Armerinayanti NW1 , Bakta IM,2
, Bakta IM,2 Alit Artha IG3
Alit Artha IG3 , Wahyuniari IAI4
, Wahyuniari IAI4 and Samuel Widodo1
and Samuel Widodo1
1Anatomic Pathology Department, Faculty of Medicine, Warmadewa University, Indonesia
2Internal Medicine Department, Faculty of Medicine Udayana University, Indonesia
3Anatomic Pathology Department, Faculty of Medicine Udayana University, Indonesia
4Histologic Department, Faculty of Medicine Udayana University, Indonesia
Corresponding Author E-mail: armerinayantipranata@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2628
Abstract
Cervical carcinoma being the second common cancer in women in Indonesia, as well as in Bali, and mostly patients are diagnosed at an advanced stage with having metastases. The lymphatic pathway (lymph nodes) is the most frequent route for cervical cancer metastases. MicroRNA is a novel invention for predicting the biological behavior of cervical carcinoma and has the potential to act as the foundation for targeted therapy for cervical cancer. Several microRNA profiles, including microRNA 21, microRNA 126, and microRNA 143, were discovered to regulate the biological activity of cervical cancer. However, no studies have established a correlation between the expression of one of these microRNAs and the incidence of lymph node metastases in cervical cancer. This study aims to analyze whether overexpression of microRNA 21 is a risk factor for lymph node metastases in cervical cell carcinoma. Collected data was descriptively analyzed using the chi-square test with a p-value<0.05 and 95% CI. The results showed that microRNA 21 was significantly overexpressed in cervical carcinomas with lymph node metastases compared to those without lymph node metastases, representing a 19-fold increased risk for lymph node metastases. This can be influenced by the activity of microRNA 21 on several signaling pathways, such as Phosphatase and Tensin Homolog (PTEN), Programmed Cell Death Protein 4 (PDCD4), and Tissue inhibitor of metalloproteinase 3 (TIMP3), that affect the progression, invasion capacity, and metastasis of tumor cells.
Keywords
Cervical Carcinoma; lymph node; metastases; microRNA 21 Overexpression; Risk Factor
Download this article as:| Copy the following to cite this article: Armerinayanti N. W, Bakta I. M, Artha I. G. A, Wahyuniari I. A. I, Widodo S. Overexpression of MicroRNA 21 in Cervical Carcinoma with Lymph Node Metastasis. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Armerinayanti N. W, Bakta I. M, Artha I. G. A, Wahyuniari I. A. I, Widodo S. Overexpression of MicroRNA 21 in Cervical Carcinoma with Lymph Node Metastasis. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3FsBgv4 |
Introduction
Cervical carcinoma is the fourth most prevalent malignancy in women worldwide and one of the top three among women under 45 years old. This number has decreased as a result of the successful implementation of the HPV vaccine program, screening and treatment of precancerous lesions, and an increase in the number of healthcare providers participating in the diagnosis and treatment of invasive cancer. In contrast, the incidence by age standardization remains high in Indonesia, particularly in Bali, with >40 instances per 100.000 women.1Cervical carcinoma originating from cervix epithelium is still a public health issue in Indonesia and Bali. Cervical squamous cell carcinoma is the most prevalent type of cervical carcinoma, comprising for around 70% of cases.2,3
In 2008, the Indonesian Cancer Foundation (YKI) reported that 52 million Indonesian women were at risk for cervical carcinoma and that 36% of all cancer patients were women with cervical carcinoma.1,2Despite undergoing treatment, the mortality rate for cervical carcinoma remains about 90%, with the majority of patients dying of metastases. Metastasis is alarming for cancer patients since it represents the tumor’s aggressiveness and is related to a poor prognosis. The lymphatic pathway (lymph nodes) is the most common route for cervical carcinoma metastases.2,3
In cervical cancer, determining the status of the inguinal lymph nodes is crucial because it serves as a prognostic indicator of distant metastases, recurrence, and appropriate therapy. However, the status of the lymph nodes cannot always be determined because clinicians do not always perform lymphadectomy. Determining the prognostic characteristics related with metastases is critical, as this will affect future treatment. Classic prognostic variables used to predict the occurrence of metastases include tumor size, grade, stage, lymphovascular invasion, and resection margins, with researchers obtaining varying findings. Therefore, new measures that correlate with the risk of metastasis in cervical cancer are required. Metastases of the lymph nodes can only be identified through histopathological examination of radical surgical tissue.1,3,4Predicting the possibility of lymph node metastases can help with the prevention of lymph node metastases and also in determining whether the clinician will perform surgery and if the patient requires radical action.5,6
Several parameters have been developed that could also assist clinicians in predicting the possibility of lymph node metastasis development.7,8 One of the most investigated biomolecular parameters is the expression profile of microRNAs (miRNAs), a class of endogenous non-coding RNAs that act as negative regulators/suppressors of protein-coding gene expression by selectively binding to the base complement 3 untranslated region (3-UTR) of messenger RNAs (mRNAs). Several miRNA profiles, including miR-21, miR-126, and miR-143, have been identified as playing a role in determining the biological behavior of cervical carcinoma.9,10
A recent study has investigated of the important role of miR-21in cervical carcinoma. In cervical carcinoma, miR-21 is classified as an oncogene miR because it stimulates cell proliferation and migration. The expression levels of miR-21 and ZEB1 (Zinc finger E-box binding homeobox 1 transcription factor) were significantly higher in cervical carcinoma tissue with lymph node metastases than in normal cervical tissue, according to previous research. In a recent study, it was discovered that the upregulation of miR-21 in cervical carcinoma tissue modulates EMT (Epithelial-Mesenchymal Transition) activation, which in turn induces lymphatic metastasis. In 2020, Sánchez conducted opinion on the overexpression of miR-21 in cervical cancer cell lines HeLa and SiHa, which led to the upregulation of mesenchymal cell markers vimentin and N-cadherin and the decreased expression of epithelial cell marker E-cadherin.11 The EMT is the ability of epithelial cells to transform from immobile to motile mesenchymal progenitor cells. This pathway is essential for development (type 1), normal tissue regeneration or pathological fibrosis (type 2), and cancer cell metastatic transformation (type 3). Type 3 EMT is essential for tumor progression to metastasis, and both reactivation and dedifferentiation as well as activated cancer cells are generated to have an invasive and motile phenotype.4,10
In addition, previous studies discovered a significant correlation between miR-21 overexpression with muscular infiltration, and parametrial invasion. Multiple studies have shown that elevated miR-21 expression is related to cell proliferation, tumor invasion, metastasis, angiogenesis, immunological response, and apoptosis inhibition in cervical cancer cases.5,6The aim of this study was to investigate the role of miR-21 overexpression of lymph node metastases in cervical carcinoma.
Materials and methods
This study used a case-control study design to explore the correlation between miR-21 overexpression and the presence of lymph node metastases in cervical carcinoma.In addition, descriptive table data was used to present other findings such as the patient’s stage and age group.
The research was conducted from July 2021 – December 2021 in the Biomolecular Laboratory of the Faculty of Medicine and Health Sciences at Warmadewa University (evaluation of preparations may be conducted at the researcher’s place).
The population of interest for this study included all cervical carcinoma samples collected at Balimed Denpasar Hospital on 2021. The research sample was obtained from samples of cervical squamous cell carcinoma from radical surgical tissue collected at the Biomolecular Laboratory of the Faculty of Medicine and Health Sciences at Warmadewa University based on inclusion and exclusion criteria. Calculation of the sample is based on the following formula:
The significance level for the 95% confidence interval (za) is 1.960 and the power value (zb) is 0.842. The p-value is determined by dividing the odds ratio which is considered clinically significant with the formula P = R/1+R so that a value of 3/4 is obtained, while the value of Q=1-P is 1/4. Based on this formula, the total minimum number of samples is 29.8 rounded up to 30. In this study, the number of samples in the group with lymph node metastases was 15 people while those with non-metastatic lymph nodes were 15 people. Overexpression of miR-21 is defined by the presence of high levels of oncogenic microRNA (oncomir), which inhibits the expression of tumor suppressor genes (TS genes) and genes that regulate cell differentiation and apoptosis on chromosome 17. Also miR-21 is considered overexpressed if its expression is at least five-fold higher than that of the reference sample (u6 s RNA)1.9Lymph node metastasis is the dissemination of tumor cells beyond the original tumor, specifically in regional lymph node tissue and beyond the pelvis.6
The data was descriptively analyzed using the chi-square test to determine the correlation between miR-21 overexpression and lymph node metastases in cervical carcinoma. The significance test was determined at p<0.05. Data precision is determined by a 95% Confident Interval (CI).
Results and Discussion
Case Distribution Based on Patient Clinical Data
The majority of cervical carcinoma cases (63.3%) were diagnosed in women younger than 50 years old, according to research data. This is congruent with findings from the International Agency for Research on Cancer (IARC) indicating that cervical carcinoma is one of the top three cancers among women aged 45. This may be due to the fact that sexual activity, a risk factor for cervical carcinoma, is most prevalent in this age group, which corresponds to the reproductive age range. Epidemiological studies indicate that HPV infection is most prevalent in women in their twenties, as determined by the detection of HPV DNA. It takes between 10 to 20 years from the presence of HPV infection to the development of cervical carcinoma, hence the prevalence of cervical cancer in the third and fourth decades is considerable.1,4,5
Table 1: Research sample characteristics
|
Characteristics |
n=30 (%) |
| Age (year) | |
| <50 | 19 (63.3%) |
| >/=50 | 11 (36.7%) |
| Tumor Size (cm) | |
| <4 | 9 (30%) |
| >/=4 | 21 (70%) |
| Lymph node metastases group | |
| 1) positive metastases | 15 (50%) |
| 2) negative metastases | 15 (50%) |
According to table 1, 70% of the tumors in this study group were larger than or equal to four centimeters. This relates to the selection of study samples that use radical surgical tissue, which is typically obtained from cervical carcinomas that are apparent to the naked eye (above stage IB1). In developing nations, such as Indonesia, cervical cancer is still diagnosed at a more advanced stage, where the size of the tumor exceeds 4 centimeters and already causes serious clinical symptoms such as profuse bleeding and spread to surrounding tissues such as the vagina and bladder.
Correlation between Overexpression of MicroRNA-21 with Patient Clinical Data
Quantitative real-time PCR showed no significant association between miR-21 overexpression and the patient’s age or tumor size (p>0.05). This is consistent with findings from prior studies indicating that miR-21 expression is unaffected by age or the aging process. The miR-21 is a small non-coding RNA molecule whose biological control is correlated to its role in immunological activity, cell differentiation, and carcinogenesis in diverse types of tumors.12,13
Table 2: Correlation between miR-21 overexpression and research sample clinical data
| Characteristics | n=30 (%) | miR-21 Overexpression (n) | p value |
| Age (year) | |||
| <50 | 19 (63.3%) | 12 | 0.592 |
| >/=50 | 11 (36.7%) | 8 | |
| Tumor Size (cm) | |||
| <4 | 9 (30%) | 6 | 0.037 |
| >/=4 | 21 (70%) | 14 |
The contrary association was noticed between tumor growth and miR-21 overexpression (p<0.05). This is consistent with the findings of numerous studies indicating that miR-21 expression is implicated in several hallmarks of carcinogenesis, including tumor proliferative activity, tumor development, and the capacity to invade and metastasis 2,3 .
The MiR-21 is associated with cell proliferation by inhibiting multiple protein targets, including Programmed Cell Death Protein 4 (PDCD4), Sprouty RTK SignallingAntagonist 2 (SPRY2), Phosphatase and Tensin Homolog (PTEN), and Reversion-Inducing Cysteine-Rich Protein with Kazal motifs (RECK) via the mechanism depicted in figure 2. In addition, several studies demonstrated that Mitogen-Activated Protein Kinase/Extracellular Receptor Kinase (MAPK/ERK) and Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/AKT) are the signalling pathways targeted by miR-21 in influencing cell proliferation.6,7
Correlation Between Overexpression of MicroRNA-21 with Lymph Node Metastasis
In this study, miR-21 expression was evaluated in both sample groups using quantitative real-time PCR (cervical carcinoma with lymph node metastases and those without lymph node metastases). Normal cervical tissue samples were used as the negative control to analyze the multiple expression of miR-21 between the two groups in this investigation, with U6 sRNA serving as the internal control.
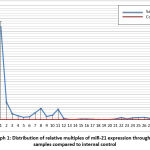 |
Graph 1: Distribution of relative multiples of miR-21 expression throughout samples compared to internal control. |
According to graph 1, both sample groups had a considerably higher multiple of expression compared to negative internal controls. In addition, miR-21 was detected in both sets of study samples, including those with lymph node metastases and those without lymph node metastases. It is known that the carcinogenesis of cervical carcinoma is consistently related to previous infections with high-risk HPV types 16 and 18. The miR-21 genomic locus resides in the FRA17B locus of chromosome 17q 23.2, which is one of the HPV 16 integration sites. MiR-21 expression in Cervical carcinoma is a marker for HPV 16 integration factor involvement. Because miR-21 is involved in the carcinogenesis process of cervical cancer, it may be deduced that, in general, cases of cervical cancer will have higher miR-21 expression than normal cervical tissue.4–6
The activation of theI3K and its target, AKT, and mTOR signalling pathways, which regulate differentiation, proliferation, and cell survival, play a role in the continuation of the cervical carcinoma carcinogenesis process. By activating the PI3K/AKT/mTOR signalling pathway, tumor cell proliferation is increased.5,14
The expression of microRNAs increased significantly in the cervical carcinoma group with lymph node metastases compared to the group without lymph node metastases (p=0.000; p<0.05). The relative expression multiples in samples with lymph node metastases were higher than five times the expression of the reference control, hence they were classified as overexpressed. miR-21 overexpression increases the risk of cervical carcinoma lymph node metastasis by 19-fold. This is congruent with results regarding the molecular mechanism of miR-21’s participation in inducing invasion and metastasis in a variety of solid carcinomas; thus, miR-21 is recognized as a metasmiR. Involved molecular mechanisms may include suppression of the activation of PDCD4, TIMP3, TPM1, SERPINB5, coding for Maspin, and PTEN, among others. If PDCD4 is blocked, there will be a decrease in JNK activation, resulting in an increase in BCL2-like (anti-apoptotic) proteins, which prevents apoptosis and eventually contributes to tumor cell proliferation and migration.14,15,16
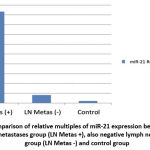 |
Graph 2: Comparison of relative multiples of miR-21 expression between positive lymph node metastases group (LN Metas +), also negative lymph node metastases group (LN Metas -) and control group |
Graph 2 further shows that PTEN activity can suppress the PI3K/PTEN/AKT signalling pathway and target AKT to modulate cellular processes such as cell growth, differentiation, proliferation, and migration. Overexpression of miR-21 can downregulate PTEN, hence maintaining the activation of the PI3K/PTEN/AKT signalling pathway, which affects tumor proliferation and progression. PTEN can also inhibit MMP-9 and MMP-2 expression by dephosphorylating FAK. Thus, inhibition of PTEN activity would result in the overexpression of matrix metalloproteinases such as MMP2 and MMP9, which promote migration, cellular invasion, and metastasis, including lymph node metastasis.1,2,5,10,17 In this study, the overexpression of miR-21 in lymph node metastasis samples is most likely influenced by the inhibition of this PTEN pathway.
Conclusion
It can be concluded that, miR-21 expression was significantly higher in cervical carcinomas with lymph node metastases compared to cervical carcinomas without lymph node metastases, furthermore, cervical carcinomas with lymph node metastases showed overexpression of miR-21. In cervical carcinoma, overexpression of miR-21 multiplies the risk of lymph node metastasis by 19-fold. This relates to the wide range of molecular roles of miR-21 in various signalling pathways of tumor progression, migration, and metastasis, such as inhibition of PTEN, which triggers anti-apoptotic activity, and activation of metalloproteinases such as MMP-9, which affect the emergence of EMT phenotypes in cervical carcinoma, thereby facilitating deeper and deeper invasion. In addition, this study also showed that miR-21 overexpression has an impact on tumor size, since there was a significant difference between tumors with a size of ³4 cm and those with a size of <4 cm with regard to miR-21 overexpression.
Acknowledgement
We would like to express our deepest appreciation to the Biomolecular Laboratory of the Faculty of Medicine and Health Sciences at Warmadewa University and The Anatomical Pathology Laboratory at Balimed Denpasar Hospital, which has given the infrastructure and facilities necessary for this research to be conducted.
Conflict of Interest
There are no Conflict of Interest
Funding Source
This research was supported by grants from institutions provided by the Faculty of Medicine and Health Sciences at Warmadewa University. The grant number is 098/Unwar/FKIK/UP2M/UR-21/IV/2020.
References
- Andrijono, et al. Panduan Penatalaksanaan Kanker Serviks. Komite Penanggulangan Kanker Nasional. 2013.pp 1-30. Available at: http://kanker.kemkes.go.id/guidelines/PPKServiks.pdf [Accessed: 29 January 2021].
CrossRef - Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). 2019.
CrossRef - Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12).
CrossRef - Endo D, Todo Y, Okamoto K, Minobe S, Kato H, Nishiyama N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: A retrospective analysis in a japanese cohort. J Gynecol Oncol. 2015;26(1).
CrossRef - Haie-Meder C, Morice P, Castiglione M. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21(SUPPL. 5).
CrossRef - Huang B, Cai J, Xu X, Guo S, Wang Z. High-grade tumor budding stratifies early-stage cervical cancer with recurrence risk. PLoS One. 2016;11(11).
CrossRef - Baeta S, dkk. 2019. The Role of Human Papillomavirus in Cervical Cancer. International Journal of Cancer and Clinical Research 6(5). Available at: http://dx.doi.org/10.23937/2378-3419/1410125 [Accessed: 29 March 2021].
CrossRef - Kumar V, Abbas A, Aster J, Turner J. Robbins & Cotran Pathologic Basis of Disease Tenth Edition, Chapter 22. The Female Genital Tract. Elsevier.pp. 1017-1037. 2015.
- Li X, Yin Y, Sheng X, Han X, Sun L, Lu C, et al. Distribution pattern of lymph node metastases and its implication in individualized radiotherapeutic clinical target volume delineation of regional lymph nodes in patients with stage IA to IIA cervical cancer. Radiation Oncology. 2015;10(1).
CrossRef - Sun T, Qin Y, Zhong W long. Epithelial-Mesenchymal Transition and its Regulation in Tumor Metastasis. In: Tumor Metastasis. vol.10, pp.217-239, 2016.
CrossRef - Bautista-Sánchez D, Arriaga-Canon C, Pedroza-Torres A, de La Rosa-Velázquez IA, González-Barrios R, Contreras-Espinosa L, et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Vol. 20, Molecular Therapy – Nucleic Acids. vol. 20, pp. 409-420, 2020.
CrossRef - Wang A, Xu Q, Sha R, Bao T, Xi X, Guo G. MicroRNA-29a inhibits cell proliferation and arrests cell cycle by modulating p16 methylation in cervical cancer. Oncol Lett. 2021;21(4).
CrossRef - Yao T, Lin Z. MiR-21 is involved in cervical squamous cell tumorigenesis and regulates CCL20. BiochimBiophys Acta Mol Basis Dis. 2012;1822(2).
CrossRef - Banno K, Iida M, Yanokura M, Kisu I, Iwata T, Tominaga E, et al. MicroRNA in cervical cancer: OncomiRs and tumor suppressor miRs in diagnosis and treatment. T Xing,B., Guo, J., Sheng, Y., Wu, G., Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Frontiers in Oncology. Vol.10; 1-8, 2021. doi: 10.3389/fonc.2020.606335.
CrossRef - Du, G., Cao, D., & Meng, L. 2017. MiR-21 inhibitor suppresses cell proliferation and colony formation through regulating the PTEN/AKT pathway and improves paclitaxel sensitivity in cervical cancer cells. Molecular Medicine Reports, 15(5), 2713–2719. https://doi.org/10.3892/mmr.2017.6340 [Accessed: 29 March 2021].
CrossRef - Deftereos, G., Corrie, S. R., Feng, Q., Morihara, J., Stern, J., Hawes, S. E., & Kiviat, N. B. 2011. Expression of MIR-21 and Mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS ONE, 6(12). https://doi.org/10.1371/journal.pone.0028423 [Accessed: 29 March 2021].
CrossRef - Feng, H.Y., Tsao, C.J. 2016. Emerging role of microRNA-21 in cancer. Biomedical Report 5: 395-402
CrossRef








