors are so grateful to the deanship of faculty of pharmacy & medical sciences; Prof. Ibrahim Al-Adham at University of Petra, the deanship of scientific research at University of Petra for their support, and Mr. Salim Al-Shawabkah from Applied Sciences University for his assistance.
Conflict of Interest
No conflict of interest is declared.
Funding Sources
The study was conducted at the expense of the authors.
References
- Kahraman, C., Arituluk, Z. C., Irem, I., & Cankaya, T. The clinical importance of herb-drug interactions and toxicological risks of plants and herbal products. Medical Toxicology, 1-31. (2020)
CrossRef - Hu, Z., Yang, X., Ho, P. C. L., Chan, S. Y., Heng, P. W. S., Chan, E., … & Zhou, S. Herb-drug interactions. Drugs, 65(9) : 1239-1282. (2005)
CrossRef - Fugh‐Berman, A., & Ernst, E. Herb–drug interactions: review and assessment of report reliability. British journal of clinical pharmacology, 52(5) : 587-595. (2001).
CrossRef - Fugh-Berman, A. Herb-drug interactions. The Lancet, 355(9198) : 134-138. (2000)
CrossRef - Samson, S. L., & Garber, A. J. Metformin and other biguanides: pharmacology and therapeutic usage. International Textbook of Diabetes Mellitus, 641-656. (2015)
CrossRef - Bailey, C. J. Metformin: historical overview. Diabetologia, 60(9) : 1566-1576. (2017)
CrossRef - Bouchoucha, M., Uzzan, B., & Cohen, R. Metformin and digestive disorders. Diabetes & metabolism, 37(2) : 90-96. (2011)
CrossRef - Adak, T., Samadi, A., Ünal, A. Z., & Sabuncuoğlu, S. A reappraisal on metformin. Regulatory Toxicology and Pharmacology, 92 : 324-332. (2018)
CrossRef - Wang, Y. W., He, S. J., Feng, X., Cheng, J., Luo, Y. T., Tian, L., & Huang, Q. Metformin: a review of its potential indications. Drug design, development and therapy, 11 : 2421. (2017)
CrossRef - Kim, G. T., Tran, N. K. S., Choi, E. H., Song, Y. J., Song, J. H., Shim, S. M., & Park, T. S. Immunomodulatory efficacy of standardized Annona muricata (Graviola) leaf extract via activation of mitogen-activated protein kinase pathways in RAW 264.7 macrophages. Evidence-Based Complementary and Alternative Medicine, 2016. (2016)
CrossRef - Qazi, A. K., Siddiqui, J. A., Jahan, R., Chaudhary, S., Walker, L. A., Sayed, Z., … & Macha, M. A. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis, 39(4) : 522-533. (2018)
CrossRef - Abu-Qatouseh, L. Sulforaphane from broccoli attenuates inflammatory hepcidin by reducing IL-6 secretion in human HepG2 cells. Journal of Functional Foods, 75 : 104210. (2020)
CrossRef - Awad, R., Mallah, E., Al Khawaja, B., Dayyih, W. A., El-Hajji, F., Matalka, K. Z., & Arafat, T. Pomegranate and licorice juices modulate metformin pharmacokinetics in rats. Neuroendocrinology Letters, 37(3) : 202-206. (2016).
- Mallah, E. M., Rayyan, W. S., Dayyih, W. A., Elhajji, F. D., Mansour, K. A., Al-Majali, I. S., & Arafat, T. A. Dose-dependent synergistic effect of pomegranate juice on the bioavailability of sildenafil in rats by using HPLC method. Latin American Journal of Pharmacy, 35 (6) : 1277-84.
(2016). - Mallah, E., Saleh, S., Rayyan, W. A., Dayyih, W. A., Elhajji, F. D., Mima, M., … &Arafat, T. The influence of Eruca sativa (Arugula) on pharmacokinetics of sildenafil in rats. Neuroendocrinology Letters, 38(4) : 101-107. (2017).
- Englund, G., Rorsman, F., Rönnblom, A., Karlbom, U., Lazorova, L., Gråsjö, J., … & Artursson, P. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. European Journal of Pharmaceutical Sciences, 29(3-4) : 269-277. (2006)
CrossRef - Awad, R., Mallah, E., Al-Ani, I., Dayyih, W. A., Zakarya, Z., & Arafat, T. Investigation of possible pharmacokinetic interaction of metformin with sugar replacement sweeteners in rats. Journal of Applied Pharmaceutical Science, 6(10) : 210-215. (2016)
CrossRef - Sambol, N. C., Chiang, J., O’Conner, M., Liu, C. Y., Lin, E. T., Goodman, A. M., … & Karam, J. H. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin‐dependent diabetes mellitus. The Journal of Clinical Pharmacology, 36(11) : 1012-1021. (1996)
CrossRef - Ali Kadhim, K., Khalil Ismael, D., Hoshi Khalaf, B., Ibrahim Hussein, K., Hashim Zalzala, M., & Abdulrahman Hussain, S. Dose-dependent relationship between serum metformin levels and glycemic control, insulin resistance and leptin levels in females newly diagnosed with type 2 diabetes mellitus. Journal of Diabetes Mellitus, 2(02) : 179-185. (2012).
- Falah, R. R., Talib, W. H., & Shbailat, S. J. Combination of metformin and curcumin targets breast cancer in mice by angiogenesis inhibition, immune system modulation and induction of p53 independent apoptosis. Therapeutic advances in medical oncology, 9(4) : 235-252. (2017)
CrossRef - Queiroz, E. A., Puukila, S., Eichler, R., Sampaio, S. C., Forsyth, H. L., Lees, S. J., … & Khaper, N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PloS one, 9(5), e98207. (2014)
CrossRef - Sahra, I. B., Laurent, K., Loubat, A., Giorgetti-Peraldi, S., Colosetti, P., Auberger, P., … & Bost, F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene, 27(25) : 3576-3586. (2008)
CrossRef - Agu, K. C., & Okolie, P. N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food science & nutrition, 5(5) : 1029-1036. (2017)
CrossRef - Awad, M. G., Ali, R. A., El-Monem, A., Dalia, D., & El-Magd, M. A. Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Molecular & Cellular Toxicology, 16(4) : 385-399. (2020)
CrossRef








