Ruaa Mohammed Ibrahim , Nabaa M. Ibrahim
, Nabaa M. Ibrahim and Thukaa Z. Abdul-jalil*
and Thukaa Z. Abdul-jalil*
Department of pharmacognosy and Medicinal plants, college of pharmacy, University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail: thukaazuhaira@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2624
Abstract
Morus alba, member of the Moraceae family, is a perennial tree utilized in folk medicine, preparing the modern drug, and considered the main food for silkworms. However, data on chemical content in the leaves is still limited; the main objective of this study is to detect the presence and determine the concentration of different polyphenolic constituents in the leaves of the Morus alba plant by reverse phase-high performance liquid chromatography (RP-HPLC) and evaluate the cytotoxic effect of ethyl acetate extract of this plant on human breast cancer (AMJ-13) cell line. Phytochemical analysis of the Morus alba leaves ethyl acetate extract led to identifying and quantification of six polyphenolic constituents designated as phenolic acids (caffeic, chlorogenic, and p-coumaric acid), catechins (epicatechin) and flavonoids (luteolin and apigenin) in which p-coumaric acid exhibited the highest concentration follow by luteolin (identified for the first time), chlorogenic acid, caffeic acid, apigenin and epicatechin as the least. According to estimates, the ethyl acetate extract with a high concentration of polyphenolic constituents gave the best findings as cytotoxic against breast cancer AMJ-13 cell line with an IC50 value of 129.5 μg/ml.
Keywords
AMJ-13 cell line; Catechins; flavonoids; HPLC; Phenolic acids
Download this article as:| Copy the following to cite this article: Ibrahim R. M, Ibrahim N. M, Abdul-Jalil T. Z. Polyphenolic Profiles and Cytotoxic Effect of Iraqi Morus alba leaves Ethyl Acetate Extract. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Ibrahim R. M, Ibrahim N. M, Abdul-Jalil T. Z. Polyphenolic Profiles and Cytotoxic Effect of Iraqi Morus alba leaves Ethyl Acetate Extract. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3HLtv3p |
Introduction
Morus alba L. (of family Moraceae) is a deciduous, monoecious tree of moderate size with 1.8 meters in width and 3 meters in height 1. It is native to Japan, China, and India and is sometimes distributed in other places like North America, Europe, and Africa. It is commonly named white mulberry. Morus alba is distributed where silkworms are elevated throughout the world. Its leaf is the main food behoof for silkworms 2. The leaves of the mulberry are used as powdered juice and tea in Japan. White mulberry is utilized in various parts of the world as a vegetable and is also grown for fruit production in European countries 1. The root bark, fruit, and leaf of white mulberry have a prolonged history in conventional medicine of Chinese. Its root bark is an expectorant, anti-inflammatory, antitussive, and diuretic agent. The mulberry fruit act as an analgesic and tonic agent. The mulberry leaves can be used for antitussive, pyrolysis treatment, blood cooling, and improvement of eye problems 3. As it is rich in minerals and contains metabolizable energy, protein, and small anti-nutritional agents such as tannic acid, mulberry leaves have been considered an alternative source of protein for poultry production. White mulberry is an excellent source of β-carotene, ascorbic acid, and antioxidant compounds like rutin 4. Also, this plant contains several active phytochemical compounds such as flavonoids, phytosterols, tannins, sitosterols, triterpenes, saponins, benzofuran derivatives, anthocyanins, anthraquinones glycosides, oleanolic acid, and others5. In addition to the nutritive value, the leaves of the mulberry are safe and normal medicinal agents reported to have antimicrobial, antidiabetic, antimutagenic, anticancer, antioxidant, anxiolytic, antistress, anthelmintic, immunomodulatory, nephroprotective, hypocholesterolemic, hepatoprotective 6-8, hypouricemic 9, neuroprotective 10, anti-inflammatory 11, and cardioprotective actions 12. To best knowledge, few reports have been published on Morus alba, so it sparked our interest in conducting scientific research on the polyphenolic composition and cytotoxic activity of Iraqi Morus alba ethyl acetate leaves extract.
 |
Figure 1: Photo of Iraqi Morus alba plant. |
Materials and Methods
Plant material
Morus alba leaves (Family Moraceae) were cultivated and assembled in Baghdad, Iraq. The plant leaves were washed with tap water (H2O), dehydrated in shadow (at room temperature), and then ground as powder by mortar and pestle.
Preparation of Morus alba extract
Extraction was made with some modifications as process mentioned by Iswantini et al. 14. Powder leaves of Morus alba (25 g) were macerated with 250 ml of n-hexane for one day and then filtrated. The plant materials left were extracted with 250 ml of aqueous ethanol (ethanol-water 85:15 v/v) by soxhlet apparatus for 12 hrs. The crude extract was filtered and reduced in volumes under vacuum to obtain a dry residue. The dried extract was hanging in the water and fractionated with ethyl acetate (100 ml x 2), as shown in figure 2. The ethyl acetate extract was dried under vacuum to give 0.408 gm, and the percentage yield was 1.632% and then stored for further examination.
 |
Figure 2: Scheme of extraction, polyphenolic determination, and cytotoxic evaluation of Iraqi Morus alba. |
Qualitative and Quantitative determination of phenolic compounds (flavonoid, phenolic acid, and catechin) by RP-HPLC
RP-HPLC technique is used in the identification, and quantitative estimation of phenolic acids (caffeic, chlorogenic, and p-coumaric acid), catechins (epicatechin), and flavonoids (luteolin and apigenin) in the Morus alba ethyl acetate extract of leaf part, in which using a mobile phase (Isocratic) consist of acetonitrile: water (1:1). The C18 Column (250 mm x 4.6 mm, with particle size 5 μm) was utilized as stationary phase. The 20 μL injection volume and 1.0 ml/min flow rate were used, and the injection concentration was 1 mg /ml. The UV detection was performed at 265 nm 15.
Cytotoxic evaluation of the ethyl acetate extract of Morus alba:
To evaluate the cytotoxic effect of Morus alba ethyl acetate extract of leaf part, breast cancer cell (AMJ13) was taken from an Iraqi patient with breast cancer and maintained in RPMI-1640, supplemented with penicillin (100 unit/mL), streptomycin (100 µg/mL), and fetal bovine (10%). The cells were passaged using Trypsin-EDTA, incubated at 37 °C, and reseed twice per 7 days at 50% confluence 16.
On 96-well plates, the MTT test was performed, and at 1×104 cells per well, the cell line was seeded 17. Cells were treated with the serial concentrations of ethyl acetate fraction (tested compound) when a confluent monolayer was done (after 24 hrs.). After treatment with 72 hrs, the viability of the cell was determined through the elimination of the intermedia, a solution of MTT (28 µL) was inserted, and the cells were incubated for about 1.5 hrs. at 37 °C. After the solution of MTT was eliminated, the residual crystal was dissolved in the wells through an addendum of Dimethyl Sulphoxide (130 µL), then incubated for about 15 min (at 37 °C) with stirring 18. The test was done in triplicate, and the absorbance was computed at 492 nm (wavelength of the test) on a microplate reader. The cell growth inhibitory rate (the cytotoxicity percentage) was determined according to the next equation 19:
% Cell viability = (absorbance of treated cell / Absorbance of non-treated cell) X 100
% Cytotoxicity = 100 – cell viability
Statistical analysis
Data obtained statistically utilizing the unpaired T-test were analyzed with Graph Pad Prism 6. For the triplicate measurements, values were given as mean ± SD 20.
Results and discussion
Qualitative and Quantitative determination of phenolic compounds by RP-HPLC
The qualitative estimation of phenolic acids, flavonoids, and catechins in the Morus alba ethyl acetate extract by RP-HPLC was made by matching the retention time of six standards (caffeic, chlorogenic, and p-coumaric acid, apigenin, luteolin, and epicatechin) with plant extract at identical chromatographic condition.
The results of RP-HPLC revealed the existence of phenolic acids (caffeic, chlorogenic, and p-coumaric acid), catechins (epicatechin), and flavonoids (luteolin and apigenin) in the plant leaves; in which the retention time of these detected compounds in the plant extract was identical with their standards, as shown in figures 3-9.
The quantitative estimation of caffeic, chlorogenic, and p-coumaric acid, apigenin, luteolin, and epicatechin were achieved by utilizing a calibration chart, which a series of diluted solutions of each standard was constructed from 100 ppm as stock solution. The concentrations of these compounds in the Morus alba ethyl acetate extract were measured by a straight-line equation obtained after plotting the concentration of serial dilutions of each standard versus the area under the curve, as shown in figures 10-15.
The results show that the concentration of p-coumaric acid was higher than the concentration of other constituents in the sample (ethyl acetate extract), and the amounts of phenolic acids are higher than that of flavonoids in Morus alba leaves, as shown in table (1).
Table 1: Concentration (ppm) of two flavonoids, three phenolic acids, and one catechin in Morus alba leaves ethyl acetate extract.
| Active constituents | Concentration (ppm) |
| Caffeic Acid | 942.31 |
| P-Coumaric Acid | 1530.29 |
| Chlorogenic Acid | 1025.36 |
| Apigenin | 494.59 |
| Luteolin | 1142.94 |
| Epicatechin | 484.21 |
 |
Figure 3: HPLC chromatogram of std. caffeic acid. |
 |
Figure 4: HPLC chromatogram of std. p-coumaric acid. |
 |
Figure 5: HPLC chromatogram of std. chlorogenic acid. |
 |
Figure 6: HPLC chromatogram of std. apigenin. |
 |
Figure 7: HPLC chromatogram of std. Luteolin. |
 |
Figure 8: HPLC chromatogram of std. epicatechin. |
 |
Figure 9: HPLC chromatogram of Morus alba ethyl acetate extract. |
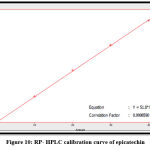 |
Figure 10: RP- HPLC calibration curve of epicatechin |
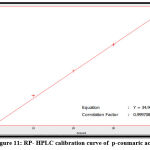 |
Figure 11: RP- HPLC calibration curve of p-coumaric acid. |
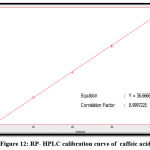 |
Figure 12: RP- HPLC calibration curve of caffeic acid. |
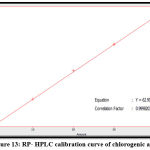 |
Figure 13: RP- HPLC calibration curve of chlorogenic acid. |
 |
Figure 14: RP- HPLC calibration curve of luteolin. |
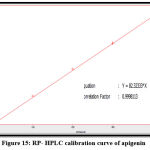 |
Figure 15: RP- HPLC calibration curve of apigenin. |
Cytotoxic assay
The cytotoxic assay was done to investigate the cytotoxicity of Morus alba leaves ethyl acetate extract on the human tumor cell line, breast ductal carcinoma AMJ-13 cells, by the MTT test.
The Morus alba leaves ethyl acetate extract revealed a cytotoxic effect verse the AMJ-13 cell line with a maximum cytotoxic activity at 1000 µg/mL and a minimum cytotoxic activity at 31.2 µg/mL as shown in figure 16 and table 2. This effect depends on the concentration.
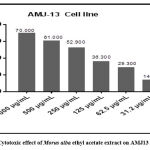 |
Figure 16: Cytotoxic effect of Morus alba ethyl acetate extract on AMJ13 cell growth. |
Table 2: Percentage of cytotoxicity (%) of Morus alba ethyl acetate extract at several concentrations.
| Concentration | 1000 µg/mL | 500 µg/mL | 250 µg/mL | 125 µg/mL | 62.5 µg/mL | 31.2 µg/mL |
| % Cytotoxicity | 70.00 | 61.00 | 52.900 | 36.300 | 29.300 | 14.30 |
The IC50 stimulating 50% inhibition of cell growth to the tested sample was129.5 μg/ml (figure 17). AMJ13 cell Morphology after treatment and before treatment with Morus alba leaves ethyl acetate extract were observed in figure 18.
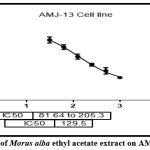 |
Figure 17: IC50 of Morus alba ethyl acetate extract on AMJ-13 cell line. |
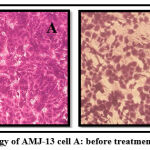 |
Figure 18: Morphology of AMJ-13 cell A: before treatment, B: After treatment. |
The phytochemical investigation revealed the existence of polyphenolic constituents in the Morus alba ethyl acetate extract. Phenolic compounds owned free radicals scavenging and antioxidant properties. It has been shown anticancer effects affecting several main elements back to apoptosis, cell proliferation, metastasis, and angiogenesis 21.
Conclusion
This study showed that the phytochemical found in leaves extract of Iraqi Morus alba could be considered an important source of medicine and vital for good health. In this study, six polyphenolic compounds have been identified, some of them for the first time, in the leaves extract of Morus alba by RP-HPLC. The result shows that the RP-HPLC method can be adopted for qualitative and quantitative determinations of phenolic acids, flavonoids, and catechins in Morus alba dried leaves extract. In addition, the dried leaves ethyl acetate extract of this plant was active against breast cancer AMJ13 cell line with IC50 value of 129.5 μg/ml, and the good cytotoxic property of white mulberry is mainly related to the existence of polyphenolic compounds and other related compounds.
Acknowledgment
I am beholden to the college of the pharmacy / University of Baghdad to award the facilities and the opportunity to do my work.
Conflict of Interest
There is no conflict of interst.
References
- Zafar MS, Muhammad F, Javed I, Akhtar M, Khaliq T, Aslam B, Waheed A, Yasmin R, and Zafar H . White mulberry (Morus alba): A brief phytochemical and pharmacological evaluations account. Int. J. Agric. Biol., 2013; 15: 612‒620.
- Yadav AV and Nade VS. anti-dopaminergic effect of the methanolic extract of Morus albaL. leaves .Indian J Pharmacol. 2008 ; 40(5): 221–226.
CrossRef - Kobayashi Y, Miyazawa M, Araki M, Kamei A, Abe K, et al. Effects of Morus alba L.(Mulberry) Leaf Extract in Hypercholesterolemic Mice on Suppression of Cholesterol Synthesis. J Pharmacogn Nat Prod, 2015; 1 (2): 113.
CrossRef - Ustundag AO and Ozdogan M. Usage Possibilities Of Mulberry Leaves In Poultry Nutrition. Scientific Papers. Series D. Animal Science.2015; 58:170- 178.
- Chen C, Liu L, Huang H, Yang M, Wang C. Mulberry extract inhibits the development of atherosclerosis in cholesterol fed rabbits. Food Chem. 2005; 91:601–7.
CrossRef - Devi B, Sharma N, Kumar D, Jeet K. Morus alba Linn: A phytopharmacological review. International Journal of Pharmacy and Pharmaceutical Sciences, 2013; 5(2): 14-18.
- Yang JY, Lee HS. Evaluation of antioxidant and antibacterial activities of morin isolated mulberry fruits (Morus alba L.). Journal of the Korean Society for Applied Biological Chemistry,2012; 55: 485-489.
CrossRef - Yang NC, Jhou KY, Tseng CY. Antihypertensive effect of mulberry leaf aqueous extract containing c-aminobutyric acid in spontaneously hypertensive rats. Food Chemistry, 2012;132: 1796-1801.
CrossRef - Cheyl L. Ethnomedicines used in Trinidad and Tobago for urinary problem and Diabetes mellitus. J Ethnobiol Ethnomedicine. 2006;13:45–51.
- Kang TH, Oh HR, Jung SM, Ryu JH, Park MW, Park YK, et al. Enhancement of neuroprotection of Mulberry leaves (Morus alba L.) prepared by the anaerobic treatment against ischemic damage. Biol Pharm Bull. 2006;29 :270–274.
CrossRef - Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH and Moon JO. “In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L.”, Journal of Pharmacy and Pharmacology, 2003;55 :1695–700.
CrossRef - Enkhmaa B, Shiwaku K, Katsube T, Kitajima K, Anuurad E, Yamasaki M, et al. Mulberry (Morus alba L.) leaves and their major flavonol Quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor deficient mice. J Nutr. 2005; 135 :729–34.
CrossRef - Gao K, Zheng C, Wang T, Zhao H, Wang J, Wang Z, Zhai X, Jia Z, Chen J, Zhou Y, Wang W. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules. 2016; 21(11):1600.
CrossRef - Iswantini D, Ramdhani TH, and Darusman LK. In vitro inhibition of celery (apium graveolens ) extract on the activity of xanthine oxidase and determination of its active compound. Indo. J. Chem., 2012, 12 (3): 247 – 254.
CrossRef
- Dubber MJ, Kanfer I. High-performance liquid chromatographic determination of selected flavonols in Ginkgo biloba solid oral dosage forms. J Pharm Pharm Sci. 2004; 7(3):303–9.
- Adil BH, Al-Shammari AM, Murbat HH. Breast cancer treatment using cold atmospheric plasma generated by the FE-DBD scheme. Clinical Plasma Medicine. 2020;19-20.
CrossRef - Abdullah SA, Al-Shammari AM, Lateef SA. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi Journal of Biological Sciences. 2020;27(3):865-72.
CrossRef - Al-Shammari AM, Jalill RDA, Hussein MF. Combined therapy of oncolytic Newcastle disease virus and rhizomes extract of Rheum ribes enhances cancer virotherapy in vitro and in vivo. Molecular Biology Reports. 2020;47(3):1691-702.
CrossRef - Al-Hussaniy HA. The Effect of MicroRNA-409-3p for Treatment and Response to Tumor Proliferation of Lung Cancer Cell Lines (In Vitro). Asian Pacific Journal of Cancer Prevention. 2022;23(9):3151-6.
CrossRef - Mohammed MS, Al-Taee MF, Al-Shammari AM. Caspase dependent and independent anti-hematological malignancy activity of AMHA1 attenuated newcastle disease virus. International Journal of Molecular and Cellular Medicine. 2019;8(3):211-22.
- Al-Ziaydi AG, Al-Shammari AM, Hamzah MI, Kadhim HS, Jabir MS. Newcastle disease virus suppress glycolysis pathway and induce breast cancer cells death. VirusDisease. 2020;31(3):341-8.
CrossRef - Basli A, Belkacem N, Amrani I. Health Benefits of Phenolic Compounds against Cancers. In Phenolic Compounds—Biological Activity; IntechOpen: London, UK, 2017;193–210.
CrossRef








