Raden Joko Kuncoroningrat Susilo1 , Dwi Winarni2
, Dwi Winarni2 , Suhailah Hayaza1
, Suhailah Hayaza1 , Sri Puji Astuti Wahyuningsih2
, Sri Puji Astuti Wahyuningsih2 , Ruey-an Doong3
, Ruey-an Doong3 , Win Darmanto2,5*
, Win Darmanto2,5* and Bilqis Inayatillah4
and Bilqis Inayatillah4
1Department of Nanotechnology Engineering, Faculty of Advance Technology and Multidiscipline, Universitas Airlangga, Surabaya 60115, Indonesia.
2Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia.
3Institute of Analytical and Environmental Sciences, National Tsing Hua University, Sec. 2 Kuang Fu Road, Hsinchu 30013, Taiwan.
4Department of Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
5Institute of Science, Technology, and Health, Jombang 61419, Indonesia
Corresponding Author E-mail: windarmanto@fst.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2588
Abstract
Liver fibrosis was regarded as result of wound healing process in chronic liver injury. The α-smooth muscle actin (α-SMA) and matrix metalloproteinase-1 (MMP1) are several indicators for extracellular matrik (ECM) production which relate in fibrosis process. This study aims to determine the effect of Ganoderma applanatum crude polysaccharide (GACP) extract on carbon tetrachloride (CCl4)-against α-smooth muscle actin and matrix metalloproteinase expressions. A total of 24 male mice strain BALB/C, 3-4 months were divided into 6 groups. control group (distilled water and olive oil), model group (distilled water and CCl4), low G. applanatum crude polysaccharide group (G. applanatum extract at the dose of 25 mg/kg BW and CCl4), middle G. applanatum crude polysaccharide group (G. applanatum extract at the dose of 50 mg/kg b.w and CCl4), high G. applanatum crude polysaccharide group (G. applanatum extract at the dose of 100 mg/kg BW and CCl4) and silymarin group (silymarin 100 mg/kg BW and CCl4). Expressions of α-SMA and MMP1 were examined by immunohistochemical methods. The observation of immunohistochemistry used an inverted microscope at 200× magnification. The results of the observation were analyzed using ImageJ software to measure the percentage of α-SMA and MMP1 expressions. All of the data were analyzed by GraphPad Prism Software. The results showed that G. applanatum extracts prevented a significant increase in α-SMA expression and decrease MMP1 expression (p < 0.05) in comparison with the model group. Therefore, G. applanatum extracts play as liver protector against chronic liver injury after successfully inhibit α-SMA expression and prevent reduction of MMP1 expression.
Keywords
α-Smooth Muscle Actin; Fibrosis; Ganoderma applanatum; Healthy Life; Liver; Matrix Metalloproteinase
Download this article as:| Copy the following to cite this article: Susilo R. J. K, Winarni D, Hayaza S, Wahyuningsih S. P. A, Doong R. Darmanto W, Inayatillah B. The Effect of Ganoderma applanatum Crude Polysaccharide Against α-Smooth Muscle Actin and Matrix Metalloproteinase-1 Expressions in Mice after Induced by Carbon Tetrachloride. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Susilo R. J. K, Winarni D, Hayaza S, Wahyuningsih S. P. A, Doong R. Darmanto W, Inayatillah B. The Effect of Ganoderma applanatum Crude Polysaccharide Against α-Smooth Muscle Actin and Matrix Metalloproteinase-1 Expressions in Mice after Induced by Carbon Tetrachloride. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3Iv0Ili |
Introduction
Liver fibrosis is one of the most deadly diseases in the world. Many fibrosis patients rarely got aid which increases fibrosis cases. Liver fibrosis is regarded as result of wound healing process in chronic liver injury. Carbon tetrachloride (CCl4) is often regarded as a key factor to stimulate fibrosis. This chemical was metabolized by the liver into radical carbon trichloromethyl (CCl3).1 Furthermore, this radical is so reactive to the cell membrane, leading to impairment in the cell membrane. This pathway caused chaos in mitochondria, hence accelerating necrotic and apoptotic cells. Death signals trigger the mobilization of macrophages into the liver cell and produce numerous pro-inflammatory cytokines and growth factors.2 This pathway leads to the transformation from hepatic stellate cells (HSCs) into myofibroblasts. At this stage, myofibroblast display α-smooth muscle actin (α-SMA) marker to start producing extracellular matrix (ECM). Fibrosis is a type of disease which have excessive ECM. The body has its mechanism to decrease ECM from tissue.3 Matrix metalloproteinases (MMPs) is degradation enzyme that plays a vital role to decrease ECM. The role of MMP is to antagonize tissue inhibitor metalloproteinases (TIMPs) which increase ECM in tissue.4
Recently, natural sources become a primary treatment to cure degenerative diseases such as cancer, fibrosis, diabetes, and Alzheimer’s. The low price, less toxicity, and no adverse side effect are several reasons for most people to use natural source treatment.5 Ganoderma applanatum is known as medicinal fungi from the Basidiomycota phylum. This mushroom has several pharmacology activities such as anti-cancer, anti-diabetic, antioxidant, anti-fibrosis, anti-inflammatory, and immunomodulatory.6-8 Mainly, G. applanatum polysaccharides are often used in cancer therapy such as lung cancer, breast cancer, liver cancer, and colon cancer. The polysaccharides of G. applanatum also act as immunomodulators and give potency to inhibit the transdifferentiation process towards myofibroblasts.9 Several studies have shown the effect of polysaccharides on liver fibrosis. However, a study with G. applanatum polysaccharides on liver fibrosis is still measly done. This study aimed to measure the effectiveness of G. applanatum crude extract polysaccharide on the production of α-SMA and MMP1 expressions in mice after induced by CCl4.
Material And Methods
Preparation of Extract
Firstly, the basidiocarp of G. applanatum was mashed until become powder. Hereafter, the powder was boiled with distilled water at 90°C (1:10) for 6 hours. After that, the boiling result was filtrated with Whatman Filter Paper. The filtrate was added with ethanol absolute (1:3) and incubated at 4°C for 24 hours. The centrifugation process was done after incubation with 3000×g for 15 minutes and the process was repeated three times based on the above method. The supernatant was disposed from the microtube and the pellet was used for the next step preparation of extract. Finally, the pellet was lyophilized to get G. applanatum crude polysaccharide extract, then the extract was kept at 25°C.
Animals
Twenty-four healthy BALB/c male mice were obtained from the Faculty of Pharmacy, Universitas Airlangga, Indonesia. All mice were acclimatized for 2 weeks at the Animal Laboratory, Faculty of Science and Technology, Universitas Airlangga, Indonesia. All mice were also got fed and beverage with ad libitum which free access to consume. The day/night cycle was also watched for 12 h at 24°C. Ethical approval of animal care has been approved by the Ethical Committee of the Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia (2.KE.168.10.2018).
Experimental Design
The study was held for 4 weeks. Inducing hepatic fibrosis, a dose of 2 mL/kg bodyweight CCl4
was injected intraperitoneally (i.p) twice a week for four weeks (dissolved in olive oil; 1:3). All mice were divided into six groups, namely the control group (only placebo), model group (only CCl4), low G.applanatum crude polysaccharide group (GACP 25 mg/kg BW and CCl4), middle G. applanatum crude polysaccharide group (GACP 50 mg/kg BW and CCl4), high G. applanatum crude polysaccharide group (GACP 100 mg/kg BW and CCl4) and silymarin group (silymarin 100 mg/kg BW and CCl4). Moreover, GACP induction was conducted every day for 4 weeks orally. The day after 4 weeks, all of the mice were dissected to get whole blood via intracardiac and liver samples for immunohistochemistry measurement.
Immunohistochemistry Analysis
The immunohistochemistry method was used to measure α-SMA and MMP1 expression. Briefly, deparaffinization and rehydration by added xylene and gradation ethanol. After that, the antigen unmasking procedure was held by rinsing in a citrate base. Blocking of endogenous peroxidase was performed by incubation and blocking solution (3% H202) for 10 minutes. Washing with PBS was done for 5 minutes. Furthermore, incubation with normal blocking serum for 20 minutes and with primary antibody (goat anti-mouse α-SMA/MMP1) for 30 minutes. Hereafter, incubation with a secondary antibody (donkey anti-goat IgG) for 30 minutes. Visualization immunoreaction with 0.1% 3,3-diaminobenzidine (DAB) for 4 minutes. Counterstaining with hematoxylin. An inverted microscope was used to observe α-SMA and MMP1 expression. All data from microscope was analyzed by Image J software to determine the expression of sample.
Statistical Analysis
All of the data were analyzed with GraphPad Prism Software (version 8). One-way ANOVA was used to determine the significance of the effect of the GACP extract. Meanwhile, the Tukey test was used to display the significant difference between the model group and treatment groups. P < 0.05 was set as statistical significance.
Results
Effect of GACP extract on α-SMA expression
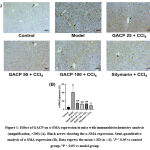
As shown in Figure 1A, there was measly α-SMA expression in normal control. However, another sight was found in the model control group which expresses α-SMA intensely. This expression was evenly distributed not only centered around of central vein but almost in between hepatocytes. Furthermore, the silymarin group showed a mild α-SMA expression in liver fibrosis and a dose of 25 mg/kg of GACP also exhibited slight α-SMA. GACP treatment in dose 50 mg/kg displayed slightly lower expression than dose 25 mg/kg. The dose of 100 mg/kg of the GACP group had a similar result to the dose of 50 mg/kg group in α-SMA expression. In a statistical evaluation from Figure 1B, CCl4 caused a significantly increased (P < 0.05) in α-SMA expression compared to the normal control group. Moreover, the model control group also produced the highest value than other groups (41.09 ± 14.07 %). The GACP and silymarin induction were significantly lower than the model control group (P < 0.05) in α-SMA expression. Especially, the silymarin group exhibited the lowest value (9.58 ± 1.31 %).
Effect of GACP extract on MMP1 expression
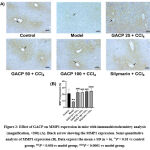
Figure 2A showed a bold expression of MMP1 obviously in the normal control group. This expression indicated normal expression in normal cells whereas no disturbance in MMP1 protein synthesis. Furthermore, the model control group displayed conversely delicate MMP1 expression. Silymarin and other GACP groups demonstrated the high expression of MMP1 as similar to with normal control group whereas the expression was spread out among hepatocytes. Figure 2B revealed that the model control group was significantly lower than the (P < 0.05) normal control group and gave the lowest value too (8.77 ± 0.75 %). Silymarin and GACP group treatment demonstrated preventing effect in comparison with the model control group which keeps the MMP1 enzyme from declining expression (P < 0.05). Moreover, the silymarin group showed the highest value in MMP1 expression (25.16 ± 3.78 %). These results confirmed that the GACP effect indirectly prevents transdifferentiation as a primary mechanism of ECM production.
Discussion
Pro-inflammatory cytokine and growth factors become key factors in the fibrosis process. Several studies about fibrosis treatment aim to decrease pro-inflammatory and growth factor levels. Natural source treatment, especially with medicinal fungi is regarded as having high efficacy to protect from fibrosis. One of the key points in fibrosis is the transdifferentiation of HSCs into myofibroblast.10 Moreover, this transdifferentiation produces high ECM such as collagen I, III, and V which are usually found in the portal stroma, liver capsule, and space of Disse. Moreover, other types of ECM such as collagen IV, XVIII, and laminin are constituents of the basal-like membrane of the sinusoid.11
This study showed an increase in α-SMA levels in the model group compared to the normal group, this indicates a transdifferentiation process from HSCs to myofibroblasts. Induction of CCl4 triggers injury to liver cells caused by lipid peroxidation in cell membranes.12 As a result of this injury, liver cells emit DAMP signals to attract immunomodulatory cells such as macrophages, NK cells, dendritic cells, and neutrophils. Furthermore, these immunomodulatory cells secrete pro-inflammatory cytokines and growth factors to accelerate the trans-differentiation process.13 Furthermore, the production of α-SMA is increasing as a marker of this process. Administration of silymarin and GACP affects protection against an increase in α-SMA marker which indicates low trans differentiation activity into myofibroblasts. The administration of GACP acts as an anti-inflammatory against the increase in pro-inflammatory cytokines derived from immunomodulatory cells. The anti-inflammatory process is derived from the failure of IKK phosphorylation which then causes the failure of NF-κB translocation.14 This failure is related to the unsuccessful pro-inflammatory cytokine gene transcription process, hence cytokines produced have low levels. Furthermore, at least pro-inflammatory cytokines are not enough to carry out the transdifferentiation process so the α-SMA marker detected does not show a higher number than fibrotic mice.15
This study also explored MMP1 in fibrotic mice where the enzyme plays a role in degrading excess ECM in liver cells. Along with the TIMP enzyme as an antagonist to MMP, the balance of these two enzymes plays a role in the production of the resulting ECM.16 This study discusses the GACP effect on the percentage of MMP1 in fibrotic mice. Based on the previous explanation, CCl4 induction can cause damage to the liver so the inflammatory process is needed as a response to the recovery of liver tissue. Furthermore, ECM is produced to patch the tissue with chronic liver injury. Increasing ECM levels, MMP1 is produced to degrade excess ECM.17 The model group showed a low percentage of MMP1 levels produced by liver tissue during the fibrosis process. This is due to high levels of MMP1 used to degrade ECM. Therefore, immunohistochemical analysis showed the least percentage of MMP1. Otherwise, GACP induction displayed a high percentage of MMP1 in liver fibrotic tissue. This allows the percentage of MMP1 available in liver tissue. The high MMP1 also indicates that less ECM is available in the GACP treatment group. The anti-inflammatory bioactivity factor of GACP is thought to be the cause of low ECM production. The anti-inflammatory activity can slow down the trans-differentiation of HSCs, hence resulting in lower ECM production.18 As a result, the percentage of available MMP1 is still in the abundant category because it has not been used to degrade ECM. The overall results of this study indicate that GACP has a high potential to inhibit α-SMA expression and prevent reduction of MMP1 expression.
Conclusion
Inducing with GACP extract inhibit elevation of α-SMA expression and prevent reduction of MMP1 expression. This study proved that GACP could take a role as protector in liver from fibrosis.
Acknowledgement
All of authors thank to pathology laboratory from Faculty of Medicine, Universitas Airlangga, Indonesia for assist in helping histology preparation.
Conflict of Interest
All of authors state that there is no conflict of interest in this study.
Funding Source
The study was funded by grant research from The Ministry of Education, Culture, Research, and Technology of Indonesia (848/UN3.14/PT/2020).
References
- Chen S, Chen Y, Chen B, Cai YJ, Zou ZL, Wang JG, et al. Plumbagin ameliorates CCl4-induced hepatic fibrosis in rats via the epidermal growth factor receptor signaling pathway. Evidence-based Complement Altern Med. 2015;2015(3).
CrossRef - Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3(2):977–1010.
CrossRef - Inoue A, Obayashi K, Sonoda Y, Nakamura A, Ueno T, Kuhara S, et al. Regulation of matrix metalloproteinase-1 and alpha-smooth muscle actin expression by interleukin-1 alpha and tumour necrosis factor alpha in hepatic stellate cells. Cytotechnology. 2017;69(3):461–8.
CrossRef - Geervliet E, Bansal R. Matrix Metalloproteinases as Potential Biomarkers. J Cells. 2020;9(1212):2–20.
CrossRef - Ahad S, Tanveer S, Malik TA. Anticoccidial activity of aqueous extract of a wild mushroom (Ganoderma applanatum) during experimentally induced coccidial infection in broiler chicken. J Parasit Dis. 2016;40(2):408–14.
CrossRef - Susilo RJK, Winarni D, Husen SA, Hayaza S, Punnapayak H, Wahyuningsih SPA, Sajidah ES, Darmanto W (2019) Hepatoprotective effect of crude polysaccharides extracted from Ganoderma lucidum against carbon tetrachloride-induced liver injury in mice, Veterinary World, 12(12):1987-1991.
CrossRef - Hayaza S, Darmanto W, Wahyuningsih SPA, Susilo RJK, Husen SA, Winarni D, Doong R. Immunomodulatory Activity of Okra Raw Polysaccharide Extract by Regulating TNF-A, IFN-G Levels, and Cell Apoptosis in DEN-induced mice. Research Journal of Pharmacy and Technology. 2022; 15(2):546-0. doi: 10.52711/0974-360X.2022.00088
CrossRef - Hayaza S, Wahyuningsih SPA, Susilo RJK, Husen SA, Winarni D, Doong RA, Darmanto W. Dual role of immunomodulation by crude polysaccharide from okra against carcinogenic liver injury in mice. Heliyon. 2021 Feb 11;7(2):e06183. doi: 10.1016/j.heliyon.2021.e06183
CrossRef - Dandapat S, Kumar M, Ranjan R, Sinaha MP. Study of Impacts of Ganoderma applanatum (Pres.) Pat. Extract on Hepatic and Renal Biochemical Parameters of Rats. Maj Obat Tradis. 2019;24(2):119.
CrossRef - Lin X, Wen J, Liu R, Gao W, Qu B, Yu M. Mv-V24-789. 2018;(December):789–800.
- Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017;60:44–65.
CrossRef - Vuda M, D’Souza R, Upadhya S, Kumar V, Rao N, Kumar V, et al. Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in rats. Exp Toxicol Pathol. 2012;64(7–8):855–9.
CrossRef - Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(SUPPL.2):89–93.
CrossRef - Zhang H, Sun SC. NF-ΚB in inflammation and renal diseases. Cell Biosci. 2015;5(1):1–12.
CrossRef - Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020;8:205031212096575.
CrossRef - Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells. 2020;9(5):1–34.
CrossRef - Cabral-Pacheco GA, Garza-Veloz I, Rosa CCD La, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):1–53.
CrossRef - McQuitty CE, Williams R, Chokshi S, Urbani L. Immunomodulatory Role of the Extracellular Matrix Within the Liver Disease Microenvironment. Front Immunol. 2020;11(November):1–33.
CrossRef