Jinan Tuma Sabah1* and Firas Rahi Alhachami2
and Firas Rahi Alhachami2
1Pathological analysis department, Faculty of sciences, Wasit university, Wasit, Iraq.
2Radiology department, college of health and medical technology, Al-Ayen university, Dhi-Qar, Iraq.
Corresponding Author E-mail: jtuma@uowasit.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2602
Abstract
Arabic frankincense is distinguished by its many medical and therapeutic benefits, as it treats many backward diseases that affect the human body. Smoking causes a long list of cancers, on top of which are lung cancer. Smoking cessation can prevent a third of cancer-related deaths. The study aimed to reveal the role of frankincense in reducing the genotoxicity of smoking on the buccal mucosa using buccal micronucleus cytome (BMCyt) assay. For this purpose, 50 smokers and 30 non-smokers participated; all of them were students of Wasit University. The study showed that smoking causes a significant increase in the level of nuclear abnormalities. Apoptosis showed the biggest change, with an about twenty-fold increase, followed by cytotoxicity (about four folds increase), and mutagenicity (about three-folds) as compared with control. A significant decrease in mutagenicity and cytotoxicity was observed (P= 0.038 and 0.051, respectively) after 4 weeks of chewing frankincense gum by smokers while increase was observed with apoptosis (P= 0.071). We conclude from this study results that chewing gum exhibited pro-apoptotic and anti-proliferative activities against cancer-damaged cells.
Keywords
Apoptosis; Buccal Micronucleus Cytome (BMCyt) assay; Carcinogenesis; Frankincense; Genotoxicity; Micronuclous; Smoking
Download this article as:| Copy the following to cite this article: Sabah J. T, Alhachami F. R. Potential Anti-Cancer Properties of Frankincese (Boswellia Sarca) Chewing Gum and its Role in Reduction of Tobacco Smoking Genotoxicity. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Sabah J. T, Alhachami F. R. Potential Anti-Cancer Properties of Frankincese (Boswellia Sarca) Chewing Gum and its Role in Reduction of Tobacco Smoking Genotoxicity. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3LJs8FY |
Introduction
Frankincense was a great traditional medicine in the Arab countries. It has been used to treat burns, bruising, digestive disorders, joint pain, and respiratory distress. It also exhibits antibacterial activity against a variety of microorganisms 1. In the family Burseraceae, the Boswellia genus of trees produce frankincense, an aromatic resin that hardens into irregular lumps of yellowish brown gum (Figure 1) 1. Alkaloids, polyphenols, penta- and tetracyclic terpenoids, tannins, saponins, essential oils, and other chemicals are found in high concentrations in frankincense resin.2,3.
Recent research has shown that frankincense extracts can inhibit the growth of tumors in animal cells and induce tumor apoptosis 4. Boswellia sacra essential oil decreases tumor aggressiveness and encourages tumor-specific apoptosis in cultured human breast cancer cells5. Boswellic acids have also been shown to have anticancer potential due to their pro-apoptotic and anti-proliferative activities several of human cancer cell lines, including leukemia cells, meningioma cells, and hepatoma cells6,7 In a naked mouse model of pancreatic cancer, boswellia sacra gum reduces tumor growth. It possesses cytotoxicity and tumor cell growth arrest in multiple types of human cancer cell lines in cultures, simultaneously activating multiple pathways leading to cancer cell-specific death 6. Additionally, it has been hypothesized that more than 50% of natural cancer therapies can trigger apoptosis.8.
Smoking tobacco is recognized as a significant public health issue with global adverse effects. The effects of tobacco smoking on health were primarily limited to industrialized nations; nevertheless, the promotion and uptake of this habit in emerging countries are currently causing a massive rise in smoking-related illness and mortality on a global scale7,9 There are an estimated more than 1 billion smokers worldwide, and smoking is thought to be the cause of about 3 million deaths each year, with this number increasing to 10 million in the next 30 to 40 years 10. According to estimates, half a billion of those alive now will pass away from a disease linked to tobacco use. 11.
Smoking-related genotoxic effects have been discovered in all eight organs that have been studied. Oral/nasal, pharynx/larynx , esophagus, lung, myeloid organs, pancreas, uterine cervix and bladder/ureter cancer are all brought on by smoking in people12. Smokers’ lung tumors display a high frequency and specific spectrum of KRAS and TP53 mutations, which point to the presence of PAHs (and possibly other substances) in the smoke 12,13. These findings are consistent with a concept of tobacco smoke carcinogenesis wherein the carcinogenic process is driven by mutations that accumulate in a field of tissue as a result of tobacco smoke’s components leading to smoking.
Over the past 20 years, there has been significant research how cigarette smoke encourages these cancers and other health issues. One method concerns the tobacco smoke’s mutagenic properties, which have been amply shown and discussed two decades ago14,15 Recent reviews have gathered research on DNA and protein adducts-related smoking in human tissues15,16 adding to the cigarette smoke exposure-related chemical biomarkers 17.
One of the best biomarkers that may be utilized to identify the genotoxicity of smoking is micronuclei (MN). Micronuclei have been used as markers for genotoxic exposure since 1937.18. Micronucleus refers to the small nucleus that originates whenever a whole chromosomes or chromosome fragments is not integrated into the main daughter nuclei during cell division. It indicates chromosomal instability and genotoxic events[19]. Besides the micronuclei, there are other nuclear abnormalities that were considered as additional biomarkers for genotoxic effects of numerous compounds, such as binucleated cells (BN), nuclear bud (NB), pyknosis (PN), karyorrhexis (KR), karyolysis (KL). 19,20
Therefore, we used the micronuclei test on buccal mucosal cells from young smokers to investigate the potential inhibitory effect of frankincense gum chewing on cytogenetic abnormalities development and risks of tumor growth.
Material and methods
Frankincense chewing gum preparation
Frankincense chewing gum was obtained from herbal stores in one of the famous markets in the wasit governorate in January 2022. The species of frankincense (Boswellia sacra) was identified by the professors who specialized in plant sciences at the Faculty of Science, Wasit University. Participants were provided with approximately 2±0.2 gm of gum and were asked to chewing gum for more than three hours per day and during smoking times.
Study group
Fifty smokers who had smoked more than ten cigarettes a day for at least a year were included in this study. They were all male students at Wasit University in Iraq, between the ages of 20 and 25. As a control sample, 30 more students who did not smoke were chosen. The following were included as exclusion criteria: the presence of a visible lesion on the oral mucosa, chronic illnesses, a history of cancer or diabetes, and recent X-ray exposure or medical treatment. Following consent from the Student Committee of Wasit, Iraq, and the Scientific Council of the Science College, university of Wasit, this study was conducted in the pathological analysis department’s labs in the college of science.
Sample collection
Both smokers and non-smokers’ buccal exfoliated cells were collected on September 3 in accordance with Thomas et al instructions with a few minor modifications. 21. Then, buccal exfoliated cells from all smokers were collected at 0 weeks and considered as control. Then the participants were asked to chew Frankincense gum daily for at least 3 hours. Buccal samples were taken weekly for four weeks and sent directly to the laboratory for examination. The sample collection was carried out in the period of 10 September – 25 October 2022. participants were asked to rinse their mouth twice with distal water before sample collection. The exfoliated buccal mucosa cells were scrapped gently using cylindrical cytological brush from both cheeks. The brush’s head was then dipped into tubes containing 3 mL of phosphate buffered saline (PBS) (Sigma-Aldrich-USA), centrifuged for 5 minutes at 800 rpm, and the supernatant was removed. The pellet was re-suspended and dropped onto microscope slides, fixed in 3:1 methanol/acetic acid (Merck, Germany) for 15 min. Then, Coded slides were dyed for 30 minutes at room temperature with Giemsa dye (Hercules, CA, USA), and examined under a light microscope (400x ).
Buccal micronucleus cytome (BMCyt) assay
A total of 1,000 cells per subjects from the control and exposed groups were examined following the method of Buajeeb et al [22]. Micronuclei were scored as a parameter of DNA damage (mutagenicity). Karyorrhexis (nuclear disintegration) and karyolysis (nuclear dissolution) were classified as apoptosis. For cytotoxicity, the following nuclear abnormalities were considered: binucleated cells, nuclear buds and pyknosis, the frequencies of nuclear abnormalities were reported in 1000 differentiated cells.
Statistical analysis
All of the biological examination results were entered into a database created in Microsoft Excel, and SPSS version 23 was used for statistical analysis. The data on genetic damage between the smokers and control groups were examined using a Poisson regression analysis. one-way analysis of variance (ANOVA) were applied to compare MN, BN, NB, KR, KL, and PN frequencies with chewing period. The frequencies of nuclear abnormalities per 1000 cells (‰) was reported, with counts indicated as mean ±standard deviation (SD). Statistical significance was defined as a P value 0.05.
Result and discussion
Characteristic of participants
This study included 50 smokers and 30 nonsmokers who were 22.9 years old. All individuals were of male gender, to avoid the influence of sex on the results. Furthermore, due to Arab cultural and religious norms, there aren’t many female smokers in the study area who are of the target age.
Genotoxic effect of smoking
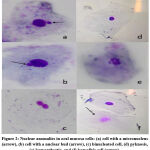
To identify the genotoxic effect of cigarette smoking and cancer development, the frequencies of nuclear anomalies in smokers buccal exfoliated cells were compared with frequencies among non-smokers students. Figure 2 shown the representative photographs of micronucleated cells and other nuclear abnormalities.
Only 0.0 to 0.9% of cells in the general population had micronuclei. Any variation in this range occurs as a result of an genotoxic effect that leads to chromosomal abnormalities [23]. In this study, the results indicated significant increase in the mutagenicity (number of micronucleated cells), Cytotoxicity (binucleated, nuclear buds and pynkosis) and Apoptosis (karyolrrhetic and karyolitic cells) in smokers as compared to non-smokers as shown in table 1. The most significant was observed for apoptosis, where smokers had an about 20-fold higher rate than non-smokers, Cytotoxicity (4 folds increase), and mutagenicity (3-folds increase). It is a clear indication of the genotoxic effect of cigarette smoking. Alkaline smoke produced by cigarettes contains free radicals which react with other combustion byproducts or living cells and cause DNA damage [24]. Genotoxicity assay appeared to be a promising biomarker for assessing the impact of carcinogens in smoke. It has been observed that the number of chromosome breaks increases when exposed to carcinogenic substances, and it has been shown that these breaks are a precursor to the cancer development. [24, 25]. The present results indicated that apoptosis eliminates the cells with genetic damage and act as a surveillance mechanism [26]. Thus, increased percentage frequencies of karyolysis may indicate of necrotic cells and genotoxic insult, as these occur in the pre-keratinization process [27].
Table 1: nuclear abnormalities (‰) in the buccal exfoliated cells of smokers and non-smokers.
| Category | Sample size | mutagenicity (mean ‰ ) | Other nuclear alterations (mean ‰ ) | |
| Cytotoxicity | Apoptosis | |||
| Control | 30 | 6.96 ± 1.38 | 2.01 | 3.24 ± -1.7 |
| smokers | 50 | 19.47 ± 4.04 | 9.85± 6.46 | 67.55±13.18 |
| P value | 0.032 | 0.026 | 0.007 | |
Potential anti-cancer activity of frankincense gum
Smokers were asked to start chewing gum for four weeks for three hours a day, preferably during the smoking period, after measuring the micronucleus and other nuclear abnormalities in their buccal mucosa (0 week). This was done to investigate the role of frankincense gum in treating and reducing cancer risks. The study showed a noticeable decline in the number of micronuclei and in the rate of Cytotoxicity with P value 0.038 and 0.051, respectively. While apoptosis showed slightly increased with insignificant difference (P=0.071) (Figure 3).
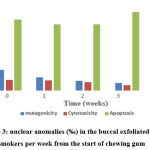 |
Figure 3: nuclear anomalies (‰) in the buccal exfoliated cells of smokers per week from the start of chewing gum. |
We found that frankincense has intense growth suppression activity and can induce apoptosis, this observation is in agreement with an earlier article that frankincense is help to treat cultured human colon, breast, bladder, and pancreatic tumor cells [1, 28]. Traditional Chinese medicine has included resins from frankincense gum as a component of anticancer medications. Many elements of frankincense essential oil’s complex chemical composition may cooperate to produce a strong anti-cancer effect.
The pro-apoptotic and anti-proliferative and effects of frankincense essential oil may work together as anti-cancer. If PI3K/Akt activation and cell cycle progression are reduced while growth arrest genes are simultaneously expressed more highly, frankincense essential oil-induced growth inhibition may result [1]. Based on caspase-3, -8, and -9 cleavages and activation, as well as poly (ADP-ribose) polymerase (PARP), in many human tumor cell, frankincense essential oil causes apoptosis [5, 28].
Conclusion
We examined the frequency of nuclear abnormalities in the exfoliated buccal cells of 50 smokers as a biomarker of DNA damage and cytotoxic effects. According to the study, smoking significantly raises the levels of apoptosis, cytotoxicity, and mutagenicity when compared to a control group (20-folds, 4 folds and 3-folds increase, respectively). Frankincense has a broad range of pharmacological potential. It showed a noticeable decline in the number of micronuclei and in the rate of cytotoxicity with P value 0.038 and 0.051, respectively. Given that frankincense has anti-cancer properties via inducing apoptosis in cancer-damaged cells, apoptosis showed a slight increase with the insignificant difference (P=0.071). All ages can benefit from using this herbal medication to improve their oral hygiene. To administer frankincense essential oil for anti-cancer therapy as effectively as possible, pharmacokinetics and pharmacodynamic investigations are also necessary.
Conflict of Interest
The author declare that there is no conflict of interests regarding the publication of this paper.
Funding Sources
The authors received no financial support for the study, authorship, and publication of this article.
References
- Frank, M.B., et al., Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complementary and Alternative Medicine, 2009. 9(1): p. 1-11.
CrossRef - Maupetit, P., New constituents in olibanum resinoid and essential oil. Perfumer & flavorist, 1984. 9(6): p. 19-37.
- Morikawa, T., H. Matsuda, and M. Yoshikawa, A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. Journal of oleo science, 2017: p. ess16149.
CrossRef - Huang, M.T., et al., Anti‐tumor and anti‐carcinogenic activities of triterpenoid, β‐boswellic acid. Biofactors, 2000. 13(1‐4): p. 225-230.
CrossRef - Ni, X., et al., Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complementary and Alternative Medicine, 2012. 12(1): p. 1-14.
CrossRef - Park, Y.S., et al., Cytotoxic action of acetyl-11-keto-β-boswellic acid (AKBA) on meningioma cells. Planta medica, 2002. 68(05): p. 397-401.
CrossRef - Liu, J.-J., et al., Keto-and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. International journal of molecular medicine, 2002. 10(4): p. 501-505.
CrossRef - Dholwani, K., et al., A review on plant-derived natural products and their analogs with anti-tumor activity. Indian journal of pharmacology, 2008. 40(2): p. 49.
CrossRef - Wong, P.K., J.J. Christie, and J.D. Wark, The effects of smoking on bone health. Clinical science, 2007. 113(5): p. 233-241.
CrossRef - Papp-Szabó, E., et al., Mutagenicity of the oral carcinogen 4-nitroquinoline-1-oxide in cultured BigBlue™ rat tongue epithelial cells and fibroblasts. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2003. 522(1-2): p. 107-117.
CrossRef - De Flora, S., et al., Modulation of cigarette smoke-related end-points in mutagenesis and carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2003. 523: p. 237-252.
CrossRef - DeMarini, D.M., Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutation Research/Reviews in Mutation Research, 2004. 567(2-3): p. 447-474.
CrossRef - Besaratinia, A. and S. Tommasi, Genotoxicity of tobacco smoke-derived aromatic amines and bladder cancer: current state of knowledge and future research directions. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 2013. 27(6): p. 2090-2100.
CrossRef - Baier, G., et al., Respiratory diseases and genotoxicity in tobacco smoke exposed children. Laryngo-rhino-otologie, 2002. 81(3): p. 217-225.
CrossRef - Maertens, R.M., et al., The genotoxicity of mainstream and sidestream marijuana and tobacco smoke condensates. Chemical research in toxicology, 2009. 22(8): p. 1406-1414.
CrossRef - Bombick, D., et al., Evaluation of the genotoxic and cytotoxic potential of mainstream whole smoke and smoke condensate from a cigarette containing a novel carbon filter. Toxicological Sciences, 1997. 39(1): p. 11-17.
CrossRef - Upadhyay, M., et al., Micronuclei in exfoliated cells: A biomarker of genotoxicity in tobacco users. Nigerian Journal of Surgery, 2019. 25(1): p. 52-59.
CrossRef - Kamath, V.V., P. Anigol, and K. Setlur, Micronuclei as prognostic indicators in oral cytological smears: A comparison between smokers and non-smokers. Clinical cancer investigation journal, 2014. 3(1): p. 49.
CrossRef - Sabah, J.T., Evaluation of genotoxic damage in buccal mucosa cytome assays in Iraqi school children exposed to air pollutants emanating from oil fields. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2021. 863: p. 503304.
CrossRef - Castañeda-Yslas, I.J., et al., Biomonitoring with micronuclei test in buccal cells of female farmers and children exposed to pesticides of Maneadero agricultural valley, Baja California, Mexico. Journal of Toxicology, 2016. 2016.
CrossRef - Thomas, P., et al., Buccal micronucleus cytome assay. Nature protocols, 2009. 4(6): p. 825-837.
CrossRef - Buajeeb, W., et al., Frequency of micronucleated exfoliated cells in oral lichen planus. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2007. 627(2): p. 191-196.
CrossRef - Naderi, N.J., S. Farhadi, and S. Sarshar, Micronucleus assay of buccal mucosa cells in smokers with the history of smoking less and more than 10 years. Indian Journal of Pathology and Microbiology, 2012. 55(4): p. 433.
CrossRef - Yerlagudda, K., V.V. Kamath, and K. Satelur, Morphological assessment of oral cytological smears before and after application of toluidine blue in smokers and nonsmokers. Int J Oral Maxillofac Pathol, 2012. 3: p. 8-14.
- Bonassi, S., et al., Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. Cancer Research, 2000. 60(6): p. 1619-1625.
- Palve, D.H. and J.V. Tupkari, Clinico-pathological correlation of micronuclei in oral squamous cell carcinoma by exfoliative cytology. Journal of Oral and Maxillofacial Pathology, 2008. 12(1): p. 2.
CrossRef - Shaul, O., et al., Mycorrhiza-induced changes in disease severity and PR protein expression in tobacco leaves. Molecular Plant-Microbe Interactions, 1999. 12(11): p. 1000-1007.
CrossRef - Suhail, M.M., et al., Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC complementary and alternative medicine, 2011. 11(1): p. 1-14.
CrossRef